Abstract
Bartonella henselae wound-associated infections suggest involvement of extracellular matrix molecules in adhesion and invasion. Pap31 was previously identified as a hemin-binding protein. Our recent studies suggest the protein is an adhesin that is recognized by the host's immune systems. In this study we examined the interactions of B. henselae Pap31 with fibronectin (Fn), heparin (Hep), and human umbilical vein endothelial cells (HUVECs). The cloned gene was expressed in Escherichia coli, and the purified Pap31 protein elicited strong antibody responses in mice and was reactive with rabbit anti-live B. henselae and mouse anti-Pap31 antibodies by Western blotting. Pap31 bound to immobilized Fn and to HUVECs in a dose-dependent manner and to Hep. Fn fragment-binding assays identified the Hep-1 and Hep-2 binding domains of human Fn and in particular the 12-13FnIII repeat module as primary binding sites for this adhesin. Furthermore, Pap31 binding to the above Fn fragments could be inhibited by Hep, suggesting a common binding site involving the 13FnIII repeat module on the Hep-2 domain of Fn. Adherence of intact B. henselae to HUVECs was inhibited by increasing concentrations of anti-Pap31 antibodies. In addition, purified Pap31 coprecipitated effectively with Fn and anti-Fn antibodies. Taken together, these data suggest that Pap31 is an Fn-binding protein mediating the B. henselae-host interaction(s), and they implicate the 13FnIII repeat module as an important binding site for this adhesin on the Fn molecule. These interactions may be important initial steps leading to bacterial attachment and colonization that promote the establishment of B. henselae infections in vivo.
The ability of bacteria to adhere to and colonize host cells is mediated by specific interactions between bacterial surface components (adhesins) and complementary host cell surface receptors including the extracellular matrix (ECM) molecules fibronectin (Fn) and heparin sulfate (HS). Fn is a glycoprotein that binds several ECM molecules and is found in soluble form in plasma and in fibrillar form in the ECM and basement membranes. Glycosaminoglycans (GAGs) such as heparin (Hep) and HS can also serve as putative receptors (1, 49). Hep and HS have common structural features and participate in the GAG-mediated recruitment of host ECM protein, a novel strategy in bacterial adherence and invasion demonstrated by Duensing et al. (15). HS, the most ubiquitous member of the GAGs, is found on the surfaces of most eukaryotic cells as part of the ECM, whereas Hep is synthesized in mast cells and basophils.
For several pathogens, the binding to ECM molecules such as Fn and Hep is an important step in pathogenesis that involves specific bacterial surface proteins (16, 26, 33, 47). This is particularly true for wound-associated infections such as those caused by Bartonella henselae. B. henselae, an opportunistic and emerging pathogen, is carried by cats and causes several distinct clinical syndromes in immunocompetent individuals and AIDS patients including cat scratch disease, bacillary angiomatosis, chronic bacteremia, and valvular endocarditis (2, 10, 24, 31, 37, 40). While several lines of evidence indicate a role for Fn in endovascular bacterial adherence during endocardial infections (21, 30, 42), other results strongly point to multifactorial events of bacterial adherence as the cause of heart valve damage (17).
B. henselae Pap31 was originally described as a bacterial surface protein that is associated with the bacteriophage-like particle from B. henselae (6). It is possible that this protein is involved in packaging or phage particle assembly, or, alternatively, it may be merely a surface protein that copurifies with the particles. Pap31 is homologous to the multigene heparin-binding protein (encoded by hbp) family in Bartonella quintana, Neisseria opacity proteins (Opa), Brucella OMP31 (a putative porin), and Agrobacterium tumefaciens OMP25 (an immunogenic surface protein) (34). HbpA, the most prominent member of the Hbp family of proteins, has been reported to play a role as a hemin receptor for B. quintana, and Opa proteins in Neisseria gonorrhoeae promote adherence (4, 8, 20, 50). The aims of the present study were to express and purify B. henselae Pap31; to characterize its binding to Fn, Hep, and host cells; and to investigate the effect of anti-Pap31 on B. henselae adherence to host cells.
MATERIALS AND METHODS
Bacteria, growth conditions, and reagents.
