Abstract
We have previously shown that vaccination with a vaccine based on the recombinant N-terminal domain of Als1p (rAls1p-N) protected BALB/c mice against disseminated infection caused by a single strain of Candida albicans (A. S. Ibrahim, B. J. Spellberg, V. Avenissian, Y. Fu, S. G. Filler, and J. E. Edwards, Jr., Infect. Immun. 73:999-1005, 2005, and B. J. Spellberg, A. S. Ibrahim, V. Avenissian, S. G. Filler, C. Myers, Y. Fu, and J. E. Edwards, Jr., Infect. Immun. 73:6191-6193, 2005). Here we show that the rAls1p-N vaccine also improves survival of outbred mice from disseminated candidiasis and that it is active against multiple virulent strains of C. albicans and non-C. albicans spp.
Candida spp. are the third most common nosocomial bloodstream isolates (15), exceeded in frequency only by coagulase-negative staphylococci and Staphylococcus aureus. Disseminated candidiasis has an attributable mortality of 40 to 50%, even with modern antifungal therapy (3, 7, 8, 14). Clearly, new strategies to prevent Candida infections are needed.
Disseminated candidiasis typically occurs after multiple weeks of hospitalization (15), affording the opportunity to intervene prior to disease onset. Furthermore, the major clinical risk factors for developing disseminated candidiasis have been well described (12), facilitating identification of at-risk patients prior to disease onset. Therefore, vaccination of targeted patient populations is an ideal strategy to prevent or ameliorate this life-threatening infection.
We have developed a vaccine based on an adhesin from C. albicans. Immunization with the recombinant N-terminal domain of Als1p (rAls1p-N) resulted in a marked improvement in survival of both immunocompetent and immunocompromised mice infected via the tail vein with an otherwise lethal inoculum of C. albicans (6, 13). We have recently reported that the mice in our murine model of hematogenously disseminated candidiasis die of septic shock (11). As candidal sepsis in humans is associated with mortality rates in excess of 55% despite antifungal therapy (9, 12), the rAls1p-N-induced survival rate of ≥50% in this model, without adjunctive antifungal therapy, is highly encouraging.
All of the in vivo rAls1p-N vaccine experiments to date have been performed in BALB/c mice, have utilized Freund's adjuvant, and have utilized a single, virulent strain of C. albicans. The current studies were performed to define the breadth of protection induced by rAls1p-N by specifically evaluating its efficacy in outbred mice, in combination with a second adjuvant, against other strains of C. albicans, and against non-C. albicans species of Candida.
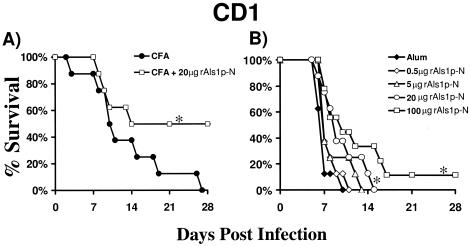
rAls1p-N improved the survival of outbred mice from disseminated candidiasis.
Outbred CD1 mice were obtained from the National Cancer Institute (Bethesda, MD). All procedures involving mice were approved by the institutional animal use and care committee, following the National Institutes of Health guidelines for animal housing and care. The mice were vaccinated with rAls1p-N plus Freund's adjuvant as previously described (6, 13). Briefly, rAls1p-N (amino acids 17 to 432 of Als1p) was produced in Saccharomyces cerevisiae and purified by gel filtration and Ni-nitrilotriacetic acid matrix affinity purification. A high degree of purity (∼90%) was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis as well as circular dichroism and Fourier transform infrared microscopy, as described previously (10). Mice were immunized by subcutaneous injection of rAls1p-N (20 μg) mixed with complete Freund's adjuvant (CFA; Sigma-Aldrich, St. Louis, MO) at day 0, followed by a booster dose in incomplete Freund's adjuvant (IFA; Sigma-Aldrich) at day 21. Control mice were immunized with CFA followed by IFA alone. Fourteen days following the boost, immunized mice were infected via the tail vein with C. albicans SC5314, as we have described previously (6, 13). Similar to our previous findings in BALB/c mice, the rAls1p-N vaccine markedly improved the survival of infected CD1 mice (Fig. 1A).
