Abstract
Little is known about the function and regulation of splenic γδ T cells during chronic Plasmodium chabaudi malaria. The splenic γδ T-cell population continues to expand, reaching levels equal to 4 times the number of splenocytes in an uninfected mouse. Splenic γδ T cells from JH−/− mice with chronic malaria expressed Vγ1+ or Vδ4+ in the same ratio as uninfected controls with Vγ1 cells dominating, but the Vγ2 ratio declined about twofold. γδ T cells from G8 mice specific for the TL antigen increased only 2-fold in number, compared with 10-fold in BALB/c controls, but G8 γδ T cells failed to express the B220 activation marker. Elimination of the parasite by drug treatment caused a slow depletion in the number of splenic γδ, CD4+, and CD8+ T cells. Following challenge, drug-cured JH−/− mice exhibited nearly identical parasitemia time courses as naïve controls. Depletion of either CD4+ T cells or γδ T cells from chronically infected JH−/− mice by monoclonal antibody treatment resulted in an immediate and significant (P < 0.05) exacerbation of parasitemia coupled with a marked decrease in splenic γδ T-cell numbers. The number of CD4+ T cells, in contrast, did not decrease in mice after anti-T-cell receptor γδ treatment. The results indicate that cell-mediated immunity against blood-stage malarial parasites during chronic malaria (i) requires the continued presence of blood-stage parasites to remain functional, (ii) is dependent upon both γδ T cells and CD4+ T cells, and (iii) lacks immunological memory.
Blood-stage parasites of the genus Plasmodium, the causative agent of malaria in both human and animal hosts, initially replicate unchecked. Subsequently, the elicited immune response controls parasite replication, reducing parasitemia to low levels (25, 27, 39). Following reports of increased numbers of γδ T cells in the peripheral blood of Plasmodium falciparum-infected human subjects (19, 33), accumulating evidence indicates that γδ T cells are a required component of the antibody-independent, cell-mediated immune response that functions to suppress acute experimental parasitemia to low levels (39, 41). A marked polyclonal expansion of the γδ T-cell population is observed in the peripheral blood of patients acutely infected with P. falciparum, with the Vγ9, Vδ2, and Vδ1 subpopulations predominating (5, 18). γδ T cells also increase in number in the peripheral blood during the paroxysms of Plasmodium vivax malaria (31).
In vitro, human γδ T cells proliferate in response to stimulation with malarial antigens (10, 39); produce various cytokines, including gamma interferon (IFN-γ) (9, 15); and are regulated by CD4+ T cells (10). γδ T cells in peripheral blood mononuclear cells depleted of CD4+ T cells fail to proliferate when stimulated with malarial antigens in vitro. However, the proliferative response of these γδ T cells in CD4+ T-cell-depleted peripheral blood mononuclear cells is restored by the addition of exogenous cytokines that signal through components of the interleukin-2 receptor (IL-2R), such as IL-2, IL-4, and IL-15 (10, 12). In addition, human γδ T cells from naïve donors inhibit the replication of P. falciparum in vitro (11, 43), possibly by granulysin-mediated mechanisms (13). Taken together these findings suggest that γδ T cells respond to malarial antigens and contribute to protection against malaria by killing blood-stage parasites.
Results from studies of mouse models of malaria also support the role of γδ T cells as effector cells, as well as their regulation by CD4+ T cells (42-44). Splenic γδ T cells increase ∼100-fold in the spleens of B-cell-deficient JH−/− mice infected with different murine species of Plasmodium (43). CD4+ T cells are required for the splenic γδ T-cell response to occur in vivo during Plasmodium chabaudi malaria in these mice (36, 47). During acute P. chabaudi malaria, the splenic γδ T-cell population begins to expand in the ascending phase of parasitemia before reaching peak values in the descending phase, and it remains elevated for weeks thereafter (43). Splenic γδ T cells from P. chabaudi-infected mice express CD25, CD44, and CD71 transiently, with maximal levels on day 10 of infection, but maintain their expression of the γδ T-cell activation marker B220 (8, 43). Cell cycle analysis indicates that once maximal expansion of the γδ T-cell population occurs, proliferation then declines markedly, with only 15% of the cells exhibiting >1N DNA content (43).
Mice depleted of γδ T cells by monoclonal antibodies (MAbs) or gene knockout develop mildly exacerbated P. chabaudi parasitemia compared with γδ T-cell-intact controls (24, 42), suggesting a minimal role for γδ T cells in protection against blood-stage malaria. However, when the function of γδ T cells is examined in the absence of masking malaria-specific antibodies, it is evident that these cells are required for cell-mediated immunity (CMI) against the parasite (42, 50). Whereas immunologically intact mice sterilize their infections, B-cell-deficient mice suppress the parasitemia of acute P. chabaudi malaria to low or subpatent levels (<5%), (16, 45, 49). However, B-cell-deficient mice depleted of γδ T cells by MAb treatment or gene knockout develop high levels of unremitting P. chabaudi parasitemia (36, 42, 50).
Although the acute response and regulation of γδ T cells during malaria have been examined, little information is available regarding the function and regulation of these cells during chronic malaria. In the present study, we therefore analyzed the regulation and function of γδ T cells during chronic P. chabaudi malaria in JH−/− mice, where, in the absence of antibodies, their function is more crucial and easier to analyze.
MATERIALS AND METHODS
Infection of mice.
Female and male C57BL/6 and BALB/c mice were purchased from Jackson Laboratories (Bar Harbor, ME) and infected at between 6 and 16 weeks of age. JH−/− mice, which fail to produce immunoglobulins due to the targeted deletion of the JH gene segments in embryonic stem cells, are devoid of surface immunoglobulin-positive (Ig+) cells in the periphery because B-cell differentiation is blocked at the large CD43+ precursor stage (6); breeding pairs of mice were provided by D. Huszar (GenPharm International, San Diego, CA).
Breeding pairs of G8 mice were kindly provided by S. Hedrick (University of California San Diego, San Diego, CA). These mice, which are on a BALB/c background, are transgenic for a rearranged γδ T-cell receptor that is allo-specific for the TL antigens of C57BL/6 mice (51). Mice were bred at the AALAC-accredited animal facility in the University of Wisconsin-Madison, and all procedures were approved by the institutional animal use and care committee.
The malarial parasite used in these studies, P. chabaudi adami 556KA, was maintained and used as described previously (4). This strain is avirulent in mice with an intact immune system but induces high levels of unremitting parasitemia in immunodeficient SCID mice (47). Frozen parasite stabilate was thawed and injected intraperitoneally (i.p.) into a BALB/c source mouse. Subsequently, blood obtained from the source mouse was used to generate inoculum for experimental animals. Experimental mice were injected i.p. on day 0 with 106 erythrocytes parasitized with P. chabaudi unless noted otherwise. During the subsequent course of infection, parasitemia was assessed by enumerating parasites in 200 to 1,000 erythrocytes on Giemsa-stained thin blood films. Groups of at least three sex- and age-matched mice between 6 and 16 weeks of age were used in each experiment. Data are presented as the percent parasitemia, calculated as number of parasitized erythrocytes/total number of erythrocytes ×100.
Flow cytometry analysis.
