Abstract
α-2,3-Sialyltransferase (Lst) is expressed on the outer membrane of Neisseria gonorrhoeae and Neisseria meningitidis and sialylates surface lipooligosaccharide (LOS), facilitating resistance to complement-mediated killing. The enzyme is constitutively expressed from a single gene (lst) and does not undergo antigenic or phase variation. We observed that Triton X-100 extracts of N. gonorrhoeae strain F62 contain about fivefold more sialyltransferase (Stase) activity than extracts of N. meningitidis strain MC58 ⊄3 a serogroup B acapsulate mutant. We confirmed and expanded upon this observation by showing that extracts of 16 random N. gonorrhoeae isolates contain various amounts of Stase activity, but, on average, 2.2-fold-more Stase activity than extracts of 16 N. meningitidis clinical isolates, representing several serogroups and nongroupable strains. Northern and real-time reverse transcription-PCR analysis of lst transcript levels in N. gonorrhoeae and N. meningitidis revealed that N. gonorrhoeae strains express more lst transcript than N. meningitidis strains. Although transcript levels correlate with average Stase activity observed in the two species, there was not a direct correlation between lst transcript levels and Stase activity among individual isolates of each species. Comparison of lst upstream (5′lst) regions of N. gonorrhoeae and N. meningitidis revealed striking sequence differences characteristic of the two pathogens. N. gonorrhoeae 5′lst regions possess 30-bp and 13-bp elements present as single elements or as tandem repeats that exist only as single elements in the 5′lst regions of N. meningitidis isolates. In addition, the 5′lst regions of N. meningitidis strains have 105-bp transposon-like Correia elements which are absent in N. gonorrhoeae. Chromosomal N. gonorrhoeae 5′lst::lacZ translational fusions expressed 4.75 ± 0.09-fold (n = 4) higher β-galactosidase (β-gal) activity than N. meningitidis 5′lst::lacZ fusions in a host-independent manner, indicating differential expression is governed at least in part by sequence variations in the 5′lst regions. Reporter fusion assays and promoter-mapping analysis revealed that N. gonorrhoeae and N. meningitidis use different promoters with different strengths to transcribe lst. In N. gonorrhoeae, a strong sigma 70 promoter 80 bp upstream of the translational start site is used to transcribe lst, whereas this promoter is inactive in N. meningitidis. In N. meningitidis, a weak sigma 70 promoter at the 3′ terminus of a 105-bp Correia repeat-enclosed element 99 bp upstream of the translational start site is used to transcribe lst. We conclude that differential Stase expression between N. gonorrhoeae and N. meningitidis is due at least in part to differential lst gene transcription.
The sialylation of lipooligosaccharide (LOS) in pathogenic Neisseria spp. is catalyzed by the outer membrane enzyme α-2,3-sialyltransferase (Lst) (15, 26). The importance of this enzyme for neisseria virulence is highlighted by the finding that Lst is found primarily in the pathogenic, as opposed to nonpathogenic, Neisseria spp.(14, 15). LOS sialylation is responsible for converting serum-sensitive strains of Neisseria gonorrhoeae to serum resistance by allowing gonococci to bind complement factor H (20). The role of LOS sialylation in mediating serum resistance of N. meningitidis is less well understood and thought to act in concert with capsule, which inhibits complement membrane attack complex insertion (19). In serum-sensitive meningococcal isolates, exogenous sialylation of LOS enhances serum resistance (8). In highly serum-resistant meningococcal disease strains, LOS sialylation appears dispensable for serum resistance (31). Thus, the need for LOS sialylation in the pathogenic Neisseria spp. varies among isolates and species.
Natural variations occur in the degree of LOS sialylation in different isolates of pathogenic Neisseria spp. (8, 15, 18, 28). The factors that could affect the degree of LOS sialylation include the availability of phase-variable terminal galactose sialylation targets (29, 30), the amount of available CMP-N-acetylneuraminic acid (CMP-NANA) (21, 34), and inherent specific activity or regulated expression of Lst. Regulation of sialyltransferase (Stase) expression has not been demonstrated within strains. In an effort to define the distribution of sialyltransferase activity among commensal and pathogenic strains of Neisseria, Mandrell et al. (15) observed that Triton X-100 extracts of N. gonorrhoeae F62 were more efficient at sialylating exogenous LOS than extracts of N. meningitidis L11 strain 7889, implying that lst may be expressed at different levels among pathogenic Neisseria spp.
In this paper, we describe differential Stase expression between N. gonorrhoeae and N. meningitidis and address the possibility that it is due to differential lst gene expression. To this end, we performed transcriptional analysis of six N. gonorrhoeae and six N. meningitidis clinical isolates and found that lst transcript levels were more abundant in N. gonorrhoeae than in N. meningitidis. By reporter fusion assays and promoter-mapping analysis, we show that N. gonorrhoeae and N. meningitidis strains use different promoters with different strengths to transcribe lst. Overall, this study indicates that expression of different levels of lst by N. meningitidis and N. gonorrhoeae is controlled at least in part at the level of transcription.
(Observations on different sialyltransferase activities, distinctive lst upstream sequences, and differential lst reporter gene expression were presented by S. V. Liu, Y.-B. Liu, and R. F. Rest at the 11th International Pathogenic Neisseria Conference, 1998, Nice, France.)
MATERIALS AND METHODS
Bacterial strains and growth conditions.
N. gonorrhoeae F62, N. meningitidis MC58 3, and Escherichia coli XL1-Blue MRF′ were obtained from P. Frederick Sparling (University of North Carolina, Chapel Hill), E. Richard Moxon (University of Oxford, Oxford, United Kingdom), and Stratagene (La Jolla, Calif.), respectively. Random clinical isolates of N. gonorrhoeae were obtained from the City of Philadelphia Public Health Laboratories, and representative N. meningitidis strains (see Table 2 and Fig. 1C) were graciously donated by David Stephens, Emory University, Atlanta, GA. N. gonorrhoeae ST01 is an lst knockout mutant of N. gonorrhoeae F62 constructed by the insertion of a kanamycin cassette in the lst open reading frame (kind gift of Michael Jennings). N. gonorrhoeae ST01 does not express lst protein or sialyltransferase activity (26). Frozen stocks of Neisseria or E. coli cells were clonally passaged for up to 1 week by growing aerobically at 37°C in a humidified 5% CO2 incubator (Forma Scientific, Marietta, Ohio) on GC agar (Difco, Detroit, Mich.) with supplement (12) or on Luria-Bertani (LB) agar, respectively.
TABLE 2.
Stase activity in Triton X-100 extracts of N. gonorrhoeae and N. meningitidis clinical isolatesa
| Strain | Mean Stase activity ± SD (cpm/μl) |
|---|---|
| N. gonorrhoeae | |
| B-9 | 685 ± 12 |
| B-23 | 945 ± 159 |
| FA1090 | 1,092 ± 117 |
| B-7 | 1,154 ± 129 |
| A-2 | 1,310 ± 508 |
| A-1 | 1,482 ± 371 |
| B-18 | 1,631 ± 88 |
| B-10 | 1,762 ± 331 |
| B-21 | 2,044 ± 133 |
| B-13 | 2,357 ± 581 |
| B-17 | 2,357 ± 458 |
| A-5 | 2,768 ± 304 |
| B-24 | 2,836 ± 896 |
| B-4 | 3,671 ± 924 |
| B-25 | 4,223 ± 473 |
| A-4 | 5,367 ± 1,915 |
| N. meningitidis (serogroup) | |
| X4 (B) | 288 ± 23 |
| X8 (C) | 330 ± 252 |
| C1 (C) | 427 ± 23 |
| X6 (U) | 611 ± 252 |
| Y2 (Y) | 642 ± 293 |
| B2 (B) | 709 ± 399 |
| Y1 (Y) | 784 ± 410 |
| X2 (29E) | 807 ± 412 |
| W1 (W-135) | 814 ± 272 |
| A1 (A) | 844 ± 357 |
| A2 (A) | 927 ± 259 |
| X9 (U) | 999 ± 267 |
| B1 (B) | 1,003 ± 259 |
| B3 (B) | 1,176 ± 280 |
| X5 (U) | 2,561 ± 783 |
| X3 (X) | 3,845 ± 731 |
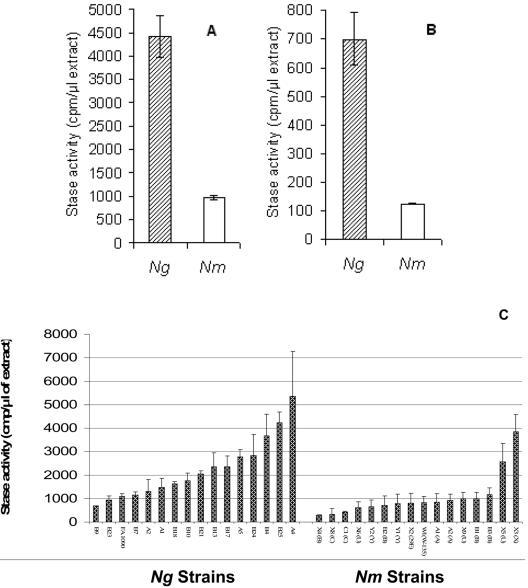
A graphical representation of the tabulated data comparing the Stase activities of N. gonorrhoeae and N. meningitidis clinical isolates is shown in Fig. 1C.
