Abstract
Ruminants often carry gastrointestinal Shiga toxin (Stx)-producing Escherichia coli (STEC). Stxs belong to a large family of ribosome-inactivating proteins (RIPs), found in many plants and some bacteria. Plant RIPs, secreted into extracellular spaces, limit the spread of viruses through plant tissues by penetrating and killing virally infected cells. Previously, we showed Stx activity against bovine leukemia virus (BLV)-infected cells in vitro and hypothesized that STEC bacteria have antiviral activity in ruminant hosts. Here, we investigated the impact of STEC on the initial phases of BLV infection in sheep. Sheep were treated with biweekly oral doses of E. coli O157:H7 (an STEC) or an isogenic stx mutant strain. A different group of sheep were similarly treated with five naturally occurring ovine STEC isolates or stx-negative E. coli. Intestinal STEC bacteria were enumerated and identified by standard fecal culture and DNA hybridization. Oral STEC treatment did not always result in carriage of STEC, although many animals consistently presented with >104 CFU/g feces. BLV viremia was assessed by spontaneous lymphocyte proliferation (SLP) in cultures of blood mononuclear cells and by syncytium formation in cocultures of the same with F-81 indicator cells. SLP was lower (P < 0.05) and syncytia were fewer (P < 0.05) in STEC-treated sheep than in untreated sheep. Both lower SLP and fewer syncytia positively correlated with fecal STEC numbers. Average weight gain post-BLV challenge was higher in STEC-treated sheep than in untreated sheep (P < 0.05). These results support the hypothesis that in ruminants, intestinal STEC bacteria have antiviral activity and mitigate BLV-induced disease.
Shiga toxin (Stx)-producing Escherichia coli (STEC) bacteria are a normal part of the gastrointestinal microbiota of domesticated and wild ruminant species, and, as such, are routinely found worldwide in regional and herd surveys (1, 5, 10, 26, 29-31, 42). STEC bacteria constitute an eclectic group of E. coli strains, diverse in genomic traits and categorized merely by the presence of genes for Shiga toxin type 1 (Stx1), Shiga toxin type 2 (Stx2), and/or Stx2 variants. Some serotypes of STEC carried by healthy cattle are highly virulent to humans (6, 10, 11), especially the O157:H7 strains, which cause hemorrhagic colitis and life-threatening sequelae, attributed to the production of Stxs by intestinal STEC (11, 14). Although the significance of the high frequency of Stx genes in strains of E. coli colonizing ruminants remains unexplained, it seems reasonable to expect that Stxs may confer an as-yet-unidentified benefit(s) to bacteria and/or to their carriers. For example, Stxs may have an impact on bovine tissues colonized by STEC: the Stx receptor, globotriaosylceramide, is present on bovine intestinal epithelium but not on vascular cells (23), and colonization of bovine intestine by O157:H7 is associated with a reduced turnover rate of enterocytes, without an increase in the apoptotic rate (35).
Stxs belong to a large family of ribosome-inactivating proteins (RIPs), found in many plants and in some bacteria (15, 47). The RIPs may be hemitoxins, comprised of only a single A chain with N-glycosidase activity specific for rRNA (15), or holotoxins of ABn structure, composed of the enzymatically active A subunit and a specific number (n) of B subunits that bind cellular toxin receptors. The N-glycosidase activity depurinates rRNA and results in an inhibition of target cell protein synthesis. Plant RIPs are primarily hemitoxins, whereas bacterial RIPs are holotoxins of AB5 structure. The plant RIPs are secreted into extracellular spaces and limit the spread of plant viruses through their tissues by penetrating and killing virally infected cells. Many plant RIPs are highly toxic to virally infected animal cells in culture at concentrations that do not affect the uninfected cells (34, 40). The virally infected plant and animal cells are thought to internalize the toxins through virus-induced permeable cell membranes (9), independent of receptor-mediated endocytosis. Thus, antiviral effects may be exerted by the enzymatic portion of an RIP molecule alone, e.g., the A subunit of ricin has activity in vitro against cells infected with the human immunodeficiency virus (55). The enzymatic A subunit of Stx1 suppresses bovine leukemia virus (BLV)-induced spontaneous lymphocyte proliferation (SLP) and reduces the output of BLV particles in bovine cell culture (3, 16, 17). Based on these results, we hypothesized that Stxs, produced by intestinal STEC, have antiviral activity in ruminant carriers and that this activity may reduce the severity of infections with BLV and other viruses or delay an onset of the acute stage of viral disease. This hypothesis was tested here by assessing BLV viremia in experimentally infected sheep treated or not treated with STEC.
BLV is an oncogenic retrovirus responsible for the enzootic form of bovine lymphosarcoma (18). In cattle, BLV infection may be asymptomatic for 1 to 7 years and then progress to a persistent lymphocytosis that is characterized by a neoplasia of B lymphocytes (45). Most cattle at this stage remain free of disease symptoms, but some may progress to a polyclonal B-cell lymphoma and then to the end stage of disease, lymphosarcoma. Although not naturally occurring, BLV infection can be experimentally induced in sheep and results in a more rapid progression of disease than it does in cattle, and lymph node pathology and other clinical signs of disease can be observed 6 to 12 months after exposure to an infectious dose of BLV (13, 28).
Because sheep, like cattle, naturally carry intestinal STEC and because sheep exhibit a relatively rapid progression of experimentally induced BLV disease, we investigated the impact of STEC on the initial phases (more than 3 months) of BLV infection in sheep. Two experiments were carried out. First, sheep were treated with repeated oral doses of STEC or an isogenic stx deletion strain. Second, a different group of sheep were similarly treated with five naturally occurring ovine STEC isolates or stx-negative E. coli. Treatments began before or 24 h after BLV challenge, and intestinal STEC bacteria were enumerated and identified by standard fecal culture and DNA hybridization. Viremia and viral load in peripheral blood mononuclear cells (PBMC) were measured by SLP in PBMC cultures and induction of syncytia in cocultures of PBMC with F-81 indicator cells. Also, weight gain was monitored as an indicator of general health.
MATERIALS AND METHODS
Experimental animals.
White-face Suffolk wethers, 3 to 4 months old, were purchased from a local supplier (Rickwood Sheep Farm, Priest River, Idaho) and acclimatized for 3 weeks before commencement of the experiment. The sheep were housed in a containment facility at the University of Idaho and given alfalfa hay once daily and water ad libitum. All animals were dewormed by oral application of Valbazen (Pfizer Inc., New York, NY) and tested negative for BLV and bluetongue virus before challenge.
Enumeration of the intestinal STEC.
Fecal samples were obtained by rectal palpation, chilled and held on ice, and transported to the laboratory within 1 h of collection. Five grams of feces was added to 45 ml peptone-Tween diluent (PT; 0.1% peptone, 1% Tween 80) and vortexed thoroughly. The fecal suspension was prefiltered through coarse, sterile, cotton gauze to remove gross debris. The gauze was flushed with 50 ml PT to maximize bacterial carriage into the prefiltrate. The prefiltrate was decimally diluted in PT, and appropriate dilutions (typically 10−1 and 10−2 of the prefiltrate) were filtered onto hydrophobic grid membrane filters (HGMFs) (Isogrid membranes; Neogen, Lansing, MI) using a spreadfilter (Filtaflex, Almonte, ON, Canada) and a vacuum manifold. HGMFs were incubated at 37°C face up on hemorrhagic colitis agar (50), modified from the original formulation, to detect all STEC bacteria by the omission of 4-methylumbelliferyl-β-d-glucuronide (MUG), 1% cefsulodin, and 0.1% potassium tellurite, as these supplements were specific to the isolation of the O157:H7 serotype. Colonies on each HGMF were replicated onto another HGMF using an HGMF replicator/inoculator system (Filtaflex). Replicate membranes were incubated as described above and stored at 4°C for isolate recovery and in case repeat hybridizations were required. Bacterial cells on original HGMFs were lysed following the method of Nizetic et al. (39) after the wetting of membranes with a pretreatment solution for 30 min (57). DNA was fixed to the membranes by exposure to UV radiation for 5 min. HGMFs with adherent DNA were hybridized with a combined probe for stx1 and stx2 produced using PCR (PCR DIG Probe synthesis kit; Roche Diagnostics, Indianapolis, IN) with the primers described by Karch and Meyer (27). Colony blot hybridization was performed using standard methods (DIG System User's Guide to Filter Hybridization; Roche) with a Robbins Scientific model 1000 hybridization oven/shaker (Sunnyvale, CA), and hybridized membranes were color developed using standard methods (DIG nucleic acid detection kit; Roche). Final STEC counts for fecal samples (in the form of CFU/g) were based on the count of positive colony blots on hybridized membranes corresponding to typical E. coli colonies on replicate membranes, multiplied by the respective dilution factor.
Detection of stx using PCR.