Flow cytometry was performed on single-cell splenocyte suspensions as described previously (46). Aliquants of splenocytes were incubated for 10 min with anti-FcγII (Fc block; Becton Dickinson, San Diego, CA). The biotinylated antibodies were anti-CD3ɛ (Boehringer Mannheim, Indianapolis, IN), anti-T-cell receptor αβ (anti-TCRαβ), anti-TCRγδ, and hamster IgG isotype control (Becton Dickinson). Aliquants of splenocytes were incubated with these biotinylated antibodies for 20 min. After the cells were washed to remove free antibody, streptavidin-phycoerythrin (PE) (Southern Biotechnology Associates, Birmingham, AL) was incubated with the cells for 20 min. to fluorescently label the biotinylated antibodies. Fluorescein isothiocyanate (FITC)-conjugated antibodies were anti-CD3, anti-CD4, and anti-CD8 (Boehringer Mannheim) and rat IgG isotype control (Becton Dickinson); these MAbs were added at the same time as streptavidin-PE and were incubated with the cells for 20 min. The cells were washed twice and resuspended in 0.5 ml phosphate-buffered saline containing 5% fetal calf serum. Propidium iodide was added 5 min before data acquisition to allow exclusion of dead cells. Data acquisition was performed on a FACSCalibur (Becton Dickinson, Mountain View, CA) using the Cellquest program. Analysis was performed using the Attractors (Becton Dickinson) program.
Assessment of expression of selected activation markers and V-region usage was performed essentially as described above. Splenic γδ T cells were fluorescence labeled with anti-TCRγδ (GL3) and with anti-CD45R (B220; Becton Dickinson). Three-color analysis was used to identify the Vγ- and Vδ-expressing γδ T cells as detailed previously (34). Anti-Vγ1 (2.11), -Vγ2 (UC3), and -Vδ4 (GL2) conjugated with FITC were kind gifts from J. Bluestone, (University of Chicago, Chicago, IL) (20). All other antibodies were purchased from Becton Dickinson. Anti-TCRγδ-PE MAbs and anti-MAC1 conjugated with allophycocyanin were added to aliquants of splenocytes, and then Vγ region-specific anti-Vγ1, -Vγ2, and -Vγ3 (FITC conjugated) were added to separate splenocyte aliquants. The anti-TCRγδ MAb labeled all γδ T cells and allowed assessment of the percentage of γδ T cells expressing Vγ1, Vγ2, and Vγ3. The anti-MAC1 MAb was used in each aliquant of splenocytes to identify and exclude phagocytes from the analysis of γδ T cells, because these cells are often a source of nonspecific labeling. Anti-Vδ9 MAb was coincubated with anti-TCRαβ-PE to exclude reaction with Vα 2 regions. Anti-Vδ4 MAb was coincubated with anti-CD3-PE because anti-Vδ4 MAb inhibits binding of anti-TCRγδ MAb.
Drug treatment.
Beginning on day 21 postinfection (p.i.) after the suppression of acute P. chabaudi parasitemia, JH−/− mice were treated for three consecutive days with 1.2 mg of sulfamethoxazole and 0.24 mg of trimethoprim/ml (TMP/S) in their drinking water or remained untreated. The drug cure was verified by the absence of parasitized erythrocytes on Giemsa-stained blood films. Spleens from drug-treated JH−/− mice and untreated controls with chronic malaria were dissected from groups of three mice each on days 6 and 32 posttreatment. Single-cell suspensions were subjected to flow cytometry analysis as described above.
In the rechallenge experiment, P. chabaudi-infected JH−/− mice and uninfected control JH−/− mice were treated with TMP/S as described above. On day 73 p.i., the drug-cured JH−/− mice and drug-treated but previously uninfected control mice were injected i.p. with 1 × 106 P. chabaudi-parasitized erythrocytes.
Antibody depletion.
Anti-CD4 (GK1.5) and anti-TCRγδ (GL3) MAbs for depletion studies were purified by high-pressure liquid chromatography from ascitic fluid generated in SCID mice as described previously (47). The proteins were >95% IgG as determined by silver staining of polyacrylamide gels. Control hamster IgG antibody was purchased from Accurate Scientific (Westbury, NY). On day 28 p.i. groups of five or six chronically P. chabaudi-infected JH−/− mice were injected i.p. with 0.5 mg/mouse of anti-TCRγδ, 1.0 mg/mouse of anti-CD4 MAb, or 0.5 mg/mouse of hamster Ig. The parasitemia of each mouse was assessed before depleting MAb injections and again 7 days later. Efficiency of the MAb depletions was verified by flow cytometric analysis of mouse splenocytes.
Detection of selected cytokine mRNA in splenic γδ and CD4+ T cells.
Spleens were dissected from chronically infected JH−/− mice at 3 weeks p.i. A single-cell suspension was made, and then the erythrocytes were lysed by hypotonic shock. The splenocytes were fluorescence labeled by incubating the cells with biotinylated anti-TCRγδ, washing, and then incubating them with streptavidin-PE and anti-CD4-FITC. After being washed twice, the cells were fluorescence-activated cell sorted on a FACSvantage instrument, and 1 × 107 CD4+ or TCRγδ T cells were obtained for mRNA analysis; the cell purity was verified by flow cytometry. The sorted cell populations were pelleted by centrifugation at 1,100 rpm, and the RNA was isolated by guanidium isothiocyanate extraction. The RNA from each cell population was reverse transcribed using random-primed reverse transcription (RT) kits (Promega, Madison, WI) and then subjected to PCR amplification. The 50-μl PCR mixture contained PCR buffer, 1.25 mM deoxynucleoside triphosphates, 10 μl reverse-transcribed product, 1.25 U DNA polymerase, and 150 nM of PCR primers. The primers used are shown in Table 1. Thirty cycles of PCR were performed on a thermal cycler (PTC-100; M-J Research, Watertown, MA). Each cycle consisted of 1 min of denaturation at 94°C, 1 min of annealing at 60°C, and 1 min of extension at 72°C. The PCR products were analyzed on a 1% agarose gel stained with ethidium bromide.
TABLE 1.
Primers used to detect mRNA for the selected cytokines by PCR
| Cytokine | Forward primer | Reverse primer |
|---|---|---|
| β-Actin | ATG GAT GAC GAT ATC GCT | ATG AGG TAG TCT GTC AGG T |
| IL-2 | CTA GGC CAC AGA ATT GAA AGA TCT | GTA GGT GGA AAT TCT AGC ATC ATC C |
| IL-4 | CTG GAG CAA GTT TTA CGG CT | CTC TGA GAA AGC CCG AAA AG |
| IL-10 | GTG AAG ACT TTC TTT CAA ACA AAG | CTG CTC CAC TGC CTT GCT CTT ATT |
| TGF-β | GGA CAC CAA CTA TTG CTT CAG CT | CCT GGA CAC GCA GTA CAG CAA G |
| TNF | TCT CAT CAG TTC TAT GGC CC | GGG AGT AGA CAA GGT ACA AC |
| IFN-γ | GCT CTG AGA CAA TGA ACG CT | AAA GAG ATA ATC TGG CTC TGC |
Statistical analysis.
Analysis of variance with the Statview program (SAS Institute, Cary, NC) was performed to statistically compare parasitemias, cell numbers, and percentages in the different groups of mice.
RESULTS
The splenic γδ T-cell population expands during chronic P. chabaudi infection of B-cell-deficient mice.