FIG. 1.
Sialyltransferase activity of N. gonorrhoeae (Ng) F62 and N. meningitidis (Nm) MC58 measured as described in Materials and Methods with LOS prepared from N. gonorrhoeae F62 (A) or N. meningitidis MC58 (B). A graphical representation of data in Table 2 comparing the Stase activities of N. gonorrhoeae and N. meningitidis clinical isolates is shown in panel C.
Measurement of Stase activity in cell extracts.
Stase activity was determined in freshly made Neisseria cell extracts using a method developed by Mandrell (15) with some modifications (17). Briefly, bacteria were harvested from overnight agar cultures, washed once, and suspended in sterile PBSGCM (phosphate-buffered saline containing 0.1% [wt/vol] gelatin, 0.1% [wt/vol] CaCl2, and 0.1% [wt/vol] MgCl2) to an optical density at 550 nm (OD550) of 0.18 (∼2 × 108 CFU/ml). Bacteria in 1.5 ml of PBSGCM were pelleted by centrifugation and suspended in 60 μl of 0.5% Triton X-100 in 0.5 mM phosphate buffer (pH 6.8). Cell suspensions were pipetted up and down at least 15 times, and cell extracts were obtained after centrifugation (10,000 × g, 4°C, 10 min). Stase activity in the cell extracts was determined by quantifying 14C-labeled NANA transferred from CMP-[14C]NANA onto purified LOS from N. gonorrhoeae F62, unless otherwise indicated after 15 min of incubation at 37°C (17).
PCR amplification of 5′lst.
PCR primers were synthesized according to sequence information derived from the lst gene (accession no. U60660) and are listed in Table 1.
TABLE 1.
Primers used in this study
| Primer | Sequence 5′→3′ | Amplification target |
|---|---|---|
| Northern blot probe PCR | ||
| Sta2 For | GCGTATGTTCAATTTGTCG | 1,140-bp product from lst of N. gonorrhoeae and N. meningitidis |
| Sta3 Rev | CGTCAAATGTCAAAATCGG | |
| Real-time PCR | ||
| LstRTF | AAACCCGCATACGAGGTATGA | 100-bp product from lst of N. gonorrhoeae and N. meningitidis |
| LstRTR | AAGCCGGTTTCAATGCGTAA | |
| 16S RT F | GCGTGGGTAGCAAACAGGAT | 100-bp product from rrs gene of N. gonorrhoeae and N. meningitidis |
| 16S RT R | CGCGTTAGCTACGCTACCAAG | |
| Cloninga | ||
| FusBam F1 | CGCTGGATCCGACATCAATATCGG | 496 bp of N. gonorrhoeae 5′lst including RBS sequence and 24 bp of lst including ATG |
| FusBam R1 | CAAAGGATCCTTTTTCAAGCCC | 586 bp of N. meningitidis 5′lst including RBS sequence and 24 bp of lst including ATG |
| Primer extension | ||
| PE1 | CGCATTCCTTTCCCCCTGATTTAC | 3′ region of primers anneals 78, 16, and 24 bp downstream of lst ATG, respectively |
| PE2 | CACAACACGGTCAAACAAGC | |
| PE3 | CAATCAGGCACAACACGGTC | |
| RPA primers | ||
| PA1 | GATCGAGCTCGTTCGATCTTGGCGTGTTTG | 292 bp of N. gonorrhoeae 5′lst excluding the RBS sequence; SacI site of PA1 is denoted by double underline |
| PA2 | GATCGGATCCCTCCATTCCGACAAATTGAAC | |
| PA3 | AATTAACCCTCACTAAAGGG | 397-bp product from pSKII including the cloned 292-bp N. gonorrhoeae 5′lst insert |
| PA4 | GTAATACGACTCACTATAGGG | |
| RT-PCR: Fig. 7 | ||
| P1 | CGGGATCCGGCTTTCCCGCGTTTGCCGG | 5′ region of the primer anneals upstream of CREE; 282 bp upstream of lst ATG |
| P2 | CGGGATCCCGCCTTGTGCCTGATGTGCG | 5′ region of the primer anneals upstream of CREE; 252 bp upstream of lst ATG |
| P3 | CGGGATCCTTTCGGTAAAATTGATTTTA | 5′ region of the primer anneals upstream of CREE; 222 bp upstream of lst ATG |
| P4 | CGGGATCCAACTGTCGGAATATCTGCTA | 5′ region of the primer anneals downstream of CREE; 91 bp upstream of lst ATG |
| P5 | CGGGATCCTTTTTCCGTCCCGGGACAC | 5′ region of the primer anneals downstream of CREE; 61 bp upstream of lst ATG |
| P6 | CGGGATCCACACTCGGGGCGTATGTTCA | 5′ region of the primer anneals downstream of CREE; 41 bp upstream of lst ATG |
| P7 | CGGGATCCAGGGATATGGGCTTGAAAAAG | 5′ region of the primer anneals downstream of CREE; 6 bp upstream of lst ATG |
| Crev | CTCCGCCATCGTCGGAAT | Common reverse primer used in conjunction with primers P1 to P7 and the 3′ region of the primer anneals 372 bp downstream of lst ATG |
| RT-PCR: Fig. 8 | ||
| IP1 | TTATTCTCTCTTGTAGGTTGG | Generates a 212-bp product of 5′icd upstream region; P4 and Crev primers used in Fig. 7 were used in Fig. 8 |
| IP2 | TGCCGCCGCACATCAGGCACA |
Primers FusBam F1 and FusBam R1 were used to include regions of the N. gonorrhoeae or N. meningitidis 5′lst region including the RBS and 24 bp of the lst gene including ATG to create translational lacZ fusions in pLES94. The BamHI restriction sites are underlined with single lines.
Construction of 5′lst::lacZ fusions.
Various lengths of Neisseria 5′lst regions were amplified by PCR using primer pairs indicated in Table 1. PCR fragments contained a putative ribosome binding site (RBS) and 24 bp of lst coding region (including ATG). Translational fusions were created using the vector pLES94, a high-copy-number plasmid containing a Neisseria uptake sequence designed to effect allelic exchange into neisserial chromosomes (27). As templates for PCRs, we used supernatants prepared from a few colonies of N. gonorrhoeae or N. meningitidis boiled for 10 min in 50 μl H2O followed by incubation at 37°C with RNase A (20 μg/ml) for 30 min. PCR-amplified 5′lst fragments were digested with BamHI and then ligated into BamHI- and shrimp alkaline phosphatase-treated pLES94. The resulting constructs were transformed by electroporation into E. coli XL1-Blue MRF′, prepared by washing log-phase E. coli in 10% glycerol at 4°C three times, and then stored at −70°C until use. Electroporation was performed at 2.5 keV, using 0.4-cm electroporation cuvettes in a Gene Pulser (Bio-Rad, Hercules, CA). The electroporated bacteria were incubated in 1 ml of super broth (25 g tryptone, 15 g yeast extract, and 5 g NaCl per liter of H2O) at 37°C for 1 to 3 h in a shaking water bath, and then the bacteria were plated onto selective media. Transformants were selected on LB agar containing 100 μg/ml ampicillin and 5-bromo-4-chloro-3-indolyl-β-galactopyranoside (X-Gal) at 40 μg/ml. After overnight incubation, blue colonies were picked and plasmids were checked for inclusion of correct inserts by PCR analysis and DNA sequencing. Plasmids containing the correct insert in the proper orientation were transformed into N. meningitidis or N. gonorrhoeae. Transformants were selected on gonococcal agar containing supplements and chloramphenicol at 1 μg/ml for N. gonorrhoeae F62 or 5 μg/ml for N. meningitidis MC58 ⊄3.