FIG. 1.
rAls1p-N vaccine improves survival of outbred, CD1 mice from hematogenously disseminated candidiasis. (A) CD1 mice (n = 8 per group) were vaccinated subcutaneously with rAls1p-N (20 μg) plus CFA or with CFA alone and infected via the tail vein with C. albicans SC5314 14 days after the boost. (B) CD1 mice (n = 8 per group) were vaccinated subcutaneously with rAls1p-N at various doses with alum or with alum alone and infected via the tail vein with C. albicans SC5314 14 days after the boost. The inoculum for panels A and B was 5 × 105 blastospores. *, P < 0.05 versus adjuvant control by log rank test.
Because Freund's adjuvant is considered to be too toxic for use in humans, we performed a dose response of rAls1p-N vaccine in alum (2% alhydrogel; Brenntag Biosector, Frederikssund, Denmark), the only vaccine adjuvant currently approved by the U.S. Food and Drug Administration (FDA) for use in humans. Vaccination with alum was performed on a schedule identical to that used for Freund's adjuvant, with immunization on day 0, boost on day 21, and infection 2 weeks later. We found that higher doses of rAls1p-N combined with alum resulted in significant improvements in survival of mice with disseminated candidiasis (Fig. 1B). There also appeared to be a dose-response relationship, with trends to improved survival at higher doses of rAls1p-N when combined with alum. Studies evaluating the efficacy of even higher doses of rAls1p-N plus alum are ongoing.
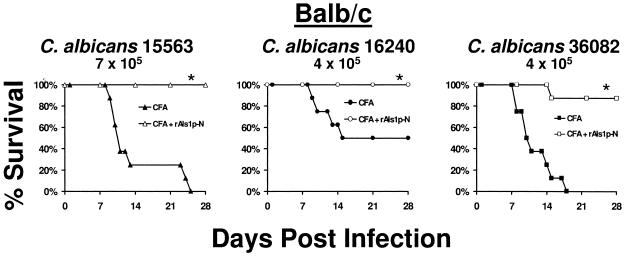
The rAls1p-N vaccine improved the survival of BALB/c mice infected with several strains of C. albicans.
For the development of an effective vaccine, it is critical for the immunogen to prime the immune system to recognize multiple strains of the target pathogen. By DNA sequence analysis, we found that the predicted amino acid sequence of the N-terminal region of Als1p was 99.9% conserved among a diverse group of clinical C. albicans isolates from bloodstream (5 strains), urine (5 strains), and oropharyngeal (10 strains) infections (data not shown). Therefore, we hypothesized that the rAls1p-N vaccine would be effective against a broad array of C. albicans strains. To determine the breadth of protection of the rAls1p-N vaccine against other strains of C. albicans, mice were vaccinated with rAls1p-N plus Freund's adjuvant as described above and infected with one of several clinical isolates of C. albicans (5). The rAls1p-N vaccine significantly improved the survival of mice infected with each of these strains (Fig. 2).
FIG. 2.
rAls1p-N vaccine improves the survival of BALB/c mice infected with one of several bloodstream isolates of C. albicans. Shown is survival of BALB/c mice immunized with rAls1p-N (20 μg) plus CFA versus immunized with CFA alone and infected via the tail vein with C. albicans 15563 (7 × 105 blastospores), 16240 (4 × 105 blastospores), or 36082 (4 × 105 blastospores) (n = 8 mice per group). *, P < 0.05 versus adjuvant control by log rank test.