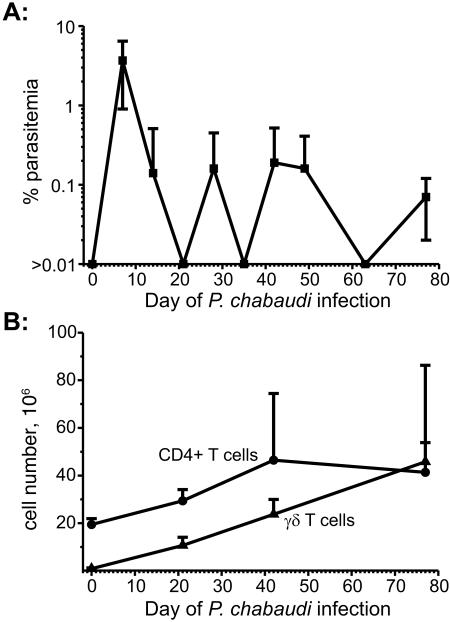
Previously we reported that both CD4+ αβ and γδ T cells proliferate in response to acute P. chabaudi malaria (43). To determine whether these cell populations continue to expand during chronic P. chabaudi infection in JH−/− mice, the spleens of three mice per group were harvested at 0, 3, 6, and 12 weeks p.i. and the numbers of CD4+ αβ and γδ T cells enumerated. Infected JH−/− mice had variable low-grade (≤1.0%) parasitemia (Fig. 1A) and exhibited increasing numbers of splenic CD4+ αβ and γδ T cells compared to uninfected JH−/− control mice throughout the observation period (Fig. 1B). Spleen cell populations in two mice with chronic P. chabaudi malaria were also analyzed at 19 months p.i.; the spleens of the P. chabaudi-infected JH−/− mice weighed 4 and 5.2 g at harvest and contained >3 ×108 CD4+ T cells and >4 × 108 γδ T cells/spleen (data not shown). Uninfected JH−/− mouse spleens weigh ∼0.08 g and contain between 3 × 107 and 5 × 107 splenocytes, of which 1 × 106 to 5 × 106 are γδ T cells (42).
FIG. 1.
(A) Chronic P. chabaudi parasitemia in JH−/− mice injected i.p with 1 × 106 parasitized erythrocytes. (B) The number of splenic CD3+ CD4+ and CD3+ TCRγδ+ cells was assessed by flow cytometry at weeks 0, 3, 6, and 12 p.i. during the course of P. chabaudi infection in groups (n = 3) of mice. The number of mice available for assessment of parasitemia was initially 12 and then declined by 3 as splenocytes were obtained from groups of mice for the flow cytometric analysis. Data represent the mean (±SD) for each time point.
The expanding populations of splenic γδ T cells express different V genes.
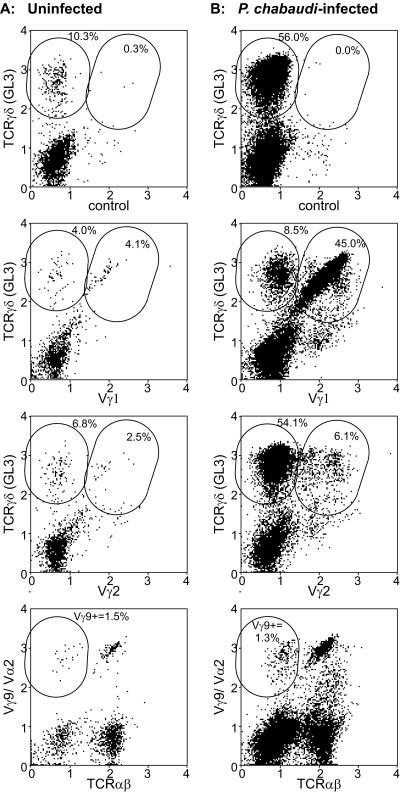
To determine whether splenic γδ T-cell subpopulations responded differentially during chronic malaria, we assessed the V-region phenotypes by flow cytometry of splenic γδ T-cell populations in groups (n = 6) of B-cell-deficient mice with chronic P. chabaudi infection (day 18 p.i.) and in uninfected controls. The results of this V-region analysis of the representative uninfected B-cell-deficient mouse (Fig. 2A) and the representative B-cell-deficient mouse with chronic P. chabaudi infection (Fig. 2B) are shown as a percentage of total live splenocytes; the averages ± standard deviations (SD) of each subset as a percentage of the γδ T-cell population are given below. Isotype antibody controls labeled 1.4% ± 1.2% of TCRγδ+ cells in uninfected mice and 0.2% ± 0.1% of TCRγδ+ cells on day 18 p.i. The improved resolution reflects the percentage of γδ T cells in splenic lymphocytes of uninfected mice (3.8% ± 1.0%) compared with infected mice (23.5% ± 7.3%).
FIG. 2.
Flow cytometric analysis of γδ T-cell subsets in an uninfected B-cell-deficient mouse (A) and a B-cell-deficient mouse with chronic P. chabaudi infection on day 18 p.i. (B). Each panel is a dot plot of the fluorescence intensity of labeling with two MAbs after exclusion with anti-MAC1 MAb labeling of macrophages and dead cells, which label nonspecifically. The antibodies used are indicated on the x and y axes; “control” represents isotype control antibodies. The values obtained for the representative mouse as a percentage of total live splenocytes are included as insets in each dot plot. The means (±SD) of percentages of the γδ T-cell population are given in the text.
γδ T cells from JH−/− mice with chronic P. chabaudi malaria were predominantly Vγ1 or Vγ2 positive (Fig. 2); Vγ1+ cells comprised 79.6% ± 5.3% of the splenic γδ T-cell population in JH−/− mice with chronic P. chabaudi malaria, and Vγ2 cells comprised 6.6% ± 3.2% the splenic γδ T-cell population. The percentage of splenic γδ T cells expressing Vγ1 or Vγ2 was 73.6% ± 9.5% and 12.1% ± 2.7%, respectively, in uninfected B-cell-deficient controls, indicating minimal preferential expansion of Vγ1 (P = 0.3) but a significant (P = 0.03) decline in the percentage of Vγ2 cells. Both Vγ1+ and Vγ2+ cells were blasts with forward scatter about 1.5-fold greater than that in uninfected control mice, and they increased markedly (∼100-fold) in number. Few (<2%) splenic γδ T cells from either B-cell-deficient mice with chronic P. chabaudi malaria or uninfected controls were positive with anti-Vγ3 (not shown) or anti-Vγ9 (Fig. 2) MAb. Cells double labeled with anti-TCRαβ MAb and anti-Vγ9/Vα2 are Vα2+ cells, whereas the anti-TCRαβ MAb-negative cells are Vγ9+ (Fig. 2). Anti-Vδ4 labeled 1.4% ± 1.1% of CD3+ T cells in B-cell-deficient mice with chronic P. chabaudi malaria and 1.1% ± 0.6% of CD3+ T cells in uninfected controls (not shown), suggesting that few Vδ4 γδ T cells were detectable in the spleen and this subset did not expand preferentially during P. chabaudi infection.
Role of T-cell antigen specificity in the expansion of the γδ T-cell subset and protection during chronic malaria.