Integration of fusions into neisserial chromosomes.
Integration of fusions into the N. gonorrhoeae F62 chromosome was done by mixing donor DNA (∼1 μg DNA in 20 μl H2O) with 20 μl of N. gonorrhoeae cell suspension (made by suspending the pellet of 3 ml of a 0.18 A550 culture in 240 μl gonococcal broth (GCB) plus 20 mM MgCl2) on GC agar plates and incubating the mixture at 37°C for 5 to 6 h. The N. gonorrhoeae cells were subsequently swabbed into 200 μl GCB and plated onto selective media. Integration of fusions into N. meningitidis MC58 ⊄3 was done by electroporation under the conditions described above for E. coli. Competent N. meningitidis cells were made by washing cells three times in ice-cold buffer containing 9% (wt/vol) sucrose and 15% (vol/vol) glycerol. After electroporation, cells were incubated in GCB with supplement at 37°C for 3 h and then plated on gonococcal agar containing chloramphenicol at 5 μg/ml. All neisserial integrants expressed levels of native Stase activity similar to those of wild-type strains (data not shown).
RNA isolation.
Total RNA was extracted from exponential-phase broth-grown bacteria using QIAGEN mini RNAeasy isolation kits according to the manufacturer's instructions. If needed, RNA preparations were concentrated by addition of 0.5× volume of 1 M LiCl (Ambion) and incubation at −20°C for 30 min. Precipitated RNA was pelleted by centrifugation at 12,000 × g and washed with cold 70% (vol/vol) ethanol, before resuspension in diethylpyrocarbonate (Sigma)-treated double-distilled H2O (ddH2O) containing 1 μl of RNasin (40 U/μl; Promega). Concentrations of RNA were determined by optical density at 260 nm. All preparations were treated twice with DNase I (6 U, RNase free; Ambion) and stored at −70°C until use.
cDNA synthesis.
cDNA was synthesized using the reverse transcriptase RNase H− SuperScript III (Invitrogen, Carlsbad, CA) following the manufacturer's instructions. Briefly, 2 μg of DNase I-treated RNA in a 10-μl volume was mixed with 1 μl (250 ng) of random hexamers (Promega) and 1 μl (10 mM) of deoxynucleoside triphosphate (dNTP) mix (Promega). This mixture was incubated at 65°C for 5 min and quickly chilled on ice. A cocktail containing 4 μl of 5× First-Strand buffer, 2 μl of 0.1 M dithiothreitol and 1 μl of RNasin were then added to each tube. After a brief centrifugation, the tubes were incubated at room temperature for 10 min. The reaction mixtures were then preincubated at 50°C for 2 min, before adding 1 μl of SuperScript. cDNA synthesis was then allowed to proceed at 50°C for 50 min, followed by incubation at 70°C for 15 min to inactivate the reaction. Nuclease-free water (1 μl) was added to reaction mixtures in place of Superscript for controls.
Real-time PCR.
The SYBR Green Master Mix kit (ABI) was used to perform real-time PCR assays. cDNA reaction mixtures (20 μl) were diluted to a final volume of 100 μl with nuclease-free ddH2O. The diluted cDNA template (2 μl), in addition to 1:2 and 1:4 dilutions thereof, was then subjected to PCR amplification in a 7700 Sequence Detector (ABI) in a total volume of 25 μl containing 12.5 μl SYBR Green Master Mix, 1 μl of each primer (0.4 μM, final concentration), and 8.5 μl ddH2O. The reactions were cycled according to the following parameters: 10 min at 95°C and then 40 cycles of 95°C for 15 s and 60°C for 1 min. Data were analyzed using the Sequence Detector v.1.7a software (ABI). The cycle threshold (CT) was defined as the cycle number corresponding to the point where the amplification plot of all samples was linear. We used the comparative CT method (ΔΔCT) for relative quantification of lst expression where 16S rRNA (rrs) expression served as the active reference control (normalizer). Quantification of relative lst expression included calculating the difference between the CT values of the normalizer (16S rRNA) and the CT values of individual samples: ΔCT = CT(16S rRNA) − CT(sample). The difference between each sample's ΔCT value and the ΔCT value of N. gonorrhoeae F62 (ΔΔCT) was then used to obtain an absolute value for the difference (fold) in lst mRNA levels between samples (2ΔΔCT). Results are expressed as arbitrary units reflecting this difference. The sequences of primers used for this analysis are given in Table 1.
Primer extension.
Primers were end labeled with [γ-32P]ATP using T4 polynucleotide kinase (Promega, Madison, WI). RNase H− SuperScript III (Gibco BRL, Rockville, MD) was used for 5′ mapping. For most reactions, 10 μl of RNA (10 to 50 μg) was mixed with 1 μl of labeled primer (2 pmol) and incubated at 70°C for 10 min. The tubes were immediately cooled on ice for 2 to 3 min for primer annealing. A cocktail containing 4 μl of 5× first-strand buffer (Gibco BRL), 2 μl of 0.1 μM dithiothreitol, 1 μl 10 mM dNTP, and 1 μl of RNasin was then added to the annealing mixture to a final volume of 19 μl. The resulting mixture was incubated at 50°C for 2 min, before adding 1 μl of Superscript, and the primer extension was performed at 50°C for 50 min. The reaction was then ethanol precipitated, and the mixture was resuspended in 4 μl of Tris-EDTA and 4 μl of gel loading buffer (Promega). Extension products were analyzed on a 6% denaturing polyacrylamide gel containing 8 M urea. The migrations of the primer extension products were compared to sequencing ladders generated by PCR (fmole DNA Cycle Sequencing Sample; Promega, Madison, Wis.) using the same primer. A constant current of 1,800 V was applied to the sequencing gel in 1× Tris-borate-EDTA buffer. After electrophoresis, the gel was dried for 1 h at 80°C in a vacuum gel dryer, and the gel was exposed to X-ray film overnight before development.
Northern blot analysis.
Northern blot analysis was performed using a 1.2% MOPS (morpholinepropanesulfonic acid)-formaldehyde agarose gel essentially as described by Maniatis et al. Before loading the agarose gel, RNA samples were mixed with 3× volumes of loading buffer (Ambion) containing 10 μg/ml ethidium bromide and heated at 68°C for 15 min. RNA was transferred using 20× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) onto Nytran N (Schleicher and Schuell, Keene, NH) and hybridized with horseradish peroxidase-labeled PCR fragments (≈1.1 kb) (North2South; Pierce) specific for lst.
DNA sequencing.
DNA fragments to be sequenced were amplified by PCR from Neisseria chromosomes with appropriate primers and run on agarose gels. Bands of the expected size were cut from gels and purified using the Wizard PCR Prep DNA purification system (Promega, Madison, Wis.). Purified DNA fragments were mixed with the appropriate primer, and nucleotide sequences were determined by direct automated fluorescent DNA sequencing at facilities at either the University of Pennsylvania or Drexel University College of Medicine.
Assay for β-Gal activity in cell extracts.
β-Galactosidase (β-Gal) activity was determined by the Miller method with minor modifications. Bacteria were grown on plates or in broth to the mid-log phase. E. coli transformants were grown in the presence of ampicillin (100 μg/ml) and tetracycline (15 μg/ml). Broth cultures or suspensions of cells harvested from plates were adjusted to an OD600 of 0.4. For Neisseria, 0.5 ml of the OD600 0.4 suspension was mixed with 0.5 ml of Z buffer. For E. coli, 0.1 ml of the OD600 0.4 suspension was mixed with 0.9 ml Z buffer. Bacterial cells were disrupted by adding 20 μl of chloroform and 10 μl of 0.1% sodium dodecyl sulfate. Cell extracts were incubated in a 28°C water bath for 5 min, and assays were started by adding 0.2 ml of O-nitrophenyl-β-d-galactopyranoside (ONPG; 4 mg/ml). Reactions were stopped when an OD420 of about 0.6 to 0.9 developed. The absorbance of reaction mixtures was determined at 420 nm. Miller units were calculated according to the formula 1,000 × OD420/[time (min) × volume (ml) × OD600].
RPAs.
RNase protection assays (RPAs) were performed using Ambion's RPA III kit following the manufacturer's directions. Briefly, 2.5 μg to 15 μg of total RNA from N. gonorrhoeae F62 or N. gonorrhoeae FA1090 was mixed with 32P- labeled antisense RNA probe (generated using Ambion's in vitro transcription kit, MAXIscript) in 10 μl of Hybridization Buffer III and incubated overnight at 42°C. Samples were digested with 1:100 dilution of RNase A/T1 mix for 30 min at 37°C. RNase was inactivated by Inactivation/Precipitation Solution III, and the protected fragments were resolved along with an end-labeled Promega ΦX174 DNA size marker by 6% polyacrylamide gel electrophoresis (PAGE) with 8 M urea.