In order to positively identify samples not harboring enough STEC bacteria to be detectable by the STEC enumeration protocol, the presence of the toxin gene stx was used as an indicator for STEC. Fecal samples were enriched by adding 1 g to 49 ml of E. coli broth (Remel, Lenexa, KS) containing 0.02 mg/ml novobiocin (Sigma-Aldrich, St. Louis, MO) and incubating them for 18 h at 37°C. Boiled cell lysates made from centrifuged pellets of enriched sample were serially washed and resuspended in 200 μl distilled H2O before being screened for the presence of stx using PCR (27). The PCR mixture included 0.25 U Taq DNA polymerase, 2 mM MgCl2, 200 μM each deoxynucleoside triphosphate, 0.1 μM of the forward and reverse primers (all reagents from Invitrogen Life Technologies, Carlsbad, CA), and 400 μg/ml bovine serum albumin (Sigma) per reaction. A 2-μl aliquot of DNA template was added to 23 μl of reaction mixture. Reactions were performed using an iCycler thermal cycler (Bio-Rad, Hercules, CA), and gel images were stored digitally using the Gene Genius imaging system (Syngene, Cambridge, United Kingdom).
Bacterial strains.
Two separate groups of STEC strains were used in the two experiments. The first experiment was performed with E. coli O157:H7 905 and its toxin-negative derivative. E. coli O157:H7 905, an Stx2-producing clinical isolate from a patient with hemolytic-uremic syndrome, and a kanamycin resistance derivative, with a deletion of stx2 made by the λ Red recombinase system (12), were kindly provided by M. Waldor (Tufts-New England Medical Center and Howard Hughes Medical Institute, Boston, Mass.) (43). Prior to use in sheep, the kanamycin resistance gene in the stx2 mutant was removed by introducing pCP20. The second experiment was performed with five naturally occurring ovine STEC isolates and stx-negative E. coli K-12. The five ovine STEC serotypes were obtained in an earlier study and included O5 stx1-negative stx2-positive (two isolates), O5 stx1-positive stx2-positive, O91 stx1-positive stx2-negative, and a nontypeable isolate, ONT stx1-positive stx2-positive (31). All bacteria were grown separately overnight at 37°C, with shaking in Luria-Bertani medium, chilled on ice, and administered orally to sheep in total doses of 5 × 1010 CFU, either singly or as a mixture of five ovine isolates, mixed immediately prior to dosing.
BLV challenge.
BLV-positive PBMC were obtained from a seropositive BLV donor, cow no. 2062, housed at the University of Idaho dairy. The donor was in the persistently lymphocytotic stage of BLV infection, as determined by elevated numbers of peripheral B lymphocytes and the occurrence of SLP in cultures of purified PBMC (data not shown). PBMC were purified from blood samples by density centrifugation on Accupaque (1.086 g/ml; Accurate Chemical and Scientific Corp., Westbury, N.Y.), followed by hypotonic lysis of erythrocytes and repeated washing in phosphate-buffered saline (PBS). To determine the optimal dose of PBMC to induce BLV infection, nine sheep were separated into three groups and received a subcutaneous injection of 1 × 106, 1 × 107, or 5 × 107 total PBMC. Control animals were injected with PBS. Based on the outcome of this challenge, 1.0 × 106 PBMC were chosen as the optimum BLV dose used in subsequent experiments. Establishment of BLV infection was determined by measuring anti-BLV antibodies by agar immunodiffusion assay at the Washington Animal Disease Diagnostic Laboratory (Washington State University, Pullman, WA).
Experimental design.
Two experiments were performed with separate groups of BLV-naive sheep. The first (experiment I) used treatment with isogenic strains of E. coli with or without stx to establish the toxin as the required feature of the E. coli strain. The second (experiment II) used naturally occurring ovine STEC strains in an effort to improve the persistence of STEC in the ovine gastrointestinal tract. In both experiments, sheep were semirandomly assigned to four treatment groups to preclude clustering of littermates and animals with high and low birth weights in the same groups. All animals were orally dosed with bacteria twice per week for the duration of the experiment, and the groups were housed without physical contact between groups.
Experiment I was a shorter-term 8-week experiment and consisted of four groups of animals: four sheep in group 1 were treated with wild-type E. coli O157:H7 905 (stx2+); four sheep in group 2 were treated with the engineered isogenic stx2 deletion mutant; three sheep in each of groups 3 and 4 were treated with toxin-negative E. coli K-12. Groups 1, 2, and 3 were challenged with BLV, and group 4 served as healthy controls with no BLV challenge.
Experiment II was a longer-term (>3 months) experiment and consisted of four groups with five sheep in each group. Groups 1 and 2 were treated with a mixture of five ovine STEC strains; groups 3 and 4 were treated with stx-negative E. coli K-12. As in experiment I, groups 1, 2, and 3 were challenged with BLV. Group 1, referred to as “pre- and post-BLV STEC,” was treated with oral STEC both prior to and post-BLV challenge, beginning 14 days before BLV challenge and ending at the conclusion of the experiment. Group 2, named “post-BLV STEC,” received stx-negative E. coli K-12 prior to BLV challenge and was treated with STEC only postchallenge, beginning 24 h after BLV challenge and continuing through the end of the experiment. Groups 1 and 2 were treated jointly in some analyses and are referred to as “STEC-treated groups.” Groups 3 (named “BLV no STEC”) and 4 (named “no BLV”) were treated with stx-negative E. coli K-12.
PBMC and serum preparation.
Blood samples were obtained 1 week before BLV challenge, weekly for the first 8 weeks post-BLV and monthly thereafter. Venous blood was collected into 20-ml vacuum tubes with 4.0 ml of acid citrate dextrose and chilled on ice. Serum samples were obtained from coagulated blood collected into untreated vacuum tubes. PBMC were purified as described previously (17). In brief, blood samples were centrifuged for 30 min at 450 × g at 4°C, and buffy coats were layered on Accupaque (1.086 g/ml) and centrifuged for 30 min at 1,000 × g at 16°C. PBMC were washed with PBS-EDTA, contaminating erythrocytes and platelets were removed by brief water lysis, and cells were rescued with 10× PBS and counted using a hemocytometer. The density of PBMC was adjusted to 1.0 × 107 cells/ml in PBS with 1% bovine serum albumin, and the cells were stored on ice until being transferred to culture vessels immediately prior to incubation.
SLP assay.
Quadruplicate PBMC samples at 5.0 × 105 cells/well in 200 μl were cultured in 96-well plates at 37°C with 6.5% CO2 in RPMI 1640 medium (Gibco BRL) supplemented with antibiotics, l-glutamine, and 20% fetal bovine serum. After 48 h, 1 μCi/well of 3H-thymidine (Moravek Biochemicals, Brea, CA) was added to cultures, and 16 to 18 h later, the cells were harvested onto glass filters using a Filtermate Packard harvester (Packard Instrument Company, Meriden, CT) and counted using a Packard scintillation counter, TopCount NXT.
Syncytium induction assay.
The syncytium induction assay was a modification of an enumeration assay for the BLV-positive PBMC from experimentally infected sheep (33). Feline epithelial F-81 cells were kept frozen in liquid nitrogen and freshly thawed for each assay. The cells were washed in PBS-EDTA-0.5% bovine serum albumin, counted using a hemocytometer, and distributed into 24-well culture plates at 5.0 × 103 or 10 × 103 viable cells/well. Viability of the cells was >95%, as determined by trypan blue exclusion. The cells were cultured overnight at 37°C with 6.5% CO2 in RPMI 1640 medium with 13% FBS, supplemented with l-glutamine and antibiotics. The next day, 1.0 × 105 or 1.0 × 106 PBMC, purified from blood samples as described above, were added to the culture wells. The cocultures were incubated for 48 h, rinsed with PBS, and cultured with fresh medium for an additional 2 to 3 days. When confluence reached 60 to 80% and well-developed syncytia could be seen, the cultures were rinsed with PBS, fixed with absolute methanol, stained with Giemsa, and examined microscopically. Syncytia with more than six nuclei were considered to be induced by BLV.
Flow cytometry.
Blood leukocytes were purified by water lysis of 200 μl of whole blood and rescue of the cells with 10× PBS. The cells were washed with PBS-EDTA and incubated (30 min at 4°C, PBS-EDTA-1% horse serum) with primary mouse monoclonal antibodies (5.0 μg/ml) to the B-cell markers B-B1 and B-B2 (BAS9A and BAQ44A, immunoglobulin M [IgM]) and to BLV protein gp51 (MW1, IgG1), from the Washington State University Monoclonal Antibody Center, Pullman, WA. The cells were washed thrice, incubated for 30 min as described above, but with goat anti-mouse polyclonal antibodies conjugated to fluorescein isothiocyanate (anti-IgM) or to Tri-Color (anti-IgG1) (Caltag Laboratories, Burlingame, CA), washed twice with PBS, and fixed with 2% formaldehyde in PBS. Flow cytometry data were acquired for 20 × 103 to 30 × 103 cells per sample, using a FACSCalibur flow cytometer equipped with an argon laser at 488 nm (BD Biosciences, San Jose, CA).
Statistical analysis.
All counts were analyzed after a square root transformation, and differences among groups were tested by analysis of variance using Tukey's method for comparisons of BLV-challenged groups and Dunnet's method for comparisons with the control. The frequencies of high and low fecal STEC counts were analyzed by the chi-square test. Pearson's correlation coefficients were calculated to assess correlation between parameters of viremia and the numbers of fecal STEC strains, and the lines of best fit were calculated when correlation was statistically significant, i.e., <0.05. Observations were clustered using McQuitty's linkage and Pearson's method for distance calculation. The average values presented in this paper are accompanied by standard errors of the means (SEMs). Statistical analysis was performed using the Minitab 13 program (Minitab Inc.).