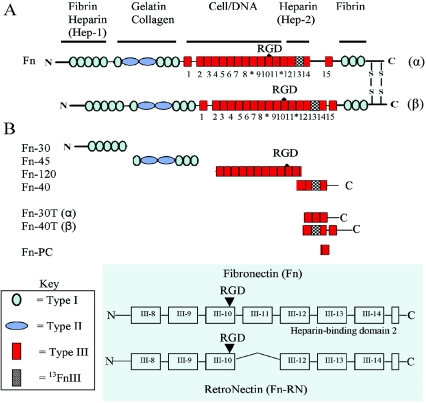
B. henselae strain Houston-1 (ATCC 49882) was grown on tryptic soy plates supplemented with 5% sheep blood in a humidified atmosphere at 37°C under 5% CO2. Escherichia coli BL21(DE3)pLysS was grown in Luria-Bertani broth. The intact human Fn and Fn fragments (Fig. 1), including the 30-kDa N-terminal heparin-binding fragment (Hep-binding domain-1 [Hep-1]), the α-chymotryptic 45-kDa collagen-binding fragment, and the synthetic peptide (KNNQKSEPLIGRKKT) derived from the C-terminal Hep-binding domain (Fn-PC), were purchased from Sigma (St. Louis, MO). The α-chymotryptic 40-kDa fragment, which contains the high-affinity Hep-binding domain and part of the V region (connecting segment-1 [CS-1] site) of Fn (C-terminal Hep-binding domain-2 [Hep-2]), and the 120-kDa cell-binding fragment were purchased from Chemicon International (Temecula, CA). RetroNectin (Fn-RN), a chimeric peptide of human Fn comprising the central cell-binding domain (type III repeat, 8 to 10), the heparin-binding domain-2 (type III repeat, modules 12 to 14), and the CS-1 site, was purchased from Takara Bio Inc. (Madison, WI) (Fig. 1).
FIG. 1.
Schematic representation of human plasma Fn, proteolytic fragments, and recombinant peptides of Fn used in this study. (A) Human plasma Fn dimer showing the α and β subunits. The domains containing the known binding sites are indicated above the diagrams. The type I, II, and III repeating structural domains of the Fn molecule are indicated as shown in the key. The 15 FnIII domain repeat modules are numbered, and the RGD cell-binding motif and the spliced V region are indicated. The checkered box within the type III domain represents repeat module 13, which contains the high-affinity Hep-binding sequence. (B) Fn fragments Fn-30, Fn-40, Fn-45, and Fn-120 represent the Hep-1, Hep-2, collagen-, and cell-binding domains, respectively. The purified Pap31 bound the Hep-1 and Hep-2 domains (Fig. 4C, bands 6 and 3, respectively) but not the collagen- or cell-binding domains. Fn-30T and Fn-40T represent the two Hep-2 thermolytic fragments to which the purified Pap31 bound in overlay experiments (Fig. 4C, bands 5 and 3). Fn-PC is the synthetic peptide located at the C-terminal end of the 14FnIII repeat module. Fn-RN (RetroNectin) is a chimeric peptide and is shown as represented on the Takara Bio Inc. website (46a), with the permission of the manufacturer.
B. henselae Pap31 expression and purification.
For expression, the mature Pap31 gene of B. henselae (without the putative promoter or leader signal peptide) was directionally subcloned downstream of an isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible T7/lac promoter and the initiation ATG of the pCRT7/NT-TOPO expression vector (Invitrogen Corporation, Carlsbad, CA) to produce recombinant Pap31 (rPap31). The recombinant plasmid was then used to transform competent E. coli BL21(DE3)pLysS as described previously (12). Expression was induced by addition of IPTG, and the fusion protein was purified using the pRSET Xpress purification system (Invitrogen) according to the manufacturer's instructions. The identity of the purified protein was confirmed by mass spectroscopy fingerprinting as described previously (12).
Abs.