Generation of 32P-labeled antisense RNA probe.
The lst upstream region of N. gonorrhoeae F62 was PCR amplified with the primer pair PA1 and PA2 and cloned into pSKII at the SacI and BamHI sites. The T7 promoter with the cloned lst fragment was PCR amplified from pSKII with the primer pair PA3 and PA4 and used as a template for in vitro transcription reactions following Ambion's MAXIscript directions. The 380-bp 32P-labeled antisense riboprobe generated from the T7 promoter was resolved by 5% PAGE with 8 M urea, and gel-purified full-size probe was used in RPAs.
RESULTS
Stase activity in Triton X-100 extracts of N. gonorrhoeae is greater than that in extracts of N. meningitidis.
Triton X-100 extracts of N. gonorrhoeae F62 and N. meningitidis MC58 ⊄3 were prepared and tested for Stase activity as described in Materials and Methods. N. gonorrhoeae F62 extracts contained 4.5-fold-more Stase activity than N. meningitidis MC58 ⊄3 extracts (N. gonorrhoeae, 4,414 ± 444 cpm/μl; N. meningitidis, 974 ± 39 cpm/μl) (Fig. 1A). The difference in Stase activity was independent of LOS source, since N. gonorrhoeae F62 extracts also had greater Stase activity than N. meningitidis MC58 ⊄3 extracts when measured using LOS purified from N. meningitidis MC58 ⊄3 (N. gonorrhoeae, 699 ± 91 cpm/μl; N. meningitidis, 124 ± 2 cpm/μl) (Fig. 1B). To extend this finding to other strains of Neisseria, Triton X-100 extracts of 16 random clinical isolates each of N. gonorrhoeae and N. meningitidis were prepared and tested for Stase activity. Triton extracts of these isolates expressed a range of Stase activities (N. gonorrhoeae, 685 to 5,367 cpm/μl; N. meningitidis, 288 to 3,845 cpm/μl) (Table 2 and Fig. 1C). On average, extracts of N. gonorrhoeae strains contained 2.2-fold more Stase activity than extracts prepared from N. meningitidis strains (Mann-Whitney U test, P < 0.001). We were interested in determining if variation in Stase activities between N. gonorrhoeae and N. meningitidis extracts was regulated at the level of transcription.
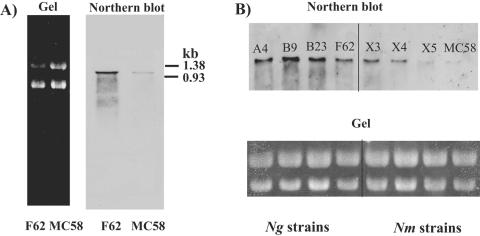
lst transcript levels are more abundant in N. gonorrhoeae than in N. meningitidis by Northern blot analysis.
We initially analyzed lst transcript levels in N. gonorrhoeae F62 and N. meningitidis MC58 ⊄3 by Northern blot analysis as described in Materials and Methods. Blots were probed with a 1.1-kb lst fragment; the N. gonorrhoeae lst gene is 1,116 bp (10). Single bands of ≈1.2 kb were detected in total RNA isolated from N. gonorrhoeae F62 and N. meningitidis MC58 ⊄3 (Fig. 2A). These bands were not detected in total RNA isolated from the lst-negative mutant N. gonorrhoeae ST01 (data not shown). Furthermore, the lst band was less intense for N. meningitidis MC58 ⊄3 than for N. gonorrhoeae F62, indicating greater lst mRNA levels in N. gonorrhoeae F62 than in N. meningitidis MC58 ⊄3. lst transcript levels were also evaluated in three additional N. gonorrhoeae and N. meningitidis isolates. The lst mRNA bands were more intense for strains of N. gonorrhoeae than for strains of N. meningitidis (Fig. 2B). Surprisingly, N. gonorrhoeae lst transcript levels were similar among all four isolates and did not reflect differences in Stase activities of Triton extracts (Fig. 2B). For example, N. gonorrhoeae A4 extracts contained 7.8-fold-more Stase activity than extracts of N. gonorrhoeae B9 (A4 = 5,367 ± 1,915 cpm/μl; B9 = 685 ± 12 cpm/μl; Table 2), but there was less than a twofold difference in the intensities of lst bands for these strains. N. meningitidis strains X3 and X4 expressed more lst mRNA than strains X5 and MC58 ⊄3. Similar to N. gonorrhoeae, the transcript levels found in N. meningitidis strains did not reflect their differences in Stase activity. For example, N. meningitidis X3 extracts contained 13.3-fold-more Stase activity than extracts of N. meningitidis X4 (X3 = 3,845 ± 731 cpm/μl, X4 = 288 ± 23 cpm/μl; Table 2), but there was less than a 2-fold difference in the intensities of lst bands for these strains. Therefore, although differences in Stase activities in extracts were the original impetus for our studies, Stase activity in Triton extracts does not directly correlate with lst transcript levels when compared between isolates of the same species. Regardless, overall, N. gonorrhoeae strains express more lst transcript than do N. meningitidis strains.
FIG. 2.
Northern analysis of lst transcripts in N. gonorrhoeae and N. meningitidis. (A) Total RNA (20 μg) isolated from N. gonorrhoeae F62 and N. meningitidis MC58 ⊄3 was fractionated on a 1.2% formaldehyde agarose gel, blotted onto Nytran N, and localized with an lst-specific horseradish peroxidase-labeled probe as described in Materials and Methods. (B) Total RNA (20 μg) prepared from N. gonorrhoeae (Ng) strains A4, B9, B23, and F62 and N. meningitidis (Nm) strains X3, X4, X5, and MC58 ⊄3 analyzed by Northern analysis. Molecular sizes are expressed in kb.
lst transcript levels are more abundant in N. gonorrhoeae than in N. meningitidis by real-time PCR.
To corroborate our Northern blot studies and for a more quantitative assessment of lst transcript levels in N. gonorrhoeae and N. meningitidis, we used semiquantitative real-time PCR. In addition to strain N. gonorrhoeae F62 (Stase activity = 4,414 ± 444 cpm/μl) and N. meningitidis MC58 (Stase activity = 974 ± 39), three additional isolates of each species exhibiting low Stase activities and two exhibiting high Stase activities were used for real-time PCR analysis: N. gonorrhoeae low, B9 = 685 ± 12, B23 = 945 ± 159, and FA1090 = 1,092 ± 117 cpm/μl, and N. gonorrhoeae high, B25 = 4,223 ± 473 and A4 = 5,367 ± 1,915 cpm/μl; and N. meningitidis low, X4 = 288 ± 23, X8 = 330 ± 252, and C1 = 427 ± 23 cpm/μl and N. meningitidis high, X5 = 2,561 ± 783 and X3 = 3,845 ± 731 cpm/μl. Expression of 16S rRNA was measured as an internal reference, and the primers used are given in Table 1. Differences in CT values (ΔCT) are given for each isolate used to calculate relative RNA levels (Fig. 3A). Consistent with results of our Northern analysis, relative differences in lst mRNA levels among strains of N. gonorrhoeae were less than 2-fold and lst transcript levels in N. meningitidis were 2.5- to 10-fold less than those in N. gonorrhoeae F62 (Fig. 3B). As with Northern analysis, variations in lst transcript levels detected in N. meningitidis strains were much more evident than in N. gonorrhoeae strains. In particular, N. meningitidis X3 produced 3.7 ± 0.3-fold more lst transcript than N. meningitidis MC58 ⊄3 and X8, and N. meningitidis C1 and X4 produced 2.2 ± 0.2-fold more lst transcript than MC58 ⊄3 and X8. Thus, real-time PCR results confirmed Northern blot analyses that Stase activity in Triton extracts does not correlate with lst transcript levels when compared within isolates of the same species (Fig. 3C).
FIG. 3.
Real-time PCR analysis of lst expression in N. gonorrhoeae (Ng) and N. meningitidis (Nm). (A) Real-time PCR was repeated on 3 days with similar results. The table contains ΔCT values representing the amount of lst sample RNA normalized to the endogenous reference, 16S rRNA. As described in Materials and Methods, relative RNA levels were determined using the ΔCT value for N. gonorrhoeae F62 as a baseline for comparison. (B) Graphical representation of RNA difference (fold) given in the table. (C) Comparison of Stase activity and lst transcript levels in N. gonorrhoeae and N. meningitidis.