RESULTS
The infecting dose of 1 × 106 PBMC from the BLV-positive cow was selected as the lowest dose that resulted in prompt seroconversion of the sheep (data not shown). Sheep did not have complications unrelated to BLV challenge (those with abnormal postmortems showed signs compatible only with BLV infection, such as lymph node enlargement and tumors), and examination of biweekly blood smears showed eosinophil counts of <5%, indicating that the animals did not carry significant worm burdens. All sheep challenged with BLV seroconverted within 1 month of challenge, and none of the control sheep became seropositive (data not shown). The naturally occurring numbers of fecal STEC bacteria, first determined within 1 week of arrival to the housing facility, varied among sheep, from undetectable to >106 CFU/g feces, and subsequently were liable to alternate between high and low values (data not shown). The presence of fecal STEC among animals that were not given oral STEC was due to these naturally occurring strains.
Stx mediates antiviral effects of STEC in sheep. (i) Experiment I.
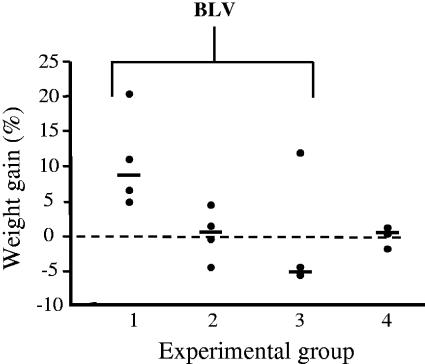
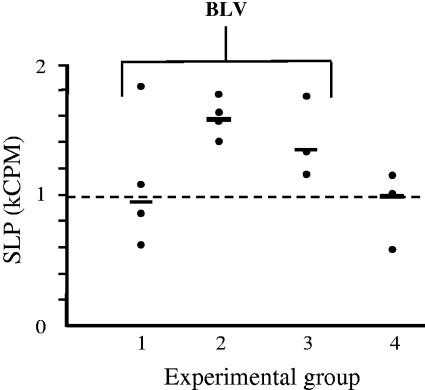
We compared the initial aftermaths of BLV challenge among three groups of sheep orally treated biweekly with 5 × 1010 CFU of wild-type E. coli O157:H7 905 (group 1), the E. coli 905 isogenic stx2 deletion mutant (group 2), or stx-negative E. coli K-12 (infection control, group 3). We observed striking differences between animals treated with STEC (group 1) and animals treated with stx-negative E. coli (groups 2 and 3) in such indicators as weight gain during the BLV incubation period (Fig. 1) and SLP of PBMC in culture (Fig. 2). Sheep treated with STEC (group 1) gained 5 to 20% of weight over the first 2 weeks post-BLV challenge, whereas only two of seven animals treated with stx-negative E. coli (groups 2 and 3) gained as much weight. These differences were correlated with better feed consumption among STEC-treated animals and indicated their general well-being. Group 4 animals were not challenged with BLV and maintained their weight as expected for this age. SLP, an indicator of viral load, increased in sheep treated with the stx2 deletion mutant and in the BLV infection control animals that received E. coli K-12 (group 3). In contrast, SLP did not increase above healthy control levels in the group of sheep treated with STEC. The improved weight gain and lower SLP values for animals in group 1 were apparently related to the stx2 in E. coli O157:H7 905. The fact that data points were similarly clustered in groups 2 and 3 (Fig. 1 and 2) indicated that responses of sheep to BLV challenge were not greatly influenced by the multitude of non-stx genes present in the stx2 deletion mutant or toxin-negative E. coli K-12.
FIG. 1.
Increased weight gain in sheep treated with STEC. Four groups of sheep received biweekly oral doses of bacteria, and three groups were challenged with BLV. Group 1 received STEC, E. coli O157:H7 strain 905, group 2 received an isogenic stx2 deletion mutant of strain 905, and groups 3 and 4 received stx-negative E. coli K-12. Data are percentages of weight changes of the sheep during first 2 weeks of bacterial treatments and post-BLV challenge. Solid circles show data for individual animals, and cross bars show median values for groups. The broken line at 0 indicates no weight change.
FIG. 2.
Oral STEC treatment prevents SLP increase in cultured PBMC. Four groups of sheep received biweekly oral doses of bacteria, and three groups were challenged with BLV. Group 1 received STEC, E. coli O157:H7 strain 905, group 2 received an isogenic stx2 deletion mutant of 905, and groups 3 and 4 received stx-negative E. coli K-12. Solid circles show mean values from four replicate cultures for individual animals, and cross bars show median values for groups. The broken line indicates the median value for the healthy control group.
During the first month post-BLV challenge, only two animals in group 1, treated with the human E. coli strain 905, presented with high total numbers of fecal STEC bacteria. These two animals had a 4-log augmentation of total fecal STEC counts, with averages of 106 CFU/g of feces compared to fecal STEC counts among animals in groups 2 and 3 that had an average of 102 CFU STEC/g feces (data not shown). The oral dosing of STEC, even without persistence of the bacteria in the gastrointestinal tract, had measurable effects. Two animals in group 1 that tested fecal STEC negative during the first 3 weeks of oral treatment showed increases in weight and low SLP (Fig. 1 and 2 and data not shown), similar to the STEC-positive animals in that group. This suggested that the Stx2 protein present in the preparations of bacteria and/or secreted during passage of inocula through the intestine contributed to the observed antiviral effect.
The absence of STEC in fecal samples from some animals in group 1 indicated a poor ability of the human isolate E. coli O157:H7 905 to persist and/or colonize the ovine intestine. This was also evident in the inability of the stx2 deletion mutant to displace naturally occurring STEC, as three animals in group 2 remained STEC positive at low levels (<103 CFU STEC/g feces) for at least 2 weeks of oral treatment (data not shown). Thus, in an effort to better augment gastrointestinal STEC by oral bacterial treatments, naturally occurring ovine STEC strains were used in experiment II.
(ii) Experiment II.
Similar to experiment I, experiment II compared the initial aftermaths of BLV challenge among groups of sheep orally treated biweekly with 5 × 1010 CFU of bacteria but employed ovine STEC isolates to more closely mimic the natural situation, in hopes of improving the persistence of the treatment strains in the gastrointestinal tract. Groups 1 and 2 received five naturally occurring ovine STEC strains, and groups 3 and 4 received stx-negative E. coli K-12. Group 1 animals were treated with STEC prior to and post-BLV challenge to increase the likelihood of the presence of STEC and/or Stxs in animals at BLV challenge. Group 2 received STEC beginning at 24 h post-BLV challenge to determine whether STEC postchallenge would affect viral disease. Group 3 was the infection control, and group 4 was a healthy control not infected with BLV.
Oral treatment with ovine bacteria had limited impact on gastrointestinal colonization by STEC.
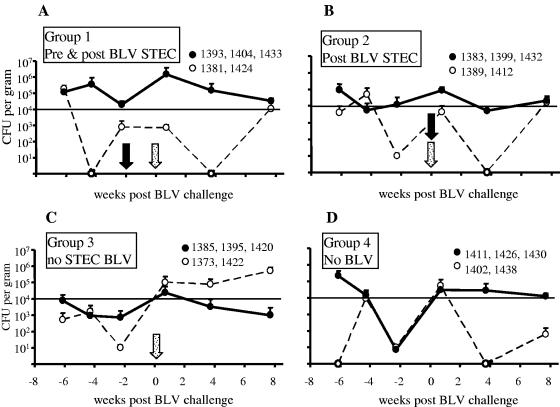
The total numbers of naturally occurring intestinal STEC bacteria in the sheep varied widely and frequently changed considerably, as can be ascertained from fecal STEC numbers obtained before and after bacterial treatment (Fig. 3). However, average carriage among untreated sheep was usually ∼104 CFU STEC/g feces. Similar to the results of experiment I, STEC treatment was not always associated with stable carriage of STEC, and some animals occasionally did not present with detectable fecal STEC in spite of the repeated applications of ovine strains. Among the two STEC-treated groups, individuals clustered in two categories: three animals in each group consistently presented with high total numbers of STEC bacteria (>104 CFU/g feces) post-BLV challenge, while STEC numbers were low in the remaining two animals in each group (Fig. 3A and B). In the BLV group without STEC, two animals spontaneously acquired and maintained high total numbers of STEC bacteria (>104 CFU/g) throughout the post-BLV-challenge period. Importantly, the STEC treatment given to animals before and after BLV challenge was associated with relatively high total numbers of fecal STEC bacteria in samples immediately preceding (average log value, 3.32) and following (average log value, 4.47) BLV challenge (Fig. 3A), whereas the counts for the group treated only postchallenge were lower before BLV challenge (average log value, 1.71) but relatively high after it (average log value, 3.91) (Fig. 3B). Just before BLV challenge, the total number of fecal STEC bacteria in animals not treated with STEC bacteria was substantially lower than that among the STEC-treated groups, with average log values of 1.46 and 0.80 (Fig. 3C and D, respectively). Thus, despite variations in STEC carriage, STEC-treated groups were consistent with the experimental design to test the effect of high numbers of STEC bacteria before and during BLV challenge or the effect of high numbers of STEC bacteria following BLV challenge, and both groups differed from the untreated controls.