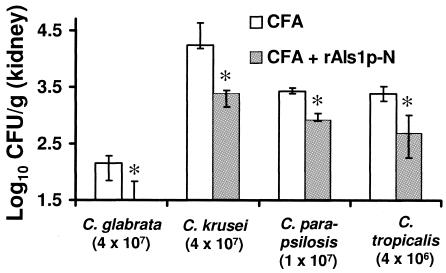
The rAls1p-N vaccine reduced tissue fungal burden in mice infected with several non-C. albicans species of Candida.
It has been previously reported that the ALS gene family is present in other Candida species, including C. dubliniensis and C. tropicalis (4). Similarly, an adhesin analogous to Als family members has been described in C. glabrata (1, 2). To determine the efficacy of the rAls1p-N vaccine against non-C. albicans species, BALB/c mice were vaccinated with rAls1p-N (20 μg) plus Freund's adjuvant as described above and infected via the tail vein with C. glabrata 31028 (a clinical bloodstream isolate from the microbiology laboratory at Harbor-University of California at Los Angeles [UCLA] Medical Center), C. krusei (91-1159; generously provided by Michael Rinaldi, San Antonio, Tex.), C. parapsilosis 22019 (clinical bloodstream isolate from Harbor-UCLA Medical Center), or C. tropicalis 4243 (clinical bloodstream isolate from Harbor-UCLA Medical Center). The rAls1p-N vaccine reduced the kidney fungal burden of mice infected with each of these species (Fig. 3).
FIG. 3.
rAls1p-N vaccine reduces tissue fungal burden in BALB/c mice infected with several non-C. albicans strains of Candida. BALB/c mice (n = 5 per group) were vaccinated with CFA or CFA plus rAls1p-N (20 μg) and infected via the tail vein with C. glabrata, C. krusei, C. parapsilosis, or C. tropicalis. Infectious inocula are shown in parentheses below the species names. Kidney fungal burden was determined on day 5 postinfection. The y axis reflects the lower limit of detection of the assay. *, P < 0.05 versus adjuvant control by nonparametric Steel test for multiple comparisons.
In summary, the rAls1p-N vaccine is a promising candidate for prevention of increasingly common and highly lethal disseminated candidiasis. The vaccine is efficacious in both inbred and outbred mice, when mixed with alum as an adjuvant, against multiple strains of C. albicans, and against several non-C. albicans species of Candida. These results support the continued development of the rAls1p-N vaccine against candidal infections.
Acknowledgments
This work was supported by NIAID/NIH grants R01 AI19990 and R01 AI063382 to J.E.E. J.E.E. is also supported by an unrestricted Freedom to Discover Grant for Infectious Disease from Bristol Myers Squibb. A.S.I. and B.J.S. are supported by NIAID grants R56 AI63503-01A1 and K08 AI060641, respectively. A.S.I. is also supported by a Burroughs Wellcome New Investigator Award in Molecular Pathogenic Mycology.