Antibodies (Abs) against B. henselae were produced by vaccinating rabbits with live B. henselae as described previously (12), and antisera against purified Pap31 were produced in mice as described previously (19). Briefly, for the production of anti-Pap31 polyclonal ascites, three CFW (Swiss Webster) female mice weighing ∼21 g each (Charles River Laboratories) were injected subcutaneously three times at 2-week intervals with 50 μg of purified protein in Freud's incomplete adjuvant. Mice were boosted by intraperitoneal injection following a satisfactory titer test. Blood was taken 3 days after the third immunization, and mice were injected with a CFW sarcoma cell line (ATCC) in the abdomen 7 days after the boost or injection. The mice were tapped at the onset of visible ascite production and then humanely euthanized. Antibody titers were assayed by enzyme-linked immunosorbent assay (ELISA) using microtiter plates precoated with purified B. henselae Pap31 (Immulon 4 HB; Dynex Technologies, Chantilly, VA) at 1 μg/ml overnight at 4°C. The following day, the plates were blocked for 1 h with phosphate-buffered saline (PBS, comprising 137 mM NaCl, 2.7 mM KCl, 0.69 mM KH2PO4, and 6.4 mM Na2HPO4 [pH 7.4]) containing 0.05% Tween 20 and 1% bovine serum albumin (PBST-BSA) and were incubated with antisera at doubling dilutions in blocking buffer, starting at 1:100 and going up to 1:12,800. Wells were subsequently washed, and antigen-antibody reactions were visualized using alkaline phosphatase-conjugated goat anti-mouse immunoglobulin G (IgG) (1:5,000 in PBST-BSA) with para-nitrophenyl phosphate (Sigma, St. Louis, MO) as the substrate. Plates were read (optical density at 450 nm) and adjusted for control wells.
Immunoblotting, proteolysis, and N-terminal amino acid sequencing.
Outer membrane proteins (OMPs) were prepared, and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis were performed as described previously (13). Limited proteolysis of human Fn with thermolysin (Sigma, St. Louis, MO) was performed as described originally by Sekiguchi and Hakomori (44). Briefly, 50 μg of human Fn was incubated with 0.25 μg of thermolysin at room temperature for 4 h, and the reaction was terminated by addition of EDTA to a final concentration of 5 mM. The fragmented Fn was subjected to SDS-PAGE and either stained with Coomassie blue or electrotransferred to a nitrocellulose membrane for ligand blotting. For ligand blotting, transblotted membranes of purified Pap31 or thermolysin-fragmented Fn were first blocked for 1 h with PBS containing 5% (wt/vol) nonfat dry milk (PBSM) and then incubated for 2 h with human Fn (80 μg/ml) or purified Pap31 (40 μg/ml), respectively, in PBSM-T (PBSM containing 0.05% [vol/vol] Tween 20). Control blots were incubated with PBSM-T only. After three washes with PBST, protein-associated Fn or Pap31 was detected with Fn- or Pap31-specific polyclonal antibodies (primary antibodies) and alkaline phosphatase-conjugated IgG as the secondary antibody. To determine surface exposure, B. henselae whole cells (1-ml culture) in the mid-logarithmic phase of growth (optical density at 600 nm, ∼0.5 to 0.6) were pelleted by centrifugation, resuspended in 250 μl of the appropriate enzyme buffer, and treated with 50 μg of protease (trypsin, chymotrypsin, or proteinase K [Sigma, St. Louis, MO]) for 1 h at 37°C as described previously (13). The cells were washed three times with PBS and subjected to SDS-PAGE and Western blot analysis using mouse anti-Pap31 antibodies. N-terminal amino acid sequence analysis was performed by the Molecular Biology Resource Facility of the University of Oklahoma Health Sciences Center (Oklahoma City) as previously described (13).
ECM molecule binding assays.
Fn-binding assays were performed by ELISA according to Bauer and Spinola (3) using 96-well microtiter plates (Immulon 4 HB; Dynex Technologies, Chantilly, VA) coated overnight at 37°C with 100 μl of a 20-μg/ml solution of Fn, Fn fragments, or fetuin, a highly glycosylated glycoprotein control in coating buffer (0.25 M NaHCO3, 0.25 M Na2CO3 [pH 9.6]). Wells were washed three times with PBST and blocked with 200 μl of PBST containing 2% BSA (Sigma, St. Louis, MO) for 2 h at 37°C. Purified Pap31 (∼10 μg/ml) in PBS was added at 100 μl per well, and the plates were incubated at 37°C for 1 h and washed three times with PBST to remove nonadherent proteins. To detect bound proteins, wells were incubated with mouse anti-Pap31 ascites (1:1,500) for 1 h at 37°C. After three washes with PBST, specific horseradish peroxidase (HRP)-conjugated secondary Abs were used in the detection of Ab binding. A colorimetric reaction was done with 3,3′,5,5′-tetramethylbenzidine at 0.1 mg/ml in conjunction with 0.01% (vol/vol) H2O2. For inhibition assays, a mixture containing 1.6 μM (29 μg/ml) of Hep (sodium salt, porcine intestinal mucosa; Sigma, St. Louis, MO) and Pap31 was added to wells precoated with Fn and Fn fragments and then incubated for 1 h at 37°C as described above. All experiments were performed twice and in triplicate. Treatment differences were analyzed using the Student t test (46).