Analysis and comparison of sequences upstream of N. gonorrhoeae and N. meningitidis lst.
Next, we wanted to identify lst upstream (5′lst) sequences that might influence lst transcription. We sequenced and compared the lst upstream regions of 28 N. gonorrhoeae and 17 N. meningitidis strains. Primers FusBam F1and FusBam R1 (Table 1) were used to amplify the upstream regions of lst genes. To our surprise, different size fragments were amplified from chromosomal DNA of N. gonorrhoeae strains, whereas an apparently constant size fragment was amplified from N. meningitidis strains (Fig. 4A). Analysis of DNA sequences revealed significant differences contained in a conserved background. Examination of the 5′lst regions revealed that N. meningitidis strains possess the 105-bp Correia repeat-enclosed element (CREE), while it is absent from the 5′lst regions of all N. gonorrhoeae strains (13). For simplicity of sequence comparison, we used N. gonorrhoeae F62 and N. meningitidis MC58 as representative strains for the respective Neisseria species. In the lst promoter region, N. gonorrhoeae F62 has two near perfect tandem repeats of 30 bp and 13 bp, whereas N. meningitidis MC58 has a single copy of each element; other single-base-pair variations also exist (Fig. 4B). In addition, 11 of the 28 N. gonorrhoeae strains had single 13- or 30-bp elements, in place of the tandem repeats observed in N. gonorrhoeae F62 (13). We also found single-base-pair differences. Overall, the 5′lst region of N. gonorrhoeae strains varied considerably, whereas the N. meningitidis 5′lst region remained relatively conserved.
FIG. 4.
Analysis and comparison of sequences upstream of N. gonorrhoeae (Ng) and N. meningitidis (Nm) lst. (A) The 5′lst upstream regions of 28 N. gonorrhoeae strains and 17 N. meningitidis strains were PCR amplified using primer pair FusBam F1 and FusBam R1, and the amplicons were resolved in a 1.5% agarose gel. (B) Nucleotide sequence comparison of the 5′lst regions of N. meningitidis MC58 and N. gonorrhoeae F62: DNA fragments absent or duplicated are indicated by dashes within or above the sequence, respectively. Individual base differences are indicated by asterisks. The −10 and −35 sequences are in boldface and underlined. The putative Shine-Dalgarno (SD) sequence for lst, and the initiation codons (IC) for lst and icd (isocitrate dehydrogenase) are italicized and in boldface. The transcriptional start sites (tsp) downstream of the −10 sites are in boldface, italic, and indicated with small arrows.
β-Galactosidase activities of lst promoter fusions of N. gonorrhoeae are higher than those of N. meningitidis.
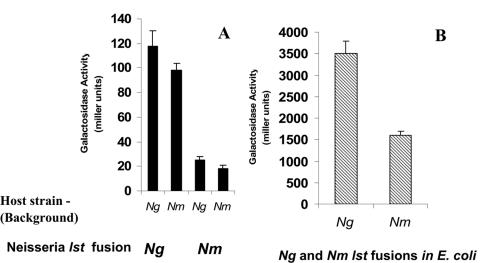
To further examine whether differences in promoter regions of N. gonorrhoeae and N. meningitidis were associated with transcript levels or enzyme activity in Triton extracts, 5′lst fragments were amplified with primers FusBam F1 and FusBam R1; incorporated into the promoterless lacZ vector, pLES94; and subsequently integrated into N. gonorrhoeae F62 and N. meningitidis MC58 ⊄3 chromosomes as described in Materials and Methods. The N. gonorrhoeae F62 5′lst::lacZ fusion expressed 4.75- or 5.5-fold-higher levels of β-Gal activity, respectively, than the N. meningitidis MC58 5′lst::lacZ fusion (Fig. 5A) when expressed in the chromosome of N. gonorrhoeae F62 (N. gonorrhoeae = 118 ± 12 Miller units, N. meningitidis = 25 ± 3 Miller units) or N. meningitidis MC58 (N. gonorrhoeae = 98 ± 6 Miller units, N. meningitidis = 18 ± 3 Miller units) (n = 4). Although to a lesser degree than when expressed in neisseria, the N. gonorrhoeae fusions also exhibited greater β-Gal activity than the N. meningitidis fusions when expressed as multicopy plasmids in E. coli (Fig. 5B) (N. gonorrhoeae = 3,498 ± 300 Miller units, N. meningitidis = 1,600 ± 100 Miller units) (n = 4). The difference was magnified when 5′lst fusions were expressed in E. coli in a low-copy plasmid (data not shown). N. gonorrhoeae 5′lst::lacZ fusions created from strains that possessed single 30-bp and 13-bp elements exhibited β-Gal activity comparable to that in strains containing these elements as tandem repeats (data not shown). All 5′lst::lacZ fusions of N. gonorrhoeae strains (A4, B9, B23, B25, and FA1090), irrespective of their Stase activities, have high β-Gal fusion activity levels similar to 5′lst::lacZ fusions of N. gonorrhoeae F62 (N. gonorrhoeae = 118 ± 12 Miller units). Similarly, all 5′lst::lacZ fusions of N. meningitidis strains (C1, X3, X4, X5, and X8) have low β-Gal activity levels comparable to 5′lst::lacZ fusions of N. meningitidis MC58 (N. meningitidis = 25 ± 3 Miller units). GenBank accession numbers for the 5′lst of six N. gonorrhoeae and N. meningitidis strains run from DQ375987 to DQ375998. Although the results of this study did not rule out a role for host-specific trans factors in modulating 5′lst promoter activity, the host independence and the similar high and low expression of 5′lst β-Gal fusion levels of N. gonorrhoeae and N. meningitidis suggest that species-specific sequence differences in the 5′lst are important for the differential 5′lst promoter activity between N. gonorrhoeae and N. meningitidis.
FIG. 5.
β-Gal activity of N. gonorrhoeae (Ng) F62 and N. meningitidis (Nm) MC58 5′lst reporter fusions. N. gonorrhoeae F62 and N. meningitidis MC58 ⊄3 5′lst::lacZ fusions were constructed with primers FusBam F1 and FusBam R1 (Table 1) as described in Materials and Methods. β-Gal activity of each fusion was determined in N. gonorrhoeae and N. meningitidis as chromosomal integrates (A) and in E. coli XL1-Blue as plasmid fusions (B).
N. gonorrhoeae lst is transcribed from a σ70 promoter.
lacZ reporter studies showed that the 5′lst regions of N. gonorrhoeae and N. meningitidis have different promoter strengths as indicated above. The size of N. gonorrhoeae and N. meningitidis lst RNA in Northern blots indicates that lst transcription starts at identical or similar sites in both species. To examine this possibility, primer extension analysis, RPAs, reverse transcription-PCR (RT-PCR), and mutational analyses were performed with N. gonorrhoeae and N. meningitidis strains to identify the lst promoter(s).
Primer extension analysis of total RNA isolated from N. gonorrhoeae F62 with primer PE1 (Table 1) produced a major band starting at an adenine residue 74 bp upstream of ATG and 6 bp downstream of the −10 sequence of the putative sigma 70 promoter (Fig. 6A and C). An equivalent band was also evident using total RNA isolated from E. coli harboring 5′lst::lacZ fusions using a lacZ-specific primer (data not shown).
FIG. 6.
Analysis of the N. gonorrhoeae lst promoter. (A) Primer extension analysis. Total RNA (20 μg) was isolated from N. gonorrhoeae strain F62, and primer extension analysis was performed using primer PE1. The extension product along with a sequencing reaction with the same primer was resolved by 6% PAGE with 8 M urea. The arrow points to the transcriptional start site. (B) RNase protection assay. 32P-labeled RNA probe was hybridized with total RNA from N. gonorrhoeae strains F62 and FA1090 or yeast RNA (negative control) and digested with a 1:100 dilution of RNase A/T1 mix. The protected fragments were resolved along with a DNA size marker by 6% PAGE with 8 M urea. (C) The 5′lst upstream sequence of N. gonorrhoeae F62. The −10 and −35 sequences of the promoter are boldface and underlined, and their positions relative to the translational start site are given. The transcriptional start site (tsp) is marked by an arrow; the putative Shine-Dalgarno (SD) sequence and translational start site are in boldface.