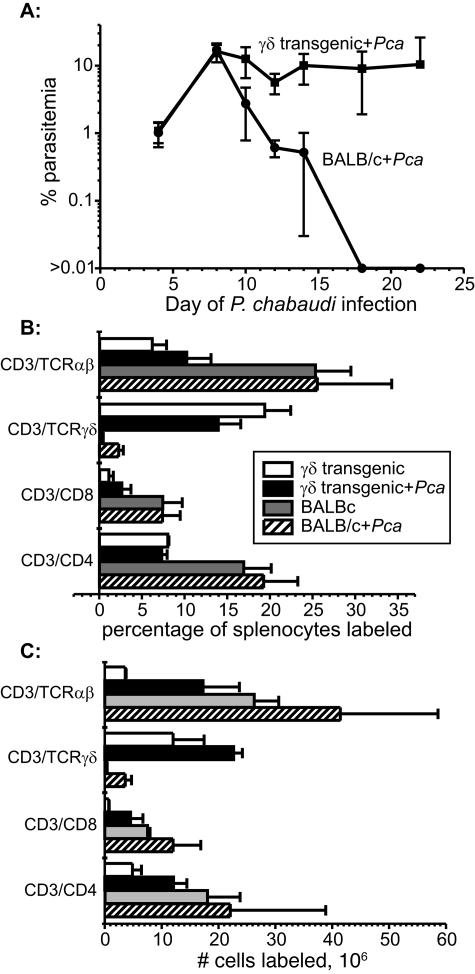
To assess whether all splenic γδ T cells are activated during P. chabaudi malaria regardless of their antigen specificity, we infected γδ T-cell-transgenic G8 mice (n = 5) on a BALB/c background with P. chabaudi and assessed the γδ T-cell response during descending parasitemia (day 10 p.i.) by flow cytometry. We previously reported that maximal activation of the γδ T-cell population occurs at this time, when the numbers and percentages of splenic γδ T cells are also maximal (18). Splenic γδ T cells from G8 mice infected with P. chabaudi failed to increase in percentage of splenocytes and did not express the activation marker CD45R (B220). On day 22 of P. chabaudi infection, the number of splenic γδ T cells had increased in parallel with splenomegaly (∼2-fold), compared to 10-fold in BALB/c mice. In addition, G8 mice failed to suppress their acute parasitemia (Fig. 3). This experiment was repeated with similar results.
FIG. 3.
(A) Time course of parasitemia in groups of G8 γδ T-cell-transgenic mice (n = 5) and BALB/c controls. (B and C) The percentage of splenocytes (B) and the number of selected populations of T cells (C) in uninfected BALB/c mice and G8 γδ T-cell-transgenic mice as well as infected groups of mice on day 22 of P. chabaudi infection. Data represent the mean (±SD) for each group.
The continued presence of viable parasites is required to maintain the expansion of the γδ T-cell population during chronic malaria.
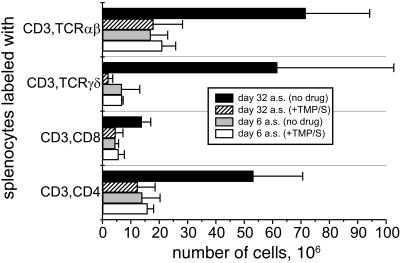
To determine whether the expansion of the γδ T-cell population detected during chronic malaria in JH−/− mice was dependent upon the continued presence of blood-stage parasites, groups of P. chabaudi-infected JH−/− mice were treated with TMP/S during the period of chronic malaria (days 21 through 23 p.i.). Infected control JH−/− mice remained untreated. TMP-treated mice were free of detectable parasites as determined by repeated negative blood films, whereas the chronic parasitemia in untreated JH−/− controls was detectable but low (<1%). The numbers of splenic γδ T cells in drug-cured JH−/− mice 1 month after drug treatment declined dramatically (>20-fold) compared with those in untreated control mice (Fig. 4). The numbers of both CD4+ T cells and CD8+ αβ T cells also declined, ∼5- and 3-fold, respectively, after drug treatment.
FIG. 4.
Average numbers ± SD of cells in splenic T-cell populations on days 6 and 32 after drug treatment with TMP/S (designated a.s. in the figure) of B-cell-deficient mice chronically infected with P. chabaudi. Parasitemia was undetectable.
Homeostasis of T cells elicited in response to P. chabaudi infection is restored in drug-cured JH−/− mice.
To determine whether the maintenance of immunity is dependent upon the continued presence of viable parasites and can be recalled, the time courses of P. chabaudi parasitemia in drug-cured JH−/− mice and uninfected controls after challenge with 1 × 106 parasitized erythrocytes were compared. Twenty-one days after the JH−/− mice had suppressed their acute infections, they and uninfected JH−/− mice were treated with TMP/S for 3 days (Fig. 5A). No parasites were detected in the Giemsa-stained thin blood films of the drug-cured JH−/− mice. Two months following drug cure, both groups of mice were challenged i.p. with 1 × 106 P. chabaudi-parasitized erythrocytes. The time course of infection in the previously infected and drug-cured JH−/− mice (secondary) was nearly identical to that in the drug-cured uninfected controls (primary) (Fig. 5B). This experiment was repeated with similar results.
FIG. 5.
(A) Experimental protocol detailed in weeks after the initial infection of the secondary group in order to compare the secondary and primary parasitemias. All infections were initiated by i.p. injection of 1 × 106 P. chabaudi-parasitized erythrocytes. (B) Time courses of primary and secondary parasitemias in groups (n = 10) of B-cell-deficient mice treated as described in panel A. Note that both groups of mice received similar drug treatments. Data represent the mean (±SD) for each time point.
CD4+ T cells are required for the maintenance of protective γδ T cells during chronic P. chabaudi malaria in B-cell-deficient mice.
To determine whether CD4+ T cells are necessary to maintain the elevated numbers of γδ T cells in the spleens of JH−/− mice during chronic malaria, we treated chronically infected mice with anti-CD4 MAb. We also treated a second group of JH−/− mice with anti-TCRγδ MAb to determine whether γδ T cells contribute to the control of parasitemia during chronic malaria. Control JH−/− mice chronically infected with P. chabaudi received hamster Ig in place of the T-cell-depleting antibodies. Previously, we have observed no detectable effect on parasitemia or immune response of Ig injection versus saline injection; consequently, we did not include both antibody controls. On the day of treatment with antibody (day 28 p.i.), all three groups of mice had similar levels of chronic parasitemia (hamster Ig, 0.5% ± 0.2%; anti-CD4, 0.6% ± 0.3%; anti-TCRγδ, 0.5% ± 0.4%).
On day 35 p.i., 7 days after treatment, parasitemia had risen significantly (P < 0.005) in anti-CD4-injected JH−/− mice compared with hamster Ig-injected control mice (Table 2). No splenic CD4+ T cells were detectable in the anti-CD4-treated mice by flow cytometry; the percentage of splenic γδ T cells within the lymphocyte region declined significantly (P < 0.005) during this period, from 33% ± 5% in hamster Ig-treated controls to 4% ± 3% in anti-CD4-treated mice (Table 2). It is unlikely that the anti-CD4 MAb depleted splenic γδ T cells, because flow cytometric analysis of splenocytes from either immunologically intact mice or B-cell-deficient infected with P. chabaudi did not detect any CD4+ or CD8+ γδ T cells (43, 44, 47).
TABLE 2.
Number of cells in CD3+ CD4+, CD3+ TCRαβ+, and CD3+ TCRγδ+ subsets and percentage of these subsets of total lymphocytes in groups of mice injected with either hamster Ig, anti-CD4 MAb, or anti-TCRγδ on day 21 after injection of antibodya
| Treatment (n) | Parasitemia at day 35 p.i.b | No. of cells (106)
|
% of lymphocytes
|
||||
|---|---|---|---|---|---|---|---|
| CD3+ CD4+ | CD3+ TCRαβ+ | CD3+ TCRγδ+ | CD3+ CD4+ | CD3+ TCRαβ+ | CD3+ TCRγδ+ | ||
| Hamster Ig (6) | 0.8 ± 0.4 | 112 ± 22 | 137 ± 27 | 89 ± 23 | 41 ± 4 | 50 ± 5 | 33 ± 5 |
| Anti-CD4 (6) | 29.1 ± 16.8c | 0 ± 0 | 64 ± 15 | 5 ± 5 | 0 ± 0 | 44 ± 7 | 4 ± 3 |
| Anti-TCRγδ (5) | 29.1 ± 10.7c | 228 ± 78 | 487 ± 237 | 1 ± 1 | 35 ± 6 | 67 ± 10 | 0 ± 0 |
Fluorescence labeling of splenocytes with isotype control antibodies was <0.2% for each animal. One mouse in the anti-CD4 MAb-injected group and one mouse in the anti-TCRγδ-injected group were indistinguishable in terms of the number and percentage of CD3+ CD4+ and CD3+ TCRγδ+ cells from hamster Ig-injected controls and were excluded. The percentage of CD3+ TCRαβ− cells was similar to the percentage of CD3+ TCRγδ+ cells, indicating that antimasking by anti-TCRγδ MAb did not affect our results (data not shown). Results are means ± SD.