For Hep-binding assays, heparin Sepharose 6 Fast flow (Amersham Biosciences, Piscataway, NJ) chromatography was used to investigate the interaction between Hep and B. henselae Pap31. Essentially, Hep Sepharose 6 Fast flow beads were equilibrated in binding buffer (10 mM sodium phosphate, pH 7.4) and incubated with Pap31 at room temperature with gentle shaking for 2 h. The mixture was then washed five times by centrifugation (6,000 × g) for 2 min at 4°C in binding buffer, and the Hep-bound proteins were eluted with 50 μl of elution buffer (10 mM sodium phosphate, pH 7.4, 1 M NaCl) and stored at −20°C until use. The eluted proteins were subjected to SDS-PAGE and stained with Coomassie blue. Fn and epidermal growth factor (EGF) (a protein with no Hep-binding capability) were used as positive and negative controls, respectively. Sepharose beads alone in binding buffer served as negative controls. Experiments were performed twice and in triplicate, and treatment differences were tested by Student's t test (46).
Binding of purified Pap31 to host cells.
Human umbilical vein endothelial cells (HUVECs) were seeded (1.4 × 104) in endothelial growth medium—10% fetal bovine serum (ATCC) in microtiter wells precoated with 0.2% gelatin and were grown to confluence in a humidified atmosphere at 37°C under 5% CO2. The following day, the seeded wells were washed three times with fetal calf serum-free M199 medium, and either live B. henselae, B. henselae OMPs, purified Pap31 protein, or a fetuin control (3 μg/well) diluted in M199 medium was added to wells and incubated for 5 h in a humidified atmosphere at 37°C under 5% CO2. The plates were washed three times as indicated above and fixed in 20% acetone (150 μl) for 10 min at room temperature. The excess acetone was removed from the wells, and the plates were allowed to dry at 37°C overnight. For immunoassays, the acetone-fixed cells were probed with rabbit anti-B. henselae (1:8,000), mouse anti-Pap31 ascites, or rabbit anti-fetuin (Sigma, St. Louis, MO; 1:1,600) diluted in PBSM-T, followed by HRP-conjugated goat anti-rabbit or anti-mouse IgG (Sigma, St. Louis, MO; 1:8,000 diluted in PBSM-T). Controls in all experiments included wells containing only cells and wells containing all ingredients except primary Abs, secondary Abs, or both. B. henselae OMPs and fetuin were used as positive and negative controls, respectively. Experiments were performed twice and in triplicate. Treatment differences were tested by Student's t test (46).
For adherence inhibition, biotinylated bacteria were first pretreated with specific antibodies for 1 h at 37°C and washed before being used to infect the monolayers as described above. Bacteria were biotinylated using the EZ-LinK Sulfo-NHS-LC-biotinylation kit (Pierce, Rockford, IL) according to the manufacturer's instructions (12). Bacteria preincubated with normal mouse serum were used as controls, and wells receiving no bacteria were used as blanks. Additional controls included wells containing all ingredients except the primary or secondary antibodies. Biotinylated bacteria binding to monolayers were detected using HRP-conjugated strepavidin (Pierce, Rockford, IL) and a substrate-chromogen mixture consisting of 0.01% hydrogen peroxide in 0.1 mg/ml of tetramethylbenzidine (Sigma, St. Louis, MO).
Immunoprecipitation experiments.