Editor: A. Casadevall
REFERENCES
- 1.Cormack, B. P., N. Ghori, and S. Falkow. 1999. An adhesin of the yeast pathogen Candida glabrata mediating adherence to human epithelial cells. Science 285:578-582. [DOI] [PubMed] [Google Scholar]
- 2.Frieman, M. B., J. M. McCaffery, and B. P. Cormack. 2002. Modular domain structure in the Candida glabrata adhesin Epa1p, a beta1,6 glucan-cross-linked cell wall protein. Mol. Microbiol. 46:479-492. [DOI] [PubMed] [Google Scholar]
- 3.Gudlaugsson, O., S. Gillespie, K. Lee, J. Vande Berg, J. Hu, S. Messer, L. Herwaldt, M. Pfaller, and D. Diekema. 2003. Attributable mortality of nosocomial candidemia, revisited. Clin. Infect. Dis. 37:1172-1177. [DOI] [PubMed] [Google Scholar]
- 4.Hoyer, L. L., R. Fundyga, J. E. Hecht, J. C. Kapteyn, F. M. Klis, and J. Arnold. 2001. Characterization of agglutinin-like sequence genes from non-albicans Candida and phylogenetic analysis of the ALS family. Genetics 157:1555-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ibrahim, A. S., F. Mirbod, S. G. Filler, Y. Banno, G. T. Cole, Y. Kitajima, J. E. Edwards, Jr., Y. Nozawa, and M. A. Ghannoum. 1995. Evidence implicating phospholipase as a virulence factor of Candida albicans. Infect. Immun. 63:1993-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ibrahim, A. S., B. J. Spellberg, V. Avenissian, Y. Fu, S. G. Filler, and J. E. Edwards, Jr. 2005. Vaccination with recombinant N-terminal domain of Als1p improves survival during murine disseminated candidiasis by enhancing cell-mediated, not humoral, immunity. Infect. Immun. 73:999-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martino, P., C. Girmenia, A. Micozzi, R. Raccah, G. Gentile, M. Venditti, and F. Mandelli. 1993. Fungemia in patients with leukemia. Am. J. Med. Sci. 306:225-232. [DOI] [PubMed] [Google Scholar]
- 8.Nucci, M., W. Pulcheri, N. Spector, A. P. Bueno, P. C. Bacha, M. J. Caiuby, A. Derossi, R. Costa, J. C. Morais, and H. P. de Oliveira. 1995. Fungal infections in neutropenic patients. A 8-year prospective study. Rev. Inst. Med. Trop. Sao Paulo 37:397-406. [DOI] [PubMed] [Google Scholar]
- 9.Opal, S. M., G. E. Garber, S. P. LaRosa, D. G. Maki, R. C. Freebairn, G. T. Kinasewitz, J. F. Dhainaut, S. B. Yan, M. D. Williams, D. E. Graham, D. R. Nelson, H. Levy, and G. R. Bernard. 2003. Systemic host responses in severe sepsis analyzed by causative microorganism and treatment effects of drotrecogin alfa (activated). Clin. Infect. Dis. 37:50-58. [DOI] [PubMed] [Google Scholar]
- 10.Sheppard, D. C., M. R. Yeaman, W. H. Welch, Q. T. Phan, Y. Fu, A. S. Ibrahim, S. G. Filler, M. Zhang, A. J. Waring, and J. E. Edwards, Jr. 2004. Functional and structural diversity in the Als protein family of Candida albicans. J. Biol. Chem. 279:30480-30489. [DOI] [PubMed] [Google Scholar]
- 11.Spellberg, B., A. S. Ibrahim, J. E. Edwards, Jr., and S. G. Filler. 2005. Mice with disseminated candidiasis die of progressive sepsis. J Infect. Dis. 192:336-343. [DOI] [PubMed] [Google Scholar]
- 12.Spellberg, B. J., and J. E. Edwards, Jr. 2002. The pathophysiology and treatment of Candida sepsis. Curr. Infect. Dis. Rep. 4:387-399. [DOI] [PubMed] [Google Scholar]
- 13.Spellberg, B. J., A. S. Ibrahim, V. Avenissian, S. G. Filler, C. Myers, Y. Fu, and J. E. J. Edwards. 2005. The anti-Candida albicans vaccine composed of the recombinant N terminus of Als1p reduces fungal burden and improves survival in both immunocompetent and immunocompromised mice. Infect. Immun. 73:6191-6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wey, S. B., M. Mori, M. A. Pfaller, R. F. Woolson, and R. P. Wenzel. 1988. Hospital-acquired candidemia. The attributable mortality and excess length of stay. Arch. Intern. Med. 148:2642-2645. [DOI] [PubMed] [Google Scholar]
- 15.Wisplinghoff, H., T. Bischoff, S. M. Tallent, H. Seifert, R. P. Wenzel, and M. B. Edmond. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 39:309-317. [DOI] [PubMed] [Google Scholar]