FIG. 3.
The effect of oral bacterial treatment on the total number of fecal STEC bacteria. The number of STEC CFU/g feces was determined by colony hybridization and PCR. Four experimental groups are shown that were treated with biweekly oral doses of 5 × 1010 CFU bacteria/animal. Two groups received STEC starting 2 weeks before BLV challenge (pre & post BLV STEC group) (A) or starting 24 h post-BLV challenge (post BLV STEC group) (B) and continuing for 16 weeks post-BLV challenge. Two groups received E. coli K-12 starting at week −2. The infection control group received BLV (BLV no STEC) (C), and the healthy control group was not challenged with BLV (no BLV) (D). Within each group, two subgroups of three (solid circles) or two (empty circles) animals were identified that had similar STEC counts (animal identifiers in key). The samples that were STEC positive by PCR but had no hybridization counts were assigned a value of 10 CFU/g feces. A solid arrow indicates commencement of biweekly STEC treatment, and a stippled arrow indicates the single injection of sheep with BLV. A horizontal solid line at log 4 separates CFU counts considered high (above log 4) and those considered low (below log 4).
Based on the results in experiment I that indicated that STEC treatment had an effect on BLV disease despite the apparent lack of bacterial colonization and that showed a difficulty in consistently changing the total number of fecal STEC bacteria, we used a two-prong approach and analyzed the results first between treatment groups, regardless of fecal STEC, and second between animals, taking into account individual total numbers of fecal STEC bacteria.
Ovine STEC treatment decreased BLV load as measured by SLP.
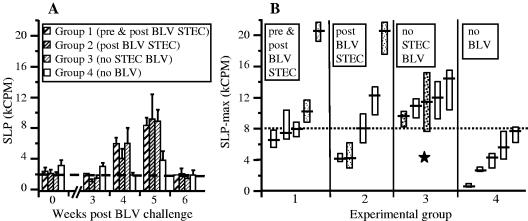
BLV-dependent SLP in cultures of PBMC from BLV-positive animals requires, and is preceded by, expression of BLV proteins by cultured cells and thus can serve as an indirect measure of BLV load (17, 51, 52). During the first 3 weeks post-BLV challenge, SLP levels in cultures from all BLV-challenged sheep were similar to those of the healthy control group and represented the incubation period of BLV infection (Fig. 4A). However, at 4 weeks post-BLV challenge, SLP in cultures from all challenged sheep increased by 130 to 880%, and group averages were higher than those of the healthy controls (P < 0.05), indicating a progression of viral disease. By 7 weeks postchallenge, SLP values returned to normal levels in all animals, consistent with the chronic asymptomatic phase of the disease. The group average SLP did not reveal differences between BLV-challenged groups because individual SLP values peaked at different times between 4 and 6 weeks post-BLV challenge, thus, individual maximal SLP counts (SLP-max) were used in further analyses. Individual SLP-max from the infection control (BLV without STEC) and the healthy control (no BLV) did not overlap (P < 0.05) (Fig. 4B). SLP-max for most sheep (three out of five) in both STEC-treated groups were within the range of the healthy control group values, and these groups differed from the BLV group without STEC (P < 0.05) after the exclusion of the single outliers (sheep 1381 and 1389).
FIG. 4.
Ovine STEC treatment prevents SLP increase in cultured PBMC. Cell proliferation was measured as the incorporation of tritiated thymidine and expressed as kCPM. Four experimental groups are shown that were treated biweekly with oral doses of 5 × 1010 CFU bacteria/animal. Two groups received STEC, starting 2 weeks before BLV challenge (pre & post BLV STEC, group 1) or starting 24 h post-BLV challenge (post BLV STEC, group 2) and continuing for 16 weeks post-BLV challenge. Two groups received E. coli K-12 starting at week −2. The infection control group received BLV (BLV no STEC, group 3), and the healthy control group was not challenged with BLV (no BLV, group 4). (A) Data are means ± SEMs of cultures from five sheep. The horizontal broken line indicates the average background proliferation in PBMC cultures prior to BLV challenge. (B) The maximal SLP values were obtained between 4 and 6 weeks post-BLV challenge. Data are median kCPM values per four culture replicates (cross bars) and 95% confidence intervals for medians (boxes). The horizontal dotted line indicates the upper range of SLP in the healthy controls (group 4), and the star indicates a statistically significant difference from this group (P < 0.05). Light stipple indicates the animals that had zero or <103 CFU/g (low) fecal STEC counts despite STEC treatment, and heavy stipple indicates animals in the untreated group that had >104 CFU/g (high) fecal STEC counts.
Fecal STEC correlated with decreased BLV load as measured by SLP.
Among all 10 STEC-treated sheep, three of the four highest SLP-max occurred in cultures from animals presenting exceptionally low total counts of fecal STEC bacteria (Fig. 4B), whereas among five sheep in the BLV group without STEC, two of the three with the lowest SLP-max occurred in animals presenting exceptionally high counts of STEC bacteria (Fig. 4B), indicating that SLP-max were inversely related to the numbers of intestinal STEC bacteria. This conjecture was confirmed by analysis of SLP-max in the context of fecal STEC numbers during BLV incubation (day 5 post-BLV challenge) and during active infection (day 26 post-BLV challenge). The six sheep in the two STEC-treated groups that presented with SLP-max within the healthy control group range (Fig. 4B) also collectively presented with >104 CFU STEC/g in 8 of 12 fecal samples (67%). Conversely, only one sample with >104 CFU/g was found among eight samples (13%) from the four remaining animals, with an SLP-max exceeding that of the control group. This suggested that a fecal STEC density of ≥104 CFU/g feces had a mitigating effect on BLV infection.
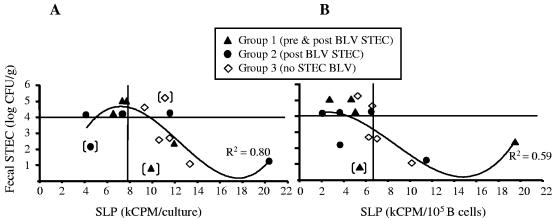
This supposition was further confirmed by plotting SLP-max against fecal STEC counts averaged over a 2-month period post-BLV challenge (Fig. 5A). Thus, SLP-max for five out of six STEC-treated animals presenting an average count of fecal STEC bacteria of ≥104 CFU/g were within the range of healthy control group SLP-max (Fig. 5A, upper left quadrant). Conversely, only one of four SLP-max from STEC-treated animals presenting average fecal STEC counts of <104 CFU/g was within the range of that of the healthy control group (Fig. 5A, lower left quadrant) (Pearson's correlation coefficient = −0.774, P = 0.003). Importantly, there was no correlation between fecal STEC counts and SLP-max among individuals of the healthy control group, indicating that in the absence of BLV infection, intestinal STEC bacteria do not influence SLP. The data points in Fig. 5A were fitted with a cuboidal polynomial line, indicating that the inverse correlation between fecal STEC numbers and SLP-max was linear for fecal STEC within a range of 1.0 to 4.5 log CFU/g.
FIG. 5.
Suppression of BLV-induced SLP correlated with the total numbers of fecal STEC bacteria. Three experimental groups are shown that were treated biweekly with oral doses of 5 × 1010 CFU bacteria/animal. Two groups received STEC, starting 2 weeks before BLV challenge (pre & post BLV STEC, group 1) or starting 24 h post-BLV challenge (post BLV STEC, group 2) and continuing for 16 weeks post-BLV challenge. The infection control group (BLV no STEC, group 3) received E. coli K-12 starting at week −2. All three groups were challenged with BLV. Fecal STEC counts, averaged over a 2-month period post-BLV challenge and expressed as the logs of CFU/g, are plotted against kCPM per culture (A) or against kCPM per 105 B cells in culture (B). Horizontal solid lines at log 4 separate CFU counts considered high (above log 4) and those considered low (below log 4). Vertical lines indicate the upper ranges of background SLP in the healthy control group. Data points were fitted with cuboidal polynomial lines (R2 values shown) after the exclusion of outliers with fecal STEC numbers inconsistent with treatment (points in brackets).
Since BLV is expressed only in B cells (37), it was expected that SLP would be proportional to the numbers of B cells in the PBMC samples. When SLP-max were expressed as kilocounts per minute (kCPM) per 105 B cells in culture, the protective effect of STEC became more apparent (Fig. 5B). Thus, B-cell-corrected SLP-max for all 8 of 15 BLV-challenged animals that presented with a high average total fecal STEC count of ≥104 CFU/g were within the range of that of the healthy control group, irrespective of STEC treatment (Fig. 5B, upper left quadrant). Conversely, among the seven remaining BLV-challenged animals presenting a low average total fecal STEC count of <104 CFU/g, four of the B-cell-corrected SLP-max were above the range of that of the healthy control group (Fig. 5B, lower right quadrant). A cuboidal polynomial line of best fit again indicated an inverse correlation between fecal STEC numbers of 1.0 to 4.5 log CFU/g and B-cell-corrected SLP-max.