Seven days after injection with antibody.
P < 0.005 compared with hamster Ig-injected controls.
Few, if any, splenic γδ T cells were detected by flow cytometry in anti-TCRγδ-treated mice, and the parasitemia in the anti-TCRγδ-injected JH−/− mice with chronic malaria increased significantly (P < 0.005) on day 35 p.i. compared to that in hamster Ig-treated JH−/− control mice during the same period (Table 2). The number of splenic CD4+ T cells was about twofold greater in the anti-TCRγδ-injected JH−/− mice with chronic P. chabaudi malaria than in hamster Ig-injected controls. Masking of the anti-TCRγδ MAb epitope by the depleting antibody did not occur, because the percentage of CD3+ TCRαβ− cells (γδ T cells) was similar to the percentage of CD3+ TCRγδ+ cells. Similar results were obtained in a replicate experiment.
γδ T cells and CD4+ αβ T cells transcribe type 1 and type 2 cytokine genes during chronic P. chabaudi malaria in B-cell-deficient mice.
To identify the cytokines produced by CD4+ and γδ T cells during chronic P. chabaudi malaria in B-cell-deficient JH−/− mice, we injected JH−/− mice i.p. with 106 parasitized erythrocytes and obtained highly purified cell populations by fluorescence-activated cell sorting from splenocytes during chronic parasitemia (day 21 p.i.). The CD4+ T-cell population contained 99.1% CD3+ CD4+ cells, and the γδ T-cell population contained 97.4% CD3+ TCRγδ+ cells. The RT-PCR analysis for cytokine mRNAs from 1 × 107 cells of these purified cell populations suggests that splenic CD4+ T cells produce the type 1 cytokines IFN-γ and tumor necrosis factor (TNF) plus the type 2 cytokines IL-4, IL-10, and transforming growth factor β (TGF-β). Splenic γδ T cells produced mRNAs for the type 1 cytokines IFN-γ and TNF plus the type 2 cytokines IL-10 and TGF-β (Fig. 6).
FIG. 6.
Highly purified splenic CD3+ CD4+ T cells and CD3+ TCRγδ+ T cells isolated from chronically infected B-cell-deficient mice produce both type 1 and type 2 cytokines. The flow cytometry analysis of the sorted CD3+ CD4+ T cells and CD3+ TCRγδ+ T cells shows the purity of the populations. The cytokine mRNAs detected by RT-PCR in CD3+CD4+ T cells and CD3+ TCRγδ+ T cells are shown in the ethidium bromide-stained agarose gels. mw mark, molecular weight marker.
DISCUSSION
A marked (100-fold) expansion of the splenic γδ T-cell population occurs in JH−/− mice during acute infection with P. chabaudi (43, 44, 49), but then the rate of increase in γδ T-cell numbers diminishes during subsequent chronic parasitemia (43). The observed increase in the number of γδ T cells as chronic P. chabaudi malaria in B-cell-deficient mice progresses (Fig. 1B) suggests that an additional slow but steady accumulation of γδ T cells occurs during chronic P. chabaudi malaria in B-cell-deficient mice. Because the percentage of proliferating cells falls after the acute phase of infection (16), γδ T cells are long-lived and are maintained by the infection. Indeed, when parasites were eliminated by drug treatment, the number of splenic γδ T cells declined over the course of a month, indicating that the continued presence of viable blood-stage parasites is required to maintain the expanding γδ T-cell population during chronic malaria in JH−/− mice.
Zinkernagel and Hengartner (52) have proposed that the persistence of antigen in the lymphohemopoeitic system, such as occurs during chronic infections, depletes antigen-reactive T cells, producing a mechanism to prevent immunopathology (52). Apoptotic T cells have been detected during acute malaria in human and animal hosts (14), but both CD4+ αβ T cells and γδ T cells continue to increase in number throughout a 6-month period p.i. in B-cell-deficient mice chronically infected with P. chabaudi, where there is continual release of antigen when the parasitized erythrocytes lyse. Despite the continual presence of antigen, our results indicate that Plasmodium-specific CD4+ T and γδ T cells are maintained in the face of persistent antigen in chronically infected B-cell-deficient mice and are required to control the parasitemia.
Our results also indicate that the Vγ1 T-cell subset maintains similar ratios in infected and uninfected mice but that the Vγ2 subpopulation declines in B-cell-deficient mice with chronic P. chabaudi infection. These two subpopulations account for the vast majority of the splenic γδ T cells, with the Vγ1 subset (∼80%) exceeding the Vγ2 subset (∼5%) by more than 15-fold. The remaining γδ T cells expressing other Vγ regions comprised less than 15% of the splenic γδ T-cell population. These results contrast with those of Seixas and Langhorne (36), who reported that the Vγ2 subpopulation expands primarily (>80% of γδ T cells) in B-cell-deficient mice infected with P. chabaudi; they used the more virulent AS strain of P. chabaudi chabaudi and the μ-MT mouse in their studies, which might explain the conflicting results.
This differential expansion of Vγ1 and Vγ2 T cells during P. chabaudi malaria suggests that the γδ T-cell response may be antigen specific. This contention is further supported by our findings with G8 mice infected with P. chabaudi. G8 mice are transgenic mice on a BALB/c background with large numbers of splenic γδ T cells that are specific for the TL antigen on C57BL/6 mice (51). The presence of rearranged Vγ and Vδ regions suppresses rearrangement of the endogenous Vγ regions in T cells, so virtually all of the γδ T cells express the transgenic receptor, with no detectable endogenous TCRγδ (51). Splenic γδ T-cell numbers in G8 mice increase markedly less in number (∼2-fold) in response to P. chabaudi infection than in BALB/c controls (∼10-fold). In contrast to γδ T cells from infected BALB/c mice, γδ T cells from infected G8 mice did not express the activation marker B220. Because there was minimal expansion of the γδ T-cell population in G8 mice and these γδ T cells did not express the activation marker B220, it is likely that responding γδ T cells are TL antigen specific and that “endogenous” γδ T-cell receptor, if any, is not sufficient to activate these cells. An alternate explanation for the minimal γδ T-cell response in G8 mice infected with P. chabaudi is that the CD4+ T-cell help in G8 mice for γδ T cells is impaired and insufficient to activate the G8 γδ T cells. The minimal expansion of G8 γδ T cells during P. chabaudi infection may represent a homestatic control mechanism due to the large numbers of cells; however, the number of G8 cells is much lower (3 × 107) than that observed in chronically B-cell-deficient mice (4 × 108), suggesting that there is minimal if any homeostatic regulation of this cell population in B-cell-deficient mice.