To confirm our primer extension results, we performed RPA analysis of different concentrations of total RNA isolated from N. gonorrhoeae strains F62 and FA1090. A protected probe fragment size of 65 bp was obtained from reaction mixtures containing total RNA from either N. gonorrhoeae F62 or N. gonorrhoeae FA1090 and was consistent with the transcriptional start site obtained from primer extension analysis (Fig. 6B): i.e., 74 bp upstream of lst ATG. Probe protection was linear with increasing concentrations of total RNA and specific for lst, since protection was absent when yeast total RNA was used in the reaction (Fig. 6B).
Finally, mutation of the sigma 70 promoter, from TAAAAT to ATTTTA in an N. gonorrhoeae 5′lst::lacZ construct abrogated β-Gal activity, confirming the function of this region as the only active promoter for lst in N. gonorrhoeae (N. gonorrhoeae = 118 ± 12 Miller units, N. gonorrhoeae TATA mutant = 5 ± 3 Miller units).
N. meningitidis lst is transcribed from a σ70 promoter present at the 3′ end of a CREE.
CREEs are repetitive elements scattered randomly in the pathogenic Neisseria spp. (2, 4, 13). CREEs act as promoters for neisserial genes like uvrB (1), drg (3) and IS1106Tip (24), or they serve as an RNA processing elements (5, 16, 23). All 17 N. meningitidis clinical isolates studied have the 105-bp CREE upstream proximal to the sigma 70 promoter that would drive lst transcription in N. gonorrhoeae (Fig. 4B). We investigated the possible modulatory role of the CREE element in lst transcription in N. meningitidis.
RT-PCR was used to map the lst promoter region in N. meningitidis. We asked whether amplification products could be obtained using a common reverse primer (Crev) in conjunction with oligonucleotide primers P1 through P7, spaced 30 to 60 bp apart (Table 1), that anneal upstream and downstream of the CREE (Fig. 7B). Amplification products were obtained only with primers that annealed downstream of the CREE (P4 to P7), suggesting that the promoter for lst transcription does not lie upstream of the CREE. An amplification product was obtained with primer P4, which anneals downstream of the CREE but upstream of the position of the N. gonorrhoeae lst promoter, suggesting that the 3′ end of the CREE is part of a promoter for lst in meningococci (Fig. 7B). The identities of the amplified products were confirmed by sequencing (data not shown).
FIG. 7.
Analysis of the N. meningitidis lst promoter. (A) Primer extension analysis. Total RNA (20 μg) was isolated from yeast (negative control) and N. meningitidis strains X4, MC58, and X3, and primer extension analyses were performed using primer PE2 (Table 1). The extension products along with a sequencing reaction with the same primer were resolved by 6% PAGE with 8 M urea. The arrow points to the transcriptional start site. The N. meningitidis −10 element is indicated as “−10,” and the inactive gonococcal −10 element is indicated as “Gc −10.” (B) RT-PCR analysis. N. meningitidis MC58 total RNA (20 μg) was reverse transcribed with and without the reverse transcriptase and was used in PCRs as template with seven forward primers (P1 to P7) and a common reverse primer (Crev). The amplified products were resolved in a 1.5% agarose gel along with DNA size markers. A diagram of the intergenic region between lst and icd is shown (not drawn to scale). The 30-bp and 13-bp elements, the 105-bp CREE, and the gonococcal −10 element are indicated as shaded boxes. The opposing dotted arrows indicate the translational start sites of lst and icd. The small arrows pointing to the right denote the forward primers (P1 to P7) and their relative position where they anneal to the lst upstream region. (C) 5′lst upstream sequence of N. meningitidis MC58. The −10 and −35 sequences of the promoter are in boldface and underlined, and their positions relative to the translational start site are given. The transcriptional start site is marked by an arrow, and a putative Shine-Dalgarno (SD) sequence and translational start site are in boldface.
It was evident from RT-PCR results that the 3′ end of CREE is part of a promoter but the transcriptional status of a second N. meningitidis promoter (the one used in N. gonorrhoeae) was unclear. To investigate the possibility that N. meningitidis strains have two lst promoters, primer extension analysis were performed on total RNA isolated from three N. meningitidis strains (MC58, X3, and X4). The extension reactions using primer PE2 (Table 1) produced a single major product that mapped to a thymine residue 92 bp upstream of ATG and 7 bp downstream of the −10 sequence of the putative sigma 70 promoter. No extension product was obtained that corresponded to the N. gonorrhoeae lst promoter (Fig. 7A). In addition, the extension product was more intense for X3 than MC58, in agreement with the amount of lst transcript found in these strains, and was specific for lst, since no band was formed when yeast RNA was used in the primer extension reaction. A single extension product was generated using a second primer, PE3 (Table 1), which was consistent with these results (data not shown). Analogous to the sigma 70 promoter 5′ of uvrB (1), the N. meningitidis lst sigma 70 promoter was also created by the insertion of the CREE (Fig. 4B).
Taken together, these data show that lst transcription in N. meningitidis proceeds from a single sigma 70 promoter present at the 3′ end of the CREE and that the N. gonorrhoeae lst promoter is inactive in N. meningitidis.
Evidence of divergent transcription from CREE ends.
The ends of CREE are complementary to each other, and DNA sequence analyses predict there may be divergent promoters directing transcription from both ends of CREEs (Fig. 8A). The 3′ terminus of the CREE found on the lst coding strand is highly similar in sequence to the 3′ terminus of CREE found on the isocitrate dehydrogenase (icd) coding strand (Fig. 8B). Since the 3′ terminus of the CREE on the lst coding strand possesses an active promoter, we speculated that the highly similar 3′ terminus of the CREE found on the icd coding strand could act as a promoter to transcribe icd. We used RT-PCR to investigate this possibility. Similar to mapping of the lst promoter, we used oligonucleotide primer pairs that anneal upstream and downstream from both the ends of CREE (i.e., with reference to the icd coding strand and the lst coding strand) (Fig. 8C). Amplified products were obtained only with primers that annealed downstream of the CREE with respect to each coding strand (Fig. 8C). The RT-PCR results suggest that the ends of the CREE possess promoters for the divergent transcription of icd and lst.
FIG. 8.
Evidence of divergent transcription from CREE ends. (A) Diagram of the intergenic region between lst and icd (not drawn to scale). The small opposing dotted arrows indicate the translational start sites of lst and icd. The number of bases denoted by dots that link sequences are given above the dots. The large open arrows pointing in the opposite directions represent inverted repeats (IRs) that are highly similar. The sequence comparison of them is shown in panel B, and individual base differences in the putative promoter regions are indicated by asterisks. (C) N. meningitidis MC58 total RNA (20 μg) was reverse transcribed with (+) and without (−) the reverse transcriptase and was used in PCRs as a template with two sets of primer pairs (IP1 and IP2 and P4 and Crev). The amplified products were resolved in 1.5% agarose gel along with DNA size markers. A diagram of the intergenic region between lst and icd is shown (not drawn to scale). The 30-bp, 13-bp, and 105-bp CREEs and the gonococcal −10 elements are indicated as hatched boxes. The opposing dotted arrows indicate the translational start sites of lst and icd. The two sets of primer pairs (IP1 and IP2 and P4 and Crev) are denoted as small arrows, and the relative positions where they anneal to the lst upstream and coding region are shown.
DISCUSSION
N. gonorrhoeae and N. meningitidis have similar and distinct systems for evading human immune factors during infection. Expression of the LOS-specific Lst is required in vitro for serum-sensitive strains of N. gonorrhoeae to resist complement-mediated killing (6, 7, 9, 34), while surface sialylation may enhance the resistance of N. meningitidis during periods of infection when capsule is not expressed (32).
Natural variations occur in the degree of LOS sialylation in different strains of pathogenic Neisseria spp. (8, 15, 18, 28). The factors that could affect the degree of LOS sialylation include the availability of phase-variable terminal galactose sialylation targets (29, 30), the amount of available CMP-NANA (21, 34), and inherent specific activity or regulated expression of Lst in these strains. In this study, we examined the regulation of lst gene transcription. We observed that extracts of N. gonorrhoeae F62 contain 5-fold more Stase activity than N. meningitidis MC58 ⊄3 and that among clinical neisseria strains that exhibit variable Stase activities, N. gonorrhoeae expresses about 2.2-fold more Stase activity than strains of N. meningitidis. Since the lst gene is 95 to 98% homologous between most strains (unpublished observation), we hypothesized that differences in transcriptional control may be the determinant for variations in Stase activities between N. gonorrhoeae and N. meningitidis. Analysis of transcript levels in N. gonorrhoeae and N. meningitidis by Northern blot analysis and real-time PCR showed that N. gonorrhoeae strains express more lst transcript than strains of N. meningitidis and correlated with Stase activity observed in the two species. However, there was not a direct correlation between levels of lst mRNA and Stase activity among strains of each species. The possible reasons for poor correlation observed between mRNA and Stase activities when comparing individual strains could be due to: (i) differences in inherent specific activity of Stases from different strains of N. gonorrhoeae or N. meningitidis due to point mutations, which we know exist from our sequencing efforts of the lst gene and recent data from others (11); (ii) Stase activity being affected by some unknown neisserial factor(s), perhaps, protein that binds either to Stase or LOS; or; (iii) the conformation of LOS and the interaction of enzyme (Stase) with its substrate (LOS) (33).