For preparation of cell lysates used in the immunoprecipitation of host cell Fn-bound purified Pap31, HUVECs exposed to purified Pap31 (3 to 6 μg/well) as described above were washed three times with PBS, resuspended in lysis buffer {0.5% 3-[(cholamidopropyl)-dimethylammonio]-1-propanesulfonate [CHAPS], 200 mM NaCl, 40 mM NaHCO3, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride, 5 μg/ml leupeptin, and 5 μg/ml pepstatin A, pH 7.5}, and then incubated on ice for 30 min with intermittent vortexing. Cells were homogenized on ice and then centrifuged at 12,000 × g for 10 min at 4°C to remove particulate matter. The supernatant was collected, transferred to fresh tubes, and stored at −70°C until use.
For immunoprecipitation, a mixture composed of 30 μl of rabbit anti-Fn polyclonal Abs (0.5-mg/ml solution; Sigma, St. Louis, MO), 30 μl of cell lysates (obtained as described above), and 210 μl of cold immunoprecipitation (IPT) buffer {0.1 M sodium phosphate [pH 7.2], 0.15 M NaCl, 2% [vol/vol] IGEPAL CA-630 [trade name for (octylphenoxy)polyethoxyethanol], 0.1% SDS, and 0.02% sodium azide} was incubated overnight at 4°C with gentle shaking. Cell lysates incubated with normal rabbit serum were used as negative controls. The following day, Reacti-Bind protein A-coated plates (Pierce, Rockford, IL) were washed three times with 200 μl of IPT buffer for 5 min each with gentle shaking. The immune complexes were then transferred to the washed protein A-coated wells and incubated at room temperature for 4 h at 4°C with gentle shaking. After incubation, the wells were washed five times with cold IPT buffer, and the bound immune complexes were eluted with a reducing sample buffer (5×; 0.3 M Tris-HCl [pH 8.0], 2.5% SDS, 10% glycerol, 100 mM dithiothreitol) and subjected to SDS-PAGE and Western blot analysis using mouse anti-Pap31 or rabbit anti-B. henselae antibodies. In parallel experiments, purified B. henselae Pap31 was preincubated with exogenous Fn at room temperature for 4 h, mixed with rabbit anti-Fn polyclonal antibodies, and used in immunoprecipitation experiments as described above. Normal rabbit serum was used as a negative control.
RESULTS
Molecular and immunological characterization of Pap31.
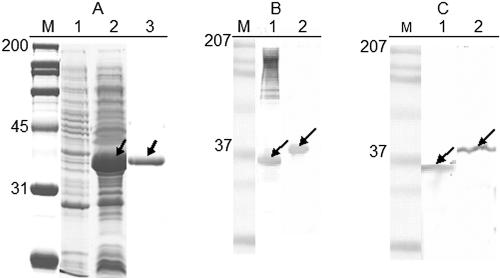
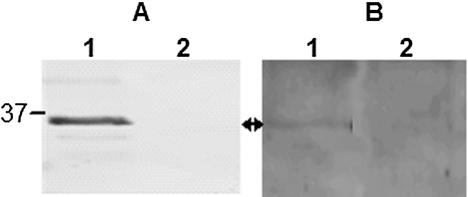
Immunoblots of protease-treated whole cells using anti-Pap31 antibodies revealed that Pap31 is susceptible to trypsin, chymotrypsin, and proteinase K digestion, indicating surface exposure (Fig. 2). Expression of the mature Pap31 gene under the control of the T7 promoter sequence (plasmid pCRT7/NT-TOPO) was achieved with high yield and purity (Fig. 3 A). The purified recombinant protein, which was confirmed to be Pap31 by mass spectrometry analysis (data not shown), was also demonstrated in Western blots with rabbit anti-B. henselae and mouse anti-Pap31 antibodies (Fig. 3B and C, respectively).
FIG. 2.