Not only are CD4+ T cells and γδ T cells essential for the suppression of P. chabaudi parasitemia during acute infection, as we reported previously (17), but both cell types are essential for the control of parasitemia during chronic infection. Depletion of either subset in chronically infected JH−/− mice resulted in an immediate and significant (P < 0.005) increase in parasitemia. Because the number of γδ T cells declines markedly and rapidly after CD4+ T-cell depletion in B-cell-deficient mice with chronic P. chabaudi infection, the γδ T-cell population responding during malaria requires CD4+ αβ T-cell help. There is a similar requirement for CD4+ T-cell help to initiate γδ T-cell activation during acute malaria (47). In the anti-CD4 MAb-injected mice with chronic P. chabaudi infection, virtually all the CD3+ TCRαβ+ cells were CD8+, but there were cell types (e.g., NK cells and monocytes) with forward and side scatter characteristics of lymphocytes that are neither T nor B cells (100% cells − 44% αβ T cells − 4% γδ T cells − 0 B cells = 52%) (Table 2). This increase in the percentage of this cell type is due in part to the removal of the CD4+ T cells, which normally comprise ∼40% of the lymphocytes in B-cell-deficient mice. Although we have not identified this cell type, their influx or proliferation in the spleen is not sufficient to control parasite replication. Although the depletion of γδ T cells by MAb treatment had no effect on the number of splenic CD4+ T cells, a significant (P < 0.005) increase in parasitemia also occurred. However, during acute parasitemia, γδ T cells affect the nature of CD4+ T-cell help, which becomes predominantly Th2 in γδ T-cell-deficient mice rather than Th1 as reported for intact control mice (35).
The mechanisms that CD4+ T cells utilize to regulate γδ T-cell numbers and function during chronic malaria remain to be elucidated but most likely involve the production of cytokines. Previously, we reported that the in vitro proliferation of human γδ T cells in response to P. falciparum antigens requires the activation of CD4+ T cells or the addition of exogenous cytokines signaling through the IL-2R (10, 12). During acute malaria, CD4+ T cells in B-cell-deficient mice produce a variety of Th1 cytokines, including IL-2, IFN-γ, TNF, and lymphotoxin (25, 39, 41). Although the results of the RNA studies are not quantitative, they suggest that CD4+ T cells from JH−/− mice with chronic P. chabaudi malaria have mRNAs for a mixture of type 1 (IFN-γ and TNF-α) and type 2 (IL-4, IL-10, and TGF-β) cytokines. Indeed, we have observed similar cytokine profiles for CD4+ T cells isolated from spleen, brain, and lung during Plasmodium berghei ANKA malaria (data not shown). We did not detect IL-2 mRNA in the CD4+ T cells isolated during chronic malaria, but other cytokines, including IL-4 and IL-15, may function to maintain the γδ T-cell population in infected B-cell-deficient mice. Indeed, most (>94%) splenic γδ T cells in P. chabaudi-infected mice express CD122 (IL-2Rβ and IL-15Rβ), but few γδ T cells express CD25 (IL-2Rα) (data not shown). Human γδ T cells stimulated with P. falciparum antigen express both type 1 and type 2 cytokines (9, 15). Therefore, it is not surprising that splenic γδ T cells during chronic P. chabaudi malaria in JH−/− mice, like CD4+ T cells, express mRNAs for both type 1 and type 2 cytokines, including IFN-γ, TNF, lymphotoxin, and IL-10. Seixas and Langhorne also detected IFN-γ mRNA in splenic γδ T cells of μMT−/− mice infected with P. chabaudi (36).
Our observation that mRNAs for both Th1 and Th2 cytokines were detected in the spleens of chronically infected JH−/− mice questions the hypothesis that chronically differentiated CD4+ T cells express either type 1 or type 2 cytokines (28, 29). Moreover it challenges the contention suggested by others that the function of terminal CD4+ T-cell differentiation during malaria is to elicit either CMI through type 1 cytokines or antibody-dependent immunity (AMI) through type 2 cytokines (30); both arms of the immune response have been reported to be capable of suppressing the parasitemia of acute P. chabaudi malaria (38, 50). Sexias et al. (35) reported that the production of type 1-dependent antibody isotypes (IgG2a and IgG2b) to Plasmodium antigens in response to primary P. chabaudi infection exceeds the type 2 dependent IgG1 antibody response. In addition, we observed that both IL-4 and IFN-γ knockout mice are protected against P. chabaudi challenge after immunization with homologous antigenic constructs, AMA-1 or MSP-1 (2), further questioning the concept that protective antibodies against blood-stage P. chabaudi are IL-4 dependent.
The T cell-mediated immune mechanisms responsible for modulating parasitemia in JH−/− mice chronically infected with P. chabaudi remain unknown. Because no cytokine has been reported to kill blood-stage malaria parasites directly (25, 27, 39), it seems likely that CD4+ T cells function in the parasite-killing effector pathway by activating other downstream cell types, which then kill the parasites. We and others have observed that human γδ T cells are capable of killing P. falciparum parasites in vitro (11, 40) by granulysin-mediated mechanisms (13). Whether similar mechanisms function in vivo remains to be determined. Both CD4+ T cells and γδ T cells from chronically infected mice expressed mRNA for IFN-γ, which plays an important role in AMI to P. chabaudi malaria and an essential role in CMI to blood-stage infection (1, 37, 48). When JH−/− mice are made IFN-γ deficient by treatment with neutralizing MAb or gene-targeted knockout, they develop noncuring high-grade parasitemia despite having the same numbers of B220+ splenic γδ T cells as infected JH−/− single-knockout mice (unpublished observations). Whether the necessary concentration of IFN-γ is provided by both T-cell populations or primarily from one has not been determined.
Previously, we reported that B-cell-deficient mice infected with normally nonlethal Plasmodium yoelii develop chronic malaria instead of dying when treated with subcurative doses of clindamycin (32). Some of the drug-treated mice were cured of their infections, but when subsequently challenged with homologous parasites, they developed fulminating fatal malaria (17). In contrast, chronically infected mice maintained a low level of parasitemia despite being challenged with 1 × 109 parasitized erythrocytes. As indicated above, the number of splenic γδ T cells declined to preinfection levels in drug-treated B-cell-deficient mice that had been infected previously. When rechallenged, these mice developed parasitemia time courses nearly identical to those of naïve mice initially infected with P. chabaudi. In contrast, B-cell-deficient mice with chronic malaria resist superinfection following rechallenge (17). These results, in addition to those of Seixas and Langhorne (36), confirm and extend our original observation that CMI against blood-stage malaria parasites not only requires the continued presence of viable parasites but lacks a memory component as well. Whether CD4+ T cells or γδ T cells or both are responsible for this lack of memory remains to be determined. It is possible that the lack of memory is due to the activation of regulatory cells, though none of those described thus far appear to alter parasitemia. Alternatively, a productive interaction between B cells and CD4+ T cells, which is lacking in B-cell-deficient mice, is needed to develop a CD4+ memory response.
It has been stated that the roles of γδ T cells in infectious diseases are enigmatic (3). Similarly, the function of γδ T cells in human malaria remains questionable. Both pathogenic and protective roles have been postulated for γδ T cells (25, 27, 39), but aside from the in vitro killing of P. falciparum (11, 13, 40), there is little evidence to support either role. Although γδ T cells increase markedly in the peripheral blood of adults suffering from acute P. falciparum malaria (19, 33), they fail to do so in parasitemic pediatric and adult African subjects living in areas where malaria is endemic, possibly because their function is obviated by the presence of AMI as we have observed in mice (7, 21). In contrast, the results of the present study of chronic malaria extend our previous findings and those of others to indicate that murine γδ T cells play a vital in vivo role in immunity to malaria. As the mechanisms of how they accomplish this are understood, it may be possible to identify similar mechanisms functioning in human γδ T cells during malaria and to modulate these to enhance parasite killing in vivo. Such efforts are currently under way to utilize γδ T cells against human immunodeficiency virus and tumors in hopes of discovering new approaches to effect disease control (22, 23, 26).