Real-time PCR analysis revealed that there was less than a 2-fold (1.6-fold) difference in lst transcript levels among the various N. gonorrhoeae strains examined, whereas transcript levels among N. meningitidis strains varied considerably; lst levels in N. meningitidis were as follows: strains MC58 ⊄3 and X8 < C1 and X4 (2.2 ± 0.2-fold more than MC58 ⊄3 and X8) < X3 (3.7 ± 0.3-fold more than MC58 ⊄3 and X8). It is possible that N. meningitidis X3 could have a mutation(s) or a host-specific factor(s) that enhances lst transcription. The observation that lst transcript levels in N. meningitidis strains vary while they are nearly unchanged in N. gonorrhoeae strains suggests that mechanisms for regulating this outer membrane protein are different between these two species. Another example of an outer membrane protein that is regulated differently between N. gonorrhoeae and N. meningitidis includes the mtrCDE-encoded efflux pump. Expression of the mtrCDE operon in N. gonorrhoeae is induced by the AraC-like protein, MtrA (22), and negatively regulated by MtrR (25). In N. meningitidis, the mtr system is subject to transcriptional regulation by integration host factor (IHF) and posttranscriptional regulation by cleavage in the inverted repeat of the Correia element (23) and not subjected to MtrR or MtrA regulatory schemes.
Sequence comparison of the 5′lst regions of N. gonorrhoeae and N. meningitidis strains reveals striking species-specific differences. The 5′lst region of N. gonorrhoeae varies, whereas the N. meningitidis 5′lst region remains relatively conserved. Some N. gonorrhoeae 5′lst regions contained a 13-bp and a 30-bp tandem repeat element that exists as single copies in other strains of N. gonorrhoeae and all strains of N. meningitidis. Most importantly, the 5′lst regions of all N. meningitidis strains have 105-bp CREEs that are absent in the 5′lst regions of all N. gonorrhoeae strains examined. To determine how these sequence differences might contribute to differential transcript levels in Neisseria spp., we created N. gonorrhoeae and N. meningitidis translational 5′lst::lacZ fusions and expressed them in the chromosomes of N. gonorrhoeae F62 and N. meningitidis MC58 ⊄3. The 5′lst region of N. gonorrhoeae was fivefold more active than that of N. meningitidis MC58 whether expressed in N. gonorrhoeae or N. meningitidis. Furthermore, this pattern of expression was observed in E. coli indicating the difference in promoter activity was predominantly a consequence of some intrinsic property of the DNA and was not caused by regulatory factors present in the host per se.
It is evident from the lacZ reporter studies that the 5′lst regions of N. gonorrhoeae and N. meningitidis have different promoter strengths. Promoter-mapping studies showed that N. gonorrhoeae and N. meningitidis use different promoters to transcribe lst under the growth conditions used in this study. In N. gonorrhoeae, a sigma 70 promoter 80 bp upstream of the translational start site is used to transcribe lst. In N. meningitidis, this promoter is inactive and is replaced by a sigma 70 promoter present at the 3′ terminus of a 105-bp CREE 99 bp upstream of the translational start site. The CREEs act as promoters for neisserial genes like uvrB (1), drg (3), and IS1106Tip (24). The promoter found at the 3′ terminus of the CREE of uvrB (1) is highly homologous to the promoter we mapped upstream of lst in N. meningitidis.
drg is transcribed only in strains that have CREE inserted in their upstream region and is silent in strains that do not have the CREE in their upstream region. Thus, regulation of drg in Neisseria spp. occurs by promoter insertion (3). Regulation of lst between the pathogenic Neisseria spp. occurs by promoter replacement. In N. gonorrhoeae, lst is transcribed from a strong sigma 70 promoter. In N. meningitidis, the strong gonococcal promoter is silent and is replaced by a weaker sigma 70 promoter formed by CREE insertion. In addition, for the first time, we provide evidence for the CREEs to act as divergent promoters directing transcription from both ends of inverted repeats. Overall, promoter-mapping studies and LacZ reporter studies show that lst transcription in N. gonorrhoeae and N. meningitidis occurs from different promoters with different strengths. We conclude that the differential Stase expression between N. gonorrhoeae and N. meningitidis is due at least in part to differential lst gene transcription.
The biological significance of the observation that N. gonorrhoeae strains transcribe more lst than N. meningitidis strains remains to be seen. One possible explanation could be that dependence of N. gonorrhoeae strains on sialylation for protection against complement-mediated killing would be higher than in N. meningitidis strains which have capsule for protection against complement-mediated killing. Sialylation of the N. gonorrhoeae lactoneotetraose (Lnt) epitope results in uniform enhanced resistance to normal human serum in serum-sensitive strains; however, sialylation of N. meningitidis Lnt does not result in enhanced serum resistance in all strains (Madico et al., abstr., p. 230, 14th Int. Pathogenic Neisseria Conf., Milwaukee, Wis., September 2004). Different lst transcript levels observed in N. gonorrhoeae and N. meningitidis and variations observed in lst transcripts among N. meningitidis strains may explain the contrasting behavior of Lnt sialylation in N. gonorrhoeae and N. meningitidis. Future experiments will address the probable role of differential sialylation of strains in protection against complement-mediated killing and association with epithelial cells. Posttranscriptional regulatory mechanisms and reasons for loss of correlation between Stase activity and lst expression among strains in each species will be examined.
Acknowledgments
We thank Michael Jennings for providing pNST-01, Virginia L. Clark for the gift of pLES94, Gi-Chung Chen for preparing LOS from N. gonorrhoeae F62, and John M. Williams for helpful suggestions. We thank Joanne Morrisey of Drexel University College of Medicine for help with technical expertise on primer extension and RPAs. We also thank Richard Kosich of the Center for Gene Therapy, MCP Hahnemann School of Medicine, for help in DNA sequencing.
This research was supported in part by grant AI33505 from the United States Public Health Service, National Institute of Allergy and Infectious Disease, to R.F.R.