Ovine STEC treatment correlated with decreased BLV load as measured by formation of syncytia.
BLV particles released from BLV-expressing cells induce syncytia in cultures of fusion-prone indicator cells, such as F-81 (53). F-81 cells were cocultured with PBMC from BLV-challenged sheep, and syncytia with more than six nuclei were enumerated as BLV induced. Smaller syncytia with fewer nuclei were not counted, as they spontaneously formed in cultures of F-81 cells alone and in cocultures with PBMC from the healthy control sheep that had no large multinucleated syncytia typical of BLV-induced cell fusions (data not shown). PBMC from BLV-challenged sheep did not induce syncytia for the first 2 weeks postchallenge, consistent with the incubation period of BLV disease. At 3 weeks post-BLV challenge, when indicator cells were cultured with low numbers of 1.0 × 105 PBMC, only one syncytium was found in all cocultures from the group treated with STEC prior to and post-BLV challenge, but seven syncytia were found in all cocultures from the group treated with STEC only post-BLV challenge, and four syncytia were found in all cocultures from the infection control group (data not shown). PBMC from the same blood samples, applied in larger numbers (1.0 × 106), induced syncytia in all cultures from BLV-challenged sheep. However, in each of the STEC-treated groups, there were only two of five cultures with at least four syncytia, whereas in the infection control group, there were four of five cultures with at least four syncytia (data not shown). At 3 months post-BLV challenge, 106 PBMC induced syncytia that were too numerous to count, indicating an increased BLV load in the sheep compared to that at 3 weeks postchallenge, but syncytia were countable when 105 PBMC were used. Thus, cultures from the group treated with STEC before and after BLV challenge showed 0 to 4 syncytia (2.2 ± 0.7), and the cultures from the group treated with STEC post-BLV challenge showed 2 to 6 syncytia (3.6 ± 0.7), but the cultures from the infection control group showed 4 to 16 syncytia (9.0 ± 2.1) (first and last groups, P < 0.05). These results suggest that STEC treatment lowered BLV load.
Analogous with SLP, it could be expected that BLV-induced syncytia would be proportional to the number of B cells in the cocultures. Accordingly, we calculated the number of B cells needed to induce one syncytium by dividing the number of B cells present in an F-81/PBMC coculture by the number of syncytia. At 3 weeks and 3 months post-BLV challenge, more B cells were needed to induce one syncytium from both STEC-treated groups than for the infection control group (Table 1). These results indicated that the BLV load was lower among sheep that received STEC treatment. By 3 months, the differences in viral load were twofold and fourfold less than that of the infection control for the group that received STEC treatment after BLV challenge and the group that received treatment before and after challenge, respectively (Table 1). This suggested that STEC treatment was the most effective in reducing BLV load when it was initiated before BLV challenge.
TABLE 1.
Number of B cells per BLV-induced syncytium in cocultures of ovine PBMC and F-81 indicator cells
| Exptl group | Time post-BLV challenge (no. of PBMC)b | No. of B cells/syncytiuma |
|---|---|---|
| Pre- and post-BLV STEC | 3 wk (106) | (2.0 ± 0.8)c × 105 |
| Post-BLV STEC | (1.9 ± 0.6) × 105 | |
| BLV without STEC | (1.4 ± 0.5)c × 105 | |
| Pre- and post-BLV STEC | 3 mo (105) | (13.0 ± 0.3)c × 103 |
| Post-BLV STEC | (8.7 ± 2.4) × 103 | |
| BLV without STEC | (3.2 ± 0.7)c × 103 |
Values are means ± SEMs of five F-81/PBMC cocultures.
Number of cells used per F-81/PBMC coculture.
These values were significantly different by analysis of variance (P < 0.05).
Fecal STEC correlated with decreased BLV load as measured by formation of syncytia.
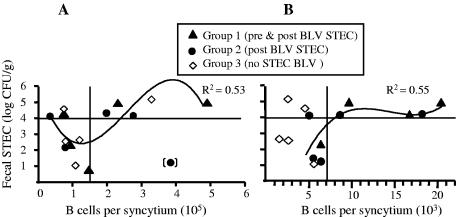
The plot of B-cell numbers per induced syncytium versus total fecal STEC numbers confirmed that B cells from sheep that presented with high STEC numbers induced fewer syncytia than B cells from sheep presenting with low STEC numbers. The optimal clustering of data points divided them among four quadrants, as shown in Fig. 6, suggesting that total fecal STEC counts of ≥104 CFU/g were required, although not always sufficient, for reduction of the numbers of syncytia. At 3 weeks post-BLV challenge, in six of seven cocultures from sheep presenting with total fecal STEC numbers of <104 CFU/g, (101 ± 10) × 103 B cells were needed to induce one syncytium (Fig. 6A, lower left quadrant). Conversely, in five of eight cocultures from sheep presenting with total fecal STEC numbers of ≥104 CFU/g, (300 ± 51) × 103 B cells were needed to induce one syncytium (Fig. 6A, upper right quadrant). The data points could be fitted with a cuboidal polynomial line similar to the one in Fig. 5A, indicating that the inverse correlation between fecal STEC numbers and the B cells needed to induce one syncytium was linear for total fecal STEC numbers within a range of 2 to 5 log CFU/g.
FIG. 6.
Suppression of BLV-induced syncytia correlated with total numbers of fecal STEC bacteria. Three experimental groups are shown that were treated biweekly with oral doses of 5 × 1010 CFU bacteria/animal. Two groups received STEC, starting 2 weeks before BLV challenge (pre & post BLV STEC, group 1) or starting 24 h post-BLV challenge (post BLV STEC, group 2) and continuing for 16 weeks post-BLV challenge. The infection control group (BLV no STEC, group 3) received E. coli K-12 starting at week −2. All groups were challenged with BLV. Fecal STEC counts, averaged over a 2-month period post-BLV challenge and expressed as logarithms of CFU/g, are plotted against numbers of B cells per syncytium. Horizontal solid lines at log 4 separate fecal STEC counts considered high (above log 4) and those considered low (below log 4). The data points were optimally clustered in four quadrants as shown, using Pearson's method to measure the distance between observations and McQuitty's linkage to measure the distance between clusters. Data points were fitted with cuboidal polynomial lines (R2 values shown) after the exclusion of the outlier with fecal STEC numbers inconsistent with treatment (point in brackets) (A) and all group 3 points (B). Results were obtained 3 weeks (A) and 3 months (B) post-BLV challenge.
At 3 months post-BLV challenge, a correlation between numbers of the fecal STEC and of B cells per syncytium was noted only in STEC-treated groups. Thus, in three cocultures from sheep presenting with total fecal STEC numbers of <104 CFU/g, (6.0 ± 0.3) × 103 B cells were needed to induce one syncytium (Fig. 6B, lower left quadrant). Conversely, in five of six cocultures from sheep presenting with total fecal STEC numbers of ≥104 CFU/g, twice that number of B cells [(14.5 ± 2.3) × 103] was needed to induce one syncytium (Fig. 6B, upper right quadrant). A cuboidal polynomial line could be fitted for the data points of STEC-treated groups, indicating that an inverse correlation between total fecal STEC numbers and the B cells needed to induce one syncytium was linear for fecal STEC within a range of 1.5 to 4.5 log CFU/g. This line reached a plateau above 4 logs, again supporting the conjecture that fecal STEC numbers of ≥104 CFU/g were necessary to reduce the BLV load in B cells. Notably, regardless of STEC treatment, PBMC obtained from all sheep presenting with >105 CFU/g at least once post-BLV challenge (sheep 1393, 1422, 1432, and 1433) induced no more than three syncytia per coculture at 3 weeks post-BLV challenge, whereas PBMC from 8 of the 12 remaining sheep that never presented with >105 CFU STEC/g feces (sheep 1373, 1385, 1399, 1395, 1404, 1412, 1420, and 1424) induced at least four syncytia per culture. These results suggest that animals that presented with total fecal STEC numbers of >105 CFU/g at least once during the 2 months post-BLV challenge had a reduced BLV load.
Oral application of STEC promoted post-BLV weight gain in sheep.
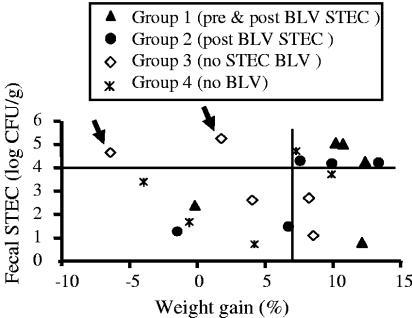
As in experiment I, weight gain was used as an indicator of general health and well-being. Between 4 and 7 weeks post-BLV challenge, the animals in the STEC-treated groups gained more weight than untreated animals. The average weight gains (kg) were 4.9 ± 1.3 in the group treated with STEC before and after BLV challenge, 3.4 ± 1.2 in the group treated after BLV challenge, 1.3 ± 1.5 in the infection control (BLV without STEC) group, and 1.9 ± 1.4 in the healthy control (no BLV) group. The difference between the group treated before and after challenge and the infection control group was significant (P < 0.05) after the exclusion of animals that lost weight. Two animals in the healthy control group lost weight between 4 and 7 weeks, indicating that weight loss could occur independently of BLV infection, and thus, a comparison was made among weight-gaining animals only. The impact of STEC treatment became apparent when weight change between 4 and 7 weeks post-BLV challenge was expressed as a percentage (Fig. 7). Four animals in the group receiving treatment prior to and post-BLV challenge and two animals in the group receiving treatment only after challenge gained 10% or more weight, while none of the animals in the infection control group gained as much (Fig. 7). Interestingly, BLV-infected and STEC-treated sheep gained more weight than the animals in the healthy control group, suggesting that STEC may promote weight gain independently of viral infection. This supposition was confirmed by analysis of the distribution of high fecal STEC counts. When all 20 animals from four groups combined were ranked by percentage of weight gain, the frequency of total fecal STEC numbers of ≥104 CFU/g post-BLV challenge was higher among the top 10 animals (15/20 or 75%) than among the bottom 10 (7/20 or 35%) (chi-square test; P < 0.05).