Acknowledgments
This work was supported by grants from the National Institutes of Health (AI 12710 and 40667).
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Batchelder, J. M., J. M. Burns, Jr., F. K. Cigel, H. Lieberg, D. D. Manning, B. J. Pepper, D. M. Yanez, H. van der Heyde, and W. P. Weidanz. 2003. Plasmodium chabaudi adami: interferon-gamma but not IL-2 is essential for the expression of cell-mediated immunity against blood-stage parasites in mice. Exp. Parasitol. 105:159-166. [DOI] [PubMed] [Google Scholar]
- 2.Burns, J. M., Jr., P. R. Flaherty, P. Nanavati, and W. P. Weidanz. 2004. Protection against Plasmodium chabaudi malaria induced by immunization with apical membrane antigen 1 and merozoite surface protein 1 in the absence of gamma interferon or interleukin-4. Infect. Immun. 72:5605-5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carding, S. R., and P. J. Egan. 2002. γδ T cells: functional plasticity and heterogeneity. Nat. Rev. Immunol. 2:336-345. [DOI] [PubMed] [Google Scholar]
- 4.Cavacini, L. A., L. A. Parke, and W. P. Weidanz. 1990. Resolution of acute malarial infections by T-cell-dependent non-antibody-mediated mechanisms of immunity. Infect. Immun. 58:2946-2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang, W. L., H. van der Heyde, D. G. Maki, M. Malkovsky, and W. P. Weidanz. 1992. Subset heterogeneity among γδ T cells found in peripheral blood during Plasmodium falciparum malaria. Immunol. Lett. 32:273-274. [DOI] [PubMed] [Google Scholar]
- 6.Chen, J., M. Trounstine, F. W. Alt, F. Young, C. Kurahara, J. F. Loring, and D. Huszar. 1993. Immunoglobulin gene rearrangement in B cell deficient mice generated by targeted deletion of the JH locus. Int. Immunol. 5:647-656. [DOI] [PubMed] [Google Scholar]
- 7.Chen, Z. W., and N. L. Letvin. 2003. Vγ2Vδ2+ T cells and anti-microbial immune responses. Microbes Infect. 5:491-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cron, R. Q., T. F. Gajewski, S. O. Sharrow, F. W. Fitch, L. A. Matis, and J. A. Bluestone. 1989. Phenotypic and functional analysis of murine CD3+,CD4−,CD8− TCR-γδ-expressing peripheral T cells. J. Immunol. 142:3754-3762. [PubMed] [Google Scholar]
- 9.Dieli, F., M. Troye-Blomberg, S. E. Farouk, G. Sirecil, and A. Salerno. 2001. Biology of γδ T cells in tuberculosis and malaria. Curr. Mol. Med. 1:437-446. [DOI] [PubMed] [Google Scholar]
- 10.Elloso, M. M., H. C. van der Heyde, A. Troutt, D. D. Manning, and W. P. Weidanz. 1996. Human γδ T cell subset-proliferative response to malarial antigen in vitro depends on CD4+ T cells or cytokines that signal through components of the IL-2R. J. Immunol. 157:2096-2102. [PubMed] [Google Scholar]
- 11.Elloso, M. M., H. C. van der Heyde, J. A. vande Waa, D. D. Manning, and W. P. Weidanz. 1994. Inhibition of Plasmodium falciparum in vitro by human γδ T cells. J. Immunol. 153:1187-1194. [PubMed] [Google Scholar]
- 12.Elloso, M. M., M. Wallace, D. D. Manning, and W. P. Weidanz. 1998. The effects of interleukin-15 on human γδ T cell responses to Plasmodium falciparum in vitro. Immunol. Lett. 64:125-132. [DOI] [PubMed] [Google Scholar]
- 13.Farouk, S. E., L. Mincheva-Nilsson, A. M. Krensky, F. Dieli, and M. Troye-Blomberg. 2004. γδ T cells inhibit in vitro growth of the asexual blood stages of Plasmodium falciparum by a granule exocytosis-dependent cytotoxic pathway that requires granulysin. Eur. J. Immunol. 34:2248-2256. [DOI] [PubMed] [Google Scholar]
- 14.Good, M. F. 2005. Vaccine-induced immunity to malaria parasites and the need for novel strategies. Trends Parasitol. 21:29-34. [DOI] [PubMed] [Google Scholar]
- 15.Goodier, M. R., C. Lundqvist, M. L. Hammarstrom, M. Troye-Blomberg, and J. Langhorne. 1995. Cytokine profiles for human Vγ9+ T cells stimulated by Plasmodium falciparum. Parasite Immunol. 17:413-423. [DOI] [PubMed] [Google Scholar]
- 16.Grun, J. L., and W. P. Weidanz. 1981. Immunity to Plasmodium chabaudi adami in the B-cell-deficient mouse. Nature 290:143-145. [DOI] [PubMed] [Google Scholar]
- 17.Grun, J. L., and W. P. Weidanz. 1983. Antibody-independent immunity to reinfection malaria in B-cell-deficient mice. Infect. Immun. 41:1197-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho, M., P. Tongtawe, J. Kriangkum, T. Wimonwattrawatee, K. Pattanapanyasat, L. Bryant, J. Shafiq, P. Suntharsamai, S. Looareesuwan, H. K. Webster, and J. F. Elliott. 1994. Polyclonal expansion of peripheral gamma delta T cells in human Plasmodium falciparum malaria. Infect. Immun. 62:855-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho, M., H. K. Webster, P. Tongtawe, K. Pattanapanyasat, and W. P. Weidanz. 1990. Increased γδ T cells in acute Plasmodium falciparum malaria. Immunol. Lett. 25:139-141. [DOI] [PubMed] [Google Scholar]
- 20.Houlden, B. A., L. A. Matis, R. Q. Cron, S. M. Widacki, G. D. Brown, C. Pampeno, D. Meruelo, and J. A. Bluestone. 1989. A TCRγδ cell recognizing a novel TL-encoded gene product. Cold Spring Harbor Symp. Quant. Biol. 54:45-55. [DOI] [PubMed] [Google Scholar]
- 21.Hviid, L., J. A. Kurtzhals, D. Dodoo, O. Rodrigues, A. Ronn, J. O. Commey, F. K. Nkrumah, and T. G. Theander. 1996. The g/d T-cell response to Plasmodium falciparum malaria in a population in which malaria is endemic. Infect. Immun. 64:4359-4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kabelitz, D., D. Wesch, E. Pitters, and M. Zoller. 2004. Potential of human γδ T lymphocytes for immunotherapy of cancer. Int. J. Cancer 112:727-732. [DOI] [PubMed] [Google Scholar]
- 23.Kato, Y., Y. Tanaka, F. Miyagawa, S. Yamashita, and N. Minato. 2001. Targeting of tumor cells for human γδ T cells by nonpeptide antigens. J. Immunol. 167:5092-5098. [DOI] [PubMed] [Google Scholar]
- 24.Langhorne, J., P. Mombaerts, and S. Tonegawa. 1995. αβ and γδ T cells in the immune response to the erythrocytic stages of malaria in mice. Int. Immunol. 7:1005-1011. [DOI] [PubMed] [Google Scholar]
- 25.Li, C., E. Seixas, and J. Langhorne. 2001. Rodent malarias: the mouse as a model for understanding immune responses and pathology induced by erythrocytic stages of the parasite. Med. Microbiol. Immunol. 189:115-126. [DOI] [PubMed] [Google Scholar]
- 26.Lopez, R. D. 2002. Human gd-T cells in adoptive immunotherapy of malignant and infectious diseases. Immunol. Res. 26:207-221. [DOI] [PubMed] [Google Scholar]
- 27.Mohan, K., and M. M. Stevenson. 1998. Acquired immunity to asexual blood-stages, p. 467-493. In I. W. Sherman (ed.), Malaria: parasite biology, pathogenesis, and protection. American Society for Microbiology, Washington, D.C.