Editor: J. N. Weiser
REFERENCES
- 1.Black, C. G., J. A. M. Fyfe, and J. K. Davies. 1995. A promoter associated with the neisserial repeat can be used to transcribe the uvrB gene from Neisseria gonorrhoeae. J. Bacteriol. 177:1952-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buisine, N., C. M. Tang, and R. Chalmers. 2002. Transposon-like Correia elements: structure, distribution and genetic exchange between pathogenic Neisseria sp. FEBS Lett. 522:52-58. [DOI] [PubMed] [Google Scholar]
- 3.Cantalupo, G., C. Bucci, P. Salvatore, C. Pagliarulo, V. Roberti, A. Lavitola, C. B. Bruni, and P. Alifano. 2001. Evolution and function of the neisserial dam-replacing gene. FEBS Lett. 495:178-183. [DOI] [PubMed] [Google Scholar]
- 4.Correia, F. F., S. Inouye, and M. Inouye. 1986. A 26-base-pair repetitive sequence specific for Neisseria gonorrhoeae and Neisseria meningitidis genomic DNA. J. Bacteriol. 167:1009-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Gregorio, E., C. Abrescia, M. S. Carlomagno, and P. P. Di Nocera. 2002. The abundant class of nemis repeats provides RNA substrates for ribonuclease III in Neisseriae. Biochim. Biophys. Acta 1576:39-44. [DOI] [PubMed] [Google Scholar]
- 6.de la Paz, H., S. J. Cooke, and J. E. Heckels. 1995. Effect of sialylation of lipopolysaccharide of Neisseria gonorrhoeae on recognition and complement-mediated killing by monoclonal antibodies directed against different outer-membrane antigens. Microbiology 141:913-920. [DOI] [PubMed] [Google Scholar]
- 7.Elkins, C., N. H. Carbonetti, V. A. Varela, D. Stirewalt, D. G. Klapper, and P. F. Sparling. 1992. Antibodies to N-terminal peptides of gonococcal porin are bactericidal when gonococcal lipopolysaccharide is not sialylated. Mol. Microbiol. 6:2617-2628. [DOI] [PubMed] [Google Scholar]
- 8.Estabrook, M. M., J. M. Griffiss, and G. A. Jarvis. 1997. Sialylation of Neisseria meningitidis lipooligosaccharide inhibits serum bactericidal activity by masking lacto-N-neotetraose. Infect. Immun. 65:4436-4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frangipane, J. V., and R. F. Rest. 1993. Anaerobic growth and cytidine 5′-monophospho-N-acetylneuraminic acid act synergistically to induce high-level serum resistance in Neisseria gonorrhoeae. Infect. Immun. 61:1657-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilbert, M., D. C. Watson, A. M. Cunningham, M. P. Jennings, N. M. Young, and W. W. Wakarchuk. 1996. Cloning of the lipooligosaccharide alpha-2,3-sialyltransferase from the bacterial pathogens Neisseria meningitidis and Neisseria gonorrhoeae. J. Biol. Chem. 271:28271-28276. [DOI] [PubMed] [Google Scholar]
- 11.Gulati, S., A. Cox, L. A. Lewis, F. St. Michael, J. Li, R. Boden, S. Ram, and P. A. Rice. 2005. Enhanced factor H binding to sialylated gonococci is restricted to the sialylated lacto-N-neotetraose lipooligosaccharide species: implications for serum resistance and evidence for a bifunctional lipooligosaccharide sialyltransferase in gonococci. Infect. Immun. 73:7390-7397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kellogg, D. S., Jr., W. L. Peacock, Jr., W. E. Deacon, L. Brown, and D. I. Pirkle. 1963. Neisseria gonorrhoeae. I. Virulence genetically linked to clonal variation. J. Bacteriol. 85:1274-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu, S. V., N. J. Saunders, A. Jeffries, and R. F. Rest. 2002. Genome analysis and strain comparison of Correia repeats and Correia repeat-enclosed elements in pathogenic Neisseria. J. Bacteriol. 184:6163-6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mandrell, R. E., and M. A. Apicella. 1993. Lipo-oligosaccharides (LOS) of mucosal pathogens: molecular mimicry and host-modification of LOS. Immunobiology 187:382-402. [DOI] [PubMed] [Google Scholar]
- 15.Mandrell, R. E., H. Smith, G. A. Jarvis, J. M. Griffiss, and J. A. Cole. 1993. Detection and some properties of the sialyltransferase implicated in the sialylation of lipopolysaccharide of Neisseria gonorrhoeae. Microb. Pathog. 14:307-313. [DOI] [PubMed] [Google Scholar]
- 16.Mazzone, M., E. De Gregorio, A. Lavitola, C. Pagliarulo, P. Alifano, and P. P. Di Nocera. 2001. Whole-genome organization and functional properties of miniature DNA insertion sequences conserved in pathogenic Neisseriae. Gene 278:211-222. [DOI] [PubMed] [Google Scholar]
- 17.McGee, D. J., and R. F. Rest. 1996. Regulation of gonococcal sialyltransferase, lipooligosaccharide, and serum resistance by glucose, pyruvate, and lactate. Infect. Immun. 64:4630-4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McQuillen, D. P., S. Gulati, S. Ram, A. K. Turner, D. B. Jani, T. C. Heeren, and P. A. Rice. 1999. Complement processing and immunoglobulin binding to Neisseria gonorrhoeae determined in vitro simulates in vivo effects. J. Infect. Dis. 179:124-135. [DOI] [PubMed] [Google Scholar]
- 19.Ram, S., F. G. Mackinnon, S. Gulati, D. P. McQuillen, U. Vogel, M. Frosch, C. Elkins, H. K. Guttormsen, L. M. Wetzler, M. Oppermann, M. K. Pangburn, and P. A. Rice. 1999. The contrasting mechanisms of serum resistance of Neisseria gonorrhoeae and group B Neisseria meningitidis. Mol. Immunol. 36:915-928. [DOI] [PubMed] [Google Scholar]
- 20.Ram, S., A. K. Sharma, S. D. Simpson, S. Gulati, D. P. McQuillen, M. K. Pangburn, and P. A. Rice. 1998. A novel sialic acid binding site on factor H mediates serum resistance of sialylated Neisseria gonorrhoeae. J. Exp. Med. 187:743-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rest, R. F., and R. E. Mandrell. 1995. Neisseria sialytransferases and their role in pathogenesis. Microb. Pathog. 19:379-390. [DOI] [PubMed] [Google Scholar]
- 22.Rouquette, C., J. B. Harmon, and W. M. Shafer. 1999. Induction of the mtrCDE-encoded efflux pump system of Neisseria gonorrhoeae requires MtrA, an AraC-like protein. Mol. Microbiol. 33:651-658. [DOI] [PubMed] [Google Scholar]
- 23.Rouquette-Loughlin, C. E., J. T. Balthazar, S. A. Hill, and W. M. Shafer. 2004. Modulation of the mtrCDE-encoded efflux pump gene complex of Neisseria meningitidis due to a Correia element insertion sequence. Mol. Microbiol. 54:731-741. [DOI] [PubMed] [Google Scholar]
- 24.Salvatore, P., C. Pagliarulo, R. Colicchio, P. Zecca, G. Cantalupo, M. Tredici, A. Lavitola, C. Bucci, C. B. Bruni, and P. Alifano. 2001. Identification, characterization, and variable expression of a naturally occurring inhibitor protein of IS1106 transposase in clinical isolates of Neisseria meningitidis. Infect. Immun. 69:7425-7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shafer, W. M., J. T. Balthazar, K. E. Hagman, and S. A. Morse. 1995. Missense mutations that alter the DNA-binding domain of the MtrR protein occur frequently in rectal isolates of Neisseria gonorrhoeae that are resistant to faecal lipids. Microbiology 141:907-911. [DOI] [PubMed] [Google Scholar]
- 26.Shell, D. M., L. Chiles, R. C. Judd, S. Seal, and R. F. Rest. 2002. The Neisseria lipooligosaccharide-specific α-2,3-sialyltransferase is a surface-exposed outer membrane protein. Infect. Immun. 70:3744-3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silver, L. E., and V. L. Clark. 1995. Construction of a translational lacZ fusion system to study gene regulation in Neisseria gonorrhoeae. Gene 166:101-104. [DOI] [PubMed] [Google Scholar]
- 28.Tsai, C.-M., and C. I. Civin. 1991. Eight lipooligosaccharides of Neisseria meningitidis react with a monoclonal antibody which binds lacto-N-neotetraose (Galβ1-4GlcNAcβ1-3Galβ1-4Glc). Infect. Immun. 59:3604-3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsai, C. M., G. Kao, and P. Zhu. 2002. Influence of the length of the lipooligosaccharide α chain on its sialylation in Neisseria meningitidis. Infect. Immun. 70:407-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Putten, J. P. 1993. Phase variation of lipopolysaccharide directs interconversion of invasive and immuno-resistant phenotypes of Neisseria gonorrhoeae. EMBO J. 12:4043-4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vogel, U., H. Claus, G. Heinze, and M. Frosch. 1999. Role of lipopolysaccharide sialylation in serum resistance of serogroup B and C meningococcal disease isolates. Infect. Immun. 67:954-957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vogel, U., S. Hammerschmidt, and M. Frosch. 1996. Sialic acids of both the capsule and the sialylated lipooligosaccharide of Neisseria meningitidis serogroup B are prerequisites for virulence of meningococci in the infant rat. Med. Microbiol. Immunol. 185:81-87. [DOI] [PubMed] [Google Scholar]
- 33.Wakarchuk, W. W., D. Watson, F. St. Michael, J. Li, Y. Wu, J. R. Brisson, N. M. Young, and M. Gilbert. 2001. Dependence of the bi-functional nature of a sialyltransferase from Neisseria meningitidis on a single amino acid substitution. J. Biol. Chem. 276:12785-12790. [DOI] [PubMed] [Google Scholar]
- 34.Wetzler, L. M., K. Barry, M. S. Blake, and E. C. Gotschlich. 1992. Gonococcal lipooligosaccharide sialylation prevents complement-dependent killing by immune sera. Infect. Immun. 60:39-43. [DOI] [PMC free article] [PubMed] [Google Scholar]