FIG. 7.
Fecal STEC numbers correlated with weight gain in sheep. Four experimental groups are shown. Two groups received STEC orally twice per week, starting 2 weeks before BLV challenge (pre & post BLV STEC, group 1) or 24 h postchallenge (post BLV STEC, group 2), until 16 weeks post-BLV challenge. The infection control group (BLV no STEC, group 3) and the healthy control group (no BLV, group 4) received E. coli K-12 starting at week −2, and the healthy control group was not challenged with BLV. Weight gains between 4 and 7 weeks post-BLV challenge are expressed as percentages and plotted against average fecal STEC numbers post-BLV challenge. Arrows indicate animals from the infection control group (no STEC BLV) that spontaneously acquired high fecal STEC counts after BLV challenge.
The animals in the infection control group gained less weight than animals in the other groups, including the healthy control sheep, suggesting that BLV challenge was associated with poor weight gain and that this effect was counteracted by STEC treatment. The protective effect appeared to be associated with total fecal STEC counts of ≥104 CFU/g. In both STEC-treated groups, all six animals that presented with high counts of fecal STEC bacteria gained >7% of weight post-BLV challenge, whereas out of the four remaining animals with lower fecal STEC numbers, three animals gained <7% of weight (Fig. 6). Taken together, these results suggest that oral application of STEC promoted weight gain in BLV-challenged sheep, especially when STEC treatment commenced before BLV challenge and when this treatment was associated with subsequent total fecal STEC counts of ≥104 CFU/g.
DISCUSSION
The goal of this work was to determine whether intestinal STEC bacteria have antiviral activity in vivo. To this end, we compared the initial stages of BLV viremia among sheep experimentally infected with BLV and treated with oral applications of STEC or stx-negative E. coli. We showed that biweekly oral STEC treatment reduced BLV viremia, although it did not always result in consistently high fecal STEC numbers. This STEC effect can be attributed to Stx and/or the presence of the stx gene because treatment with an isogenic stx deletion mutant did not have an antiviral effect. Also, the severity of viremia was reduced in proportion to the total number of fecal STEC bacteria, whether naturally occurring or from treatment. These results support the hypothesis that in ruminants, intestinal STEC bacteria have activity against BLV and possibly other related viruses.
We chose to investigate the in vivo effects of STEC against BLV because a substantial amount of previous work demonstrates that Stx suppresses BLV replication in vitro (3, 16, 17). The ovine model of BLV infection is commonly used in studies of BLV biology and of host responses to this viral infection. Major characteristics of BLV infection, such as incubation period, disease symptoms, and cellular tropism of the virus, are similar for cattle and sheep (22, 24, 45, 46). The noticeable differences, including a faster progression of BLV-induced disease in sheep, susceptibility of sheep to lower challenge doses (36, 48), and certain phenotypic differences between ovine and bovine B-cell subpopulations undergoing BLV-induced expansion in vivo (45), do not diminish the applicability of experimental BLV infection in sheep to studies of viral disease and host-virus interactions. Two clear advantages of the sheep model are faster disease progression (months rather than years) and easier animal handling.
BLV expression levels in vivo are similarly repressed in cattle and sheep, presumably by the immune response. The clinical picture before persistent lymphocytosis is that <1% of the B cells are expressing viral proteins, the virus is predominantly in a provirus form, and the amount of free virus in the blood circulation is very small. However, viral expression is rapidly derepressed in ex vivo cultures of purified PBMC from infected animals (32, 33). To assess BLV viremia, we used two well-established methods: measurements of SLP in PBMC cultures and induction of syncytia in cocultures of PBMC and F-81 indicator cells. Induction of syncytia by BLV is often used to detect BLV (pro)virus in blood PBMC and is a more direct method of viral measurement than SLP assessment (48, 53). The tendency of purified PBMC from BLV-positive cattle to spontaneously proliferate in culture is a hallmark of this infection (18, 49). BLV-induced SLP requires BLV expression by cultured cells (51), and cell proliferation rates roughly correlate with the concentration of BLV particles, since the process can be inhibited in a dose-dependent manner by monoclonal antibody to a structural protein of BLV (17, 52). Although the SLP rate may be affected by various factors (49), SLP of cultures from infected sheep can be considered a measure of BLV viremia.
Intestinal flora is a complex system of microbial communities characterized by host and spatial specificity with remarkable stability and is refractory to change by external interventions (21, 44). Therefore, it may not be surprising that oral treatments with STEC, although substantial, had a limited impact on the fecal STEC numbers in sheep and, in some cases, even failed to bring a change in host status from fecal STEC negative to fecal STEC positive. This inability to universally maintain fecal STEC numbers by oral treatment was not dependent on bacterial strain and was observed with a human isolate of E. coli O157:H7 (experiment I) and also with five ovine STEC strains (experiment II). Such outcomes were consistent with attempts by others to modify the composition of the intestinal microbiota with oral bacteria that usually result only in transient changes (20).
Our data show that the fecal concentration of naturally occurring STEC (i.e., in untreated animals) could exceed 104 CFU/g feces, consistent with published reports on the high prevalence of intestinal STEC in sheep (4, 7, 31). The fact that sheep can naturally carry STEC strains as transient members of their normal intestinal microbiota was a complicating factor in the design of our experiments and likely reduced the observed differences in aftermaths of BLV challenge between groups and confounded the analysis of the results. In spite of that, STEC treatment had a clear, measurable, and often statistically significant effect on BLV viremia. Notably, a reduction of SLP and inhibition of syncytium formation correlated with total fecal STEC numbers and were especially prominent in STEC-treated sheep that presented with fecal STEC counts of ≥104 CFU/g. Thus, the issue of naturally occurring STEC could be addressed with an experimental design that foregoes treatment with STEC and simply correlates fecal STEC numbers with reduced viremia. Although little information is available regarding the correlation between the total numbers of STEC bacteria in feces and of bacteria expressing Stx in the intestine, these results are consistent with such an assumption. Our finding that the total numbers of naturally occurring STEC bacteria in sheep may reach concentrations of 106 CFU/g, whereas concentrations of 105 to 108 CFU/g are not uncommon in cattle (19), indicates that ruminant animals are likely to carry intestinal STEC at sufficient numbers for an antiviral effect to occur.
A review of the literature uncovers a puzzling feature of BLV-induced disease in experimentally infected sheep, namely, that animals challenged with equal doses of infectious BLV often present dissimilar B-cell alterations (46) and widely different BLV loads in blood (8, 32, 56), even when littermates are compared (33). Also, seroconversion times for infected sheep may range from as little as 1 week (36) to 1 year (24). These inconsistencies of experimental results may be related to the fact that BLV-challenged sheep in the same experimental groups are likely to carry different numbers and/or strains of intestinal STEC. Accordingly, our results suggest that the number and persistence of intestinal STEC bacteria may account for this variability and that determinations of fecal STEC numbers should be considered for experiments involving viral infections in animals that routinely carry STEC.
Both Stx1 and Stx2 are able to translocate, via transcellular transport, through the intact layer formed by cells of epithelial intestinal cell lines (25, 41). Thus, it can be expected that the toxins produced by intestinal STEC can gain access to the intestinal lamina propria and to the systemic circulation. We attribute the in vivo antiviral effect of STEC to the action of Stxs translocating through the intestinal wall, entering highly permeable, BLV-expressing cells, and selectively eliminating these cells, as shown in vitro (3, 16). The toxin(s) may also stimulate the lymphocytes and other immunocytes present in the intestinal wall, systemic circulation, and lymphoid tissues. Thus, it is conceivable that the mechanism of antiviral effect observed in vivo may involve some indirect, toxin-mediated immune responses. However, the fact that treatment with an stx-negative isogenic mutant failed to induce responses comparable to those from treatment with wild-type bacteria suggests that it is unlikely that products of STEC, other than Stxs, are responsible for antiviral effects of STEC treatment. In vitro, virally infected and Stx-intoxicated cells undergo a more rapid demise than virally infected and not intoxicated cells (unpublished data). This indicates that the antiviral effect mediated by Stxs occurs at the whole-animal level and involves the elimination of virus-expressing cells, rather than rescue of cells from viral infection by direct antiviral action or by induction of an interferon system and other cellular antiviral remedies. The mechanism of Stx antiviral effects was beyond the scope of this study, but antiviral properties of RIPs are not necessarily limited to rRNA depurination. For example, some RIPs have activity against reverse transcriptase and other viral proteins (2, 38, 54), but such activities have yet to be described for Stxs.