- 28.Mosmann, T. R., H. Cherwinski, M. W. Bond, M. A. Giedlin, and R. L. Coffman. 1986. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 136:2348-2357. [PubMed] [Google Scholar]
- 29.Mosmann, T. R., and R. L. Coffman. 1989. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7:145-173. [DOI] [PubMed] [Google Scholar]
- 30.O'Garra, A., and K. Murphy. 1994. Role of cytokines in determining T-lymphocyte function. Curr. Opin. Immunol. 6:458-466. [DOI] [PubMed] [Google Scholar]
- 31.Perera, M. K., R. Carter, R. Goonewardene, and K. N. Mendis. 1994. Transient increase in circulating γ/δ T cells during Plasmodium vivax malarial paroxysms. J Exp. Med. 179:311-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts, D. W., and W. P. Weidanz. 1979. T-cell immunity to malaria in the B-cell deficient mouse. Am. J. Trop. Med. Hyg. 28:1-3. [DOI] [PubMed] [Google Scholar]
- 33.Roussilhon, C., M. Agrapart, J. J. Ballet, and A. Bensussan. 1990. T lymphocytes bearing the γδ T cell receptor in patients with acute Plasmodium falciparum malaria. J. Infect. Dis. 162:283-285. [DOI] [PubMed] [Google Scholar]
- 34.Sandor, M., A. I. Sperling, G. A. Cook, J. V. Weinstock, R. G. Lynch, and J. A. Bluestone. 1995. Two waves of γδ T cells expressing different Vδ genes are recruited into schistosome-induced liver granulomas. J. Immunol. 155:275-284. [PubMed] [Google Scholar]
- 35.Seixas, E., L. Fonseca, and J. Langhorne. 2002. The influence of γδ T cells on the CD4+ T cell and antibody response during a primary Plasmodium chabaudi chabaudi infection in mice. Parasite Immunol. 24:131-140. [DOI] [PubMed] [Google Scholar]
- 36.Seixas, E. M., and J. Langhorne. 1999. γδ T cells contribute to control of chronic parasitemia in Plasmodium chabaudi infections in mice. J. Immunol. 162:2837-2841. [PubMed] [Google Scholar]
- 37.Su, Z., and M. M. Stevenson. 2000. Central role of endogenous gamma interferon in protective immunity against blood-stage Plasmodium chabaudi AS infection. Infect. Immun. 68:4399-4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor-Robinson, A. W., R. S. Phillips, A. Severn, S. Moncada, and F. Y. Liew. 1993. The role of TH1 and TH2 cells in a rodent malaria infection. Science 260:1931-1934. [DOI] [PubMed] [Google Scholar]
- 39.Troye-Blomberg, M., W. P. Weidanz, and H. C. van der Heyde. 1999. Immunity to malaria, p. 403-438. In M. Wahlgren and P. Perlman (ed.), Malaria: molecular and clinical aspects. Charwood Academic Publishers, Reading, Berkshire, United Kingdom.
- 40.Troye-Blomberg, M., S. Worku, P. Tangteerawatana, R. Jamshaid, K. Soderstrom, G. ElGhazali, L. Moretta, M. Hammarstrom, and L. Mincheva-Nilsson. 1999. Human γδ T cells that inhibit the in vitro growth of the asexual blood stages of the Plasmodium falciparum parasite express cytolytic and proinflammatory molecules. Scand. J. Immunol. 50:642-650. [DOI] [PubMed] [Google Scholar]
- 41.van der Heyde, H. C., W. L. Chang, and W. P. Weidanz. 1997. Specific immunity to malaria and the pathogenesis of disease, p. 195-226. In S. H. E. Kaufmann (ed.), Host response to intracellular pathogens. R.G. Landes Company Biomedical Publishers, Austin, Tex.
- 42.van der Heyde, H. C., M. M. Elloso, W. L. Chang, M. Kaplan, D. D. Manning, and W. P. Weidanz. 1995. γδ T cells function in cell-mediated immunity to acute blood-stage Plasmodium chabaudi adami malaria. J. Immunol. 154:3985-3990. [PubMed] [Google Scholar]
- 43.van der Heyde, H. C., M. M. Elloso, W. L. Chang, B. J. Pepper, J. Batchelder, and W. P. Weidanz. 1996. Expansion of the γδ T cell subset in vivo during bloodstage malaria in B cell-deficient mice. J. Leukoc. Biol. 60:221-229. [DOI] [PubMed] [Google Scholar]
- 44.van der Heyde, H. C., M. M. Elloso, D. C. Roopenian, D. D. Manning, and W. P. Weidanz. 1993. Expansion of the CD4−, CD8− γδ T cell subset in the spleens of mice during non-lethal blood-stage malaria. Eur. J. Immunol. 23:1846-1850. [DOI] [PubMed] [Google Scholar]
- 45.van der Heyde, H. C., D. Huszar, C. Woodhouse, D. D. Manning, and W. P. Weidanz. 1994. The resolution of acute malaria in a definitive model of B cell deficiency, the JHD mouse. J. Immunol. 152:4557-4562. [PubMed] [Google Scholar]
- 46.van der Heyde, H. C., D. D. Manning, D. C. Roopenian, and W. P. Weidanz. 1993. Resolution of blood-stage malarial infections in CD8+ cell-deficient β2-m0/0 mice. J. Immunol. 151:3187-3191. [PubMed] [Google Scholar]
- 47.van der Heyde, H. C., D. D. Manning, and W. P. Weidanz. 1993. Role of CD4+ T cells in the expansion of the CD4−, CD8− γδ T cell subset in the spleens of mice during blood-stage malaria. J. Immunol. 151:6311-6317. [PubMed] [Google Scholar]
- 48.van der Heyde, H. C., B. Pepper, J. Batchelder, F. Cigel, and W. P. Weidanz. 1997. The time course of selected malarial infections in cytokine-deficient mice. Exp. Parasitol. 85:206-213. [DOI] [PubMed] [Google Scholar]
- 49.von der Weid, T., N. Honarvar, and J. Langhorne. 1996. Gene-targeted mice lacking B cells are unable to eliminate a blood stage malaria infection. J. Immunol. 156:2510-2516. [PubMed] [Google Scholar]
- 50.Weidanz, W. P., J. R. Kemp, J. M. Batchelder, F. K. Cigel, M. Sandor, and H. C. van der Heyde. 1999. Plasticity of immune responses suppressing parasitemia during acute Plasmodium chabaudi malaria. J. Immunol. 162:7383-7388. [PubMed] [Google Scholar]
- 51.Weintraub, B. C., M. R. Jackson, and S. M. Hedrick. 1994. γδ T cells can recognize nonclassical MHC in the absence of conventional antigenic peptides. J. Immunol. 153:3051-3058. [PubMed] [Google Scholar]
- 52.Zinkernagel, R. M., and H. Hengartner. 2001. Regulation of the immune response by antigen. Science 293:251-253. [DOI] [PubMed] [Google Scholar]