The observation that STEC-treated animals infected with BLV had better weight gain than the BLV-free control animals not receiving STEC indicated that intestinal STEC bacteria may confer physiological benefits to animal carriers. At least three possible mechanisms may be involved: i) STEC may synthesize nutritional microcomponents, ii) STEC may stimulate the host via interaction with the intestinal wall or compounds delivered to the circulation, and iii) STEC may suppress growth of intestinal competitors detrimental to the host. Since weight gain was unexpected, its mechanisms were not investigated here but will be considered in future experiments.
Taken together, the results support the hypothesis that Stxs produced by STEC in animal carriers have anti-BLV activities. The fact that antiviral effects of STEC were especially prominent when fecal STEC counts exceeded 104 CFU/g indicates that intestinal colonization by high total numbers of STEC bacteria was a strong factor in STEC-mediated antiviral effects. It is conceivable that association between STEC strains and their ruminant hosts confers a selective advantage for the latter, or for both, possibly by prolonging the life of a host and thereby enhancing the dissemination of STEC in the environment. Such advantageous association could be a factor in the high prevalence of stx in strains of E. coli found in ruminant carriers. The results presented here are limited to the early postchallenge manifestation of BLV infection and the beginning of a chronic stage of the disease. Although it is clear that intestinal STEC bacteria mitigate this initial phase of BLV-induced disease, it remains to be determined whether STEC can prevent or slow the disease progression to the lymphosarcoma end stage and whether the described effect is applicable to infections with other viruses.
Acknowledgments
This work was supported in part by the Idaho Agriculture Experiment Station, the National Research Initiative of the USDA Cooperative State Research, Education and Extension Service, grants 99-35201-8539 and 04-04562, Public Health Service grants NO1-HD-0-3309, U54-AI-57141, P20-RR16454, and P20-RR15587 from the National Institutes of Health, and grants from the United Dairymen of Idaho and the Idaho Beef Council.
We thank Lonie Austin for taking care of the sheep, collecting blood samples, and observing sheep weight and appetite, Karol Gliniewicz for technical assistance, Jessie Holcomb for animal care, and Robert Adair for performing colony hybridization assays.
Editor: A. D. O'Brien
REFERENCES
- 1.Asakura, H., S. Makino, T. Shirahata, T. Tsukamoto, H. Kurazono, T. Ikeda, and K. Takeshi. 1998. Detection and genetical characterization of Shiga toxin-producing Escherichia coli from wild deer. Microbiol. Immunol. 42:815-822. [DOI] [PubMed] [Google Scholar]
- 2.Au, T. K., R. A. Collins, T. L. Lam, T. B. Ng, W. P. Fong, and D. C. Wan. 2000. The plant ribosome inactivating proteins luffin and saporin are potent inhibitors of HIV-1 integrase. FEBS Lett. 471:169-172. [DOI] [PubMed] [Google Scholar]
- 3.Basu, I., W. A. Ferens, D. M. Stone, and C. J. Hovde. 2003. Antiviral activity of Shiga toxin requires enzymatic activity and is associated with increased permeability of the target cells. Infect. Immun. 71:327-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bettelheim, K. A., J. C. Bensink, and H. S. Tambunan. 2000. Serotypes of verotoxin-producing (Shiga toxin-producing) Escherichia coli isolated from healthy sheep. Comp. Immunol. Microbiol. Infect. Dis. 23:1-7. [DOI] [PubMed] [Google Scholar]
- 5.Beutin, L., D. Geier, H. Steinruck, S. Zimmermann, and F. Scheutz. 1993. Prevalence and some properties of verotoxin (Shiga-like toxin)-producing Escherichia coli in seven different species of healthy domestic animals. J. Clin. Microbiol. 31:2483-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanco, M., J. E. Blanco, J. Blanco, A. Mora, C. Prado, M. P. Alonso, M. Mourino, C. Madrid, C. Balsalobre, and A. Juarez. 1997. Distribution and characterization of faecal verotoxin-producing Escherichia coli (VTEC) isolated from healthy cattle. Vet. Microbiol. 54:309-319. [DOI] [PubMed] [Google Scholar]
- 7.Blanco, M., J. E. Blanco, A. Mora, J. Rey, J. M. Alonso, M. Hermoso, J. Hermoso, M. P. Alonso, G. Dahbi, E. A. González, M. I. Bernárdez, and J. Blanco. 2003. Serotypes, virulence genes, and intimin types of Shiga toxin (verotoxin)-producing Escherichia coli isolates from healthy sheep in Spain. J. Clin. Microbiol. 41:1351-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burkhardt, H., S. Rosenthal, H. A. Rosenthal, E. Karge, and E. De Clercq. 1989. Treatment of bovine leukaemia virus-infected sheep with suramin: an animal model for the development of antiretroviral compounds. Acta Virol. 33:305-313. [PubMed] [Google Scholar]
- 9.Carrasco, L. 1995. Modification of membrane permeability by animal viruses. Adv. Virus Res. 45:61-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cerqueira, A. M., B. E. Guth, R. M. Joaquim, and J. R. Andrade. 1999. High occurrence of Shiga toxin-producing Escherichia coli (STEC) in healthy cattle in Rio de Janeiro State, Brazil. Vet. Microbiol. 70:111-121. [DOI] [PubMed] [Google Scholar]
- 11.Cray, W. C., Jr., L. A. Thomas, R. A. Schneider, and H. W. Moon. 1996. Virulence attributes of Escherichia coli isolated from dairy heifer feces. Vet. Microbiol. 53:369-374. [DOI] [PubMed] [Google Scholar]
- 12.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Djilali, S., A. L. Parodi, D. Levy, and G. L. Cockerell. 1987. Development of leukemia and lymphosarcoma induced by bovine leukemia virus in sheep: a hematopathological study. Leukemia 1:777-781. [PubMed] [Google Scholar]
- 14.Elder, R. O., J. E. Keen, G. R. Siragusa, G. A. Barkocy-Gallagher, M. Koohmaraie, and W. W. Laegreid. 2000. Correlation of enterohemorrhagic Escherichia coli O157 prevalence in feces, hides, and carcasses of beef cattle during processing. Proc. Natl. Acad. Sci. USA 97:2999-3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Endo, Y., K. Mitsui, M. Motizuki, and K. Tsurugi. 1987. The mechanism of action of ricin and related toxic lectins on eukaryotic ribosomes. The site and the characteristics of the modification in 28 S ribosomal RNA caused by the toxins. J. Biol. Chem. 262:5908-5912. [PubMed] [Google Scholar]
- 16.Ferens, W. A., L. J. Grauke, and C. J. Hovde. 2004. Shiga toxin 1 targets bovine leukemia virus-expressing cells. Infect. Immun. 72:1837-1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferens, W. A., and C. J. Hovde. 2000. Antiviral activity of Shiga toxin 1: suppression of bovine leukemia virus-related spontaneous lymphocyte proliferation. Infect. Immun. 68:4462-4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrer, J. F. 1980. Bovine lymphosarcoma. Adv. Vet. Sci. Comp. Med. 24:1-68. [PubMed] [Google Scholar]
- 19.Fukushima, H., and R. Seki. 2004. High numbers of Shiga toxin-producing Escherichia coli found in bovine faeces collected at slaughter in Japan. FEMS Microbiol. Lett. 238:189-197. [DOI] [PubMed] [Google Scholar]
- 20.Gibson, G. R., and M. B. Roberfroid. 1995. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr. 125:1401-1412. [DOI] [PubMed] [Google Scholar]
- 21.Gordon, J. I., L. V. Hooper, M. S. McNevin, M. Wong, and L. Bry. 1997. Epithelial cell growth and differentiation. III. Promoting diversity in the intestine: conversations between the microflora, epithelium, and diffuse GALT. Am. J. Physiol. 273:G565-G570. [DOI] [PubMed] [Google Scholar]
- 22.Grundboeck, M., E. Buzala, M. Szczotka, and J. Rulka. 1991. Experimental infection of sheep with bovine leukemia virus (BLV). Pol. Arch. Weter. 31:5-13. [PubMed] [Google Scholar]
- 23.Hoey, D. E., C. Currie, R. W. Else, A. Nutikka, C. A. Lingwood, D. L. Gally, and D. G. Smith. 2002. Expression of receptors for verotoxin 1 from Escherichia coli O157 on bovine intestinal epithelium. J. Med. Microbiol. 51:143-149. [DOI] [PubMed] [Google Scholar]
- 24.Hoss, H. E., and C. Olson. 1974. Infectivity of bovine C-type (leukemia) virus for sheep and goats. Am. J. Vet. Res. 35:633-637. [PubMed] [Google Scholar]
- 25.Hurley, B. P., M. Jacewicz, C. M. Thorpe, L. L. Lincicome, A. J. King, G. T. Keusch, and D. W. Acheson. 1999. Shiga toxins 1 and 2 translocate differently across polarized intestinal epithelial cells. Infect. Immun. 67:6670-6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaddu-Mulindw, D. H., T. Aisu, K. Gleier, S. Zimmermann, and L. Beutin. 2001. Occurrence of Shiga toxin-producing Escherichia coli in fecal samples from children with diarrhea and from healthy zebu cattle in Uganda. Int J. Food Microbiol. 66:95-101. [DOI] [PubMed] [Google Scholar]
- 27.Karch, H., and T. Meyer. 1989. Single primer pair for amplifying segments of distinct Shiga-like-toxin genes by polymerase chain reaction. J. Clin. Microbiol. 27:2751-2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kenyon, S. J., J. F. Ferrer, R. A. McFeely, and D. C. Graves. 1981. Induction of lymphosarcoma in sheep by bovine leukemia virus. JNCI 67:1157-1163. [PubMed] [Google Scholar]
- 29.Khan, A., S. Yamasaki, T. Sato, T. Ramamurthy, A. Pal, S. Datta, N. R. Chowdhury, S. C. Das, A. Sikdar, T. Tsukamoto, S. K. Bhattacharya, Y. Takeda, and G. B. Nair. 2002. Prevalence and genetic profiling of virulence determinants of non-O157 Shiga toxin-producing Escherichia coli isolated from cattle, beef, and humans, Calcutta, India. Emerg. Infect. Dis. 8:54-62. [PubMed] [Google Scholar]
- 30.Kobayashi, H., J. Shimada, M. Nakazawa, T. Morozumi, T. Pohjanvirta, S. Pelkonen, and K. Yamamoto. 2001. Prevalence and characteristics of Shiga toxin-producing Escherichia coli from healthy cattle in Japan. Appl. Environ. Microbiol. 67:484-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kudva, I. T., P. G. Hatfield, and C. J. Hovde. 1997. Characterization of Escherichia coli O157:H7 and other Shiga toxin-producing E. coli serotypes isolated from sheep. J. Clin. Microbiol. 35:892-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lagarias, D. M., and K. Radke. 1989. Transcriptional activation of bovine leukemia virus in blood cells from experimentally infected, asymptomatic sheep with latent infections. J. Virol. 63:2099-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lagarias, D. M., and K. Radke. 1990. Transient increases of blood mononuclear cells that could express bovine leukemia virus early after experimental infection of sheep. Microb. Pathog. 9:147-158. [DOI] [PubMed] [Google Scholar]
- 34.Lee-Huang, S., P. L. Huang, H. F. Kung, B. Q. Li, P. Huang, H. I. Huang, and H. C. Chen. 1991. TAP 29: an anti-human immunodeficiency virus protein from Trichosanthes kirilowii that is nontoxic to intact cells. Proc. Natl. Acad. Sci. USA 88:6570-6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Magnuson, B. A., M. Davis, S. Hubele, P. R. Austin, I. T. Kudva, C. J. Williams, C. W. Hunt, and C. J. Hovde. 2000. Ruminant gastrointestinal cell proliferation and clearance of Escherichia coli O157:H7. Infect. Immun. 68:3808-3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mammerickx, M., D. Portetelle, K. de Clercq, and A. Burny. 1987. Experimental transmission of enzootic bovine leukosis to cattle, sheep and goats: infectious doses of blood and incubation period of the disease. Leuk. Res. 11:353-358. [DOI] [PubMed] [Google Scholar]
- 37.Mirsky, M. L., C. A. Olmstead, Y. Da, and H. A. Lewin. 1996. The prevalence of proviral bovine leukemia virus in peripheral blood mononuclear cells at two subclinical stages of infection. J. Virol. 70:2178-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ng, T. B., and H. Wang. 2001. Panaxagin, a new protein from Chinese ginseng possesses anti-fungal, anti-viral, translation-inhibiting and ribonuclease activities. Life Sci. 68:739-749. [DOI] [PubMed] [Google Scholar]
- 39.Nizetic, D., R. Drmanac, and H. Lehrach. 1991. An improved bacterial colony lysis procedure enables direct DNA hybridisation using short (10, 11 bases) oligonucleotides to cosmids. Nucleic Acids Res. 19:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olson, M. C., S. Ramakrishnan, and R. Anand. 1991. Ribosomal inhibitory proteins from plants inhibit HIV-1 replication in acutely infected peripheral blood mononuclear cells. AIDS Res. Hum. Retrovir. 7:1025-1030. [DOI] [PubMed] [Google Scholar]
- 41.Philpott, D. J., C. A. Ackerley, A. J. Kiliaan, M. A. Karmali, M. H. Perdue, and P. M. Sherman. 1997. Translocation of verotoxin-1 across T84 monolayers: mechanism of bacterial toxin penetration of epithelium. Am. J. Physiol. 273:G1349-G1358. [DOI] [PubMed] [Google Scholar]
- 42.Pradel, N., V. Livrelli, C. De Champs, J.-B. Palcoux, A. Reynaud, F. Scheutz, J. Sirot, B. Joly, and C. Forestier. 2000. Prevalence and characterization of Shiga toxin-producing Escherichia coli isolated from cattle, food, and children during a one-year prospective study in France. J. Clin. Microbiol. 38:1023-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ritchie, J. M., C. M. Thorpe, A. B. Rogers, and M. K. Waldor. 2003. Critical roles for stx2, eae, and tir in enterohemorrhagic Escherichia coli-induced diarrhea and intestinal inflammation in infant rabbits. Infect. Immun. 71:7129-7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Savage, D. C. 1987. Microorganisms associated with epithelial surfaces and stability of the indigenous gastrointestinal microflora. Nahrung 31:383-395. [DOI] [PubMed] [Google Scholar]
- 45.Schwartz, I., A. Bensaid, B. Polack, B. Perrin, M. Berthelemy, and D. Levy. 1994. In vivo leukocyte tropism of bovine leukemia virus in sheep and cattle. J. Virol. 68:4589-4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwartz-Cornil, I., N. Chevallier, C. Belloc, D. Le Rhun, V. Laine, M. Berthelemy, A. Mateo, and D. Levy. 1997. Bovine leukaemia virus-induced lymphocytosis in sheep is associated with reduction of spontaneous B cell apoptosis. J. Gen. Virol. 78:153-162. [DOI] [PubMed] [Google Scholar]
- 47.Stirpe, F., L. Barbieri, M. G. Battelli, M. Soria, and D. A. Lappi. 1992. Ribosome-inactivating proteins from plants: present status and future prospects. Bio/Technology 10:405-412. [DOI] [PubMed] [Google Scholar]
- 48.Stirtzinger, T., V. E. Valli, and J. M. Miller. 1988. The role of virus dose in experimental bovine leukemia virus infection in sheep. Can. J. Vet. Res. 52:222-228. [PMC free article] [PubMed] [Google Scholar]
- 49.Stone, D. M., L. K. Norton, and W. C. Davis. 1997. Modulation of bovine leukemia virus-associated spontaneous lymphocyte proliferation by monoclonal antibodies to lymphocyte surface molecules. Clin. Immunol. Immunopathol. 83:156-164. [DOI] [PubMed] [Google Scholar]
- 50.Szabo, R. A., E. C. D. Todd, and A. Jean. 1986. Method to isolate Escherichia coli O157:H7 from food. J. Food Prot. 49:768-772. [DOI] [PubMed] [Google Scholar]
- 51.Thorn, R. M., P. Gupta, S. J. Kenyon, and J. F. Ferrer. 1981. Evidence that the spontaneous blastogenesis of lymphocytes from bovine leukemia virus-infected cattle is viral antigen specific. Infect. Immun. 34:84-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trueblood, E. S., W. C. Brown, G. H. Palmer, W. C. Davis, D. M. Stone, and T. F. McElwain. 1998. B-lymphocyte proliferation during bovine leukemia virus-induced persistent lymphocytosis is enhanced by T-lymphocyte-derived interleukin-2. J. Virol. 72:3169-3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van der Maaten, M. J., and J. M. Miller. 1980. Use of a continuous feline cell line for virologic and serologic investigations of bovine leukemia virus infections. Am. J. Vet. Res. 41:1785-1788. [PubMed] [Google Scholar]
- 54.Wang, H., and T. B. Ng. 2001. Isolation and characterization of velutin, a novel low-molecular-weight ribosome-inactivating protein from winter mushroom (Flammulina velutipes) fruiting bodies. Life Sci. 68:2151-2158. [DOI] [PubMed] [Google Scholar]
- 55.Wang, P., and N. E. Tumer. 2000. Virus resistance mediated by ribosome inactivating proteins. Adv. Virus Res. 55:325-355. [DOI] [PubMed] [Google Scholar]
- 56.Weber, A. F., M. J. Schmerr, D. K. Sorensen, C. Bingham, K. Pomeroy, J. M. Miller, and M. J. Van Der Maaten. 1983. Infectivity in sheep of blood lymphocytes from bovine leukemia virus-infected cows with different nuclear pocket prevalences. Am. J. Vet. Res. 44:1912-1915. [PubMed] [Google Scholar]
- 57.Yan, W., M. N. Malik, P. I. Peterkin, and A. N. Sharpe. 1996. Comparison of the hydrophobic grid-membrane filter DNA probe method and the Health Protection Branch standard method for the detection of Listeria monocytogenes in foods. Int. J. Food Microbiol. 30:379-384. [DOI] [PubMed] [Google Scholar]