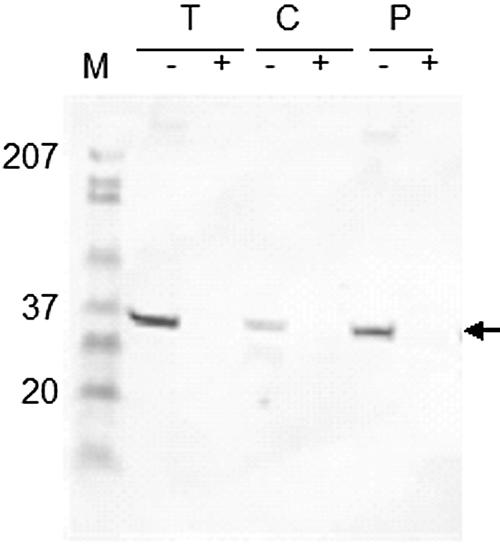
Immunoblot of B. henselae whole cells that were either left untreated (−) or treated (+) with trypsin (T), chymotrypsin (C), or proteinase K (P). Protease-treated B. henselae whole cells were subjected to SDS-PAGE and Western-blot analysis using mouse anti-Pap31 antibodies as described in Materials and Methods.
FIG. 3.
(A) Coomassie blue-stained SDS-PAGE gel of the expressed and purified recombinant Pap31. Lane 1, E. coli BL21(DE3)pLYsS (pCRT7NT-TOPO); lane 2, E. coli BL21(DE3)pLYsS (prPap31), induced with IPTG; lane 3, purified rPap31. (B) Reactivities of B. henselae OMPs (lane 1) and purified rPap31(lane 2) with rabbit antibodies against live B. henselae. (C) Reactivities of B. henselae whole cells (lane 1) and purified rPap31 (lane 2) with mouse anti-Pap31. Arrows indicate native and recombinant Pap31.
Binding of Pap31 to Fn and mapping of Fn binding sites.
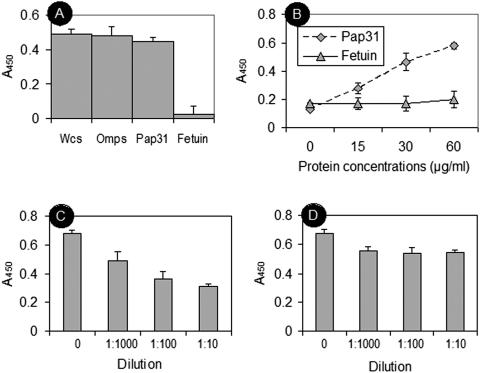
Ligand blotting revealed that Fn bound to purified Pap31 and to a band in B. henselae OMPs identified as Pap31 by mass spectrometric analysis (Fig. 4A). To further assess the affinity of purified Pap31 for the Fn molecule, increasing amounts of the purified protein were added to wells coated with Fn or a fetuin control. Purified Pap31 bound to immobilized Fn in a dose-dependent manner, and the levels of binding were consistently higher for Fn than for the fetuin control (Fig. 5A).
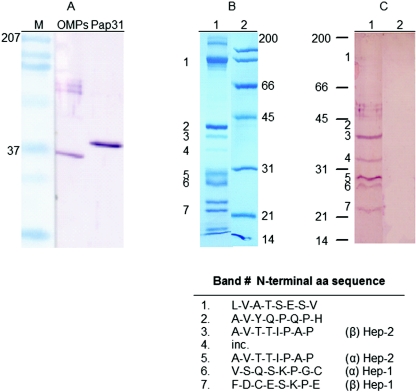
FIG. 4.
Binding of Pap31 to Fn. (A) Ligand blotting showing binding of B. henselae OMPs and purified Pap31 to Fn. (B and C) Binding of purified Pap31 to thermolysin fragments. (B) Intact human Fn was digested with thermolysin for 4 h, resolved by SDS-PAGE, and Coomassie blue stained (lane 1). Lane 2 contains broad-range molecular weight standards. (C) A duplicate gel was transferred to a nitrocellulose membrane and incubated with purified Pap31 (40 μg/ml) overnight at room temperature. Following washes, the bound Pap31 was detected with mouse anti-Pap31 antibodies. Positive bands with apparent molecular masses of 40, 34, 30, 29, and 24 kDa were detected (lane 1, bands 3, 4, 5, 6, and 7, respectively). No corresponding bands were found in a similar blot incubated with PBSM-T buffer alone (lane 2). The N-terminal amino acid sequences of thermolytic Fn fragments 1, 2, 3, 4, 5, 6, and 7, with apparent molecular masses of 120, 43, 40, 34, 30, 29, and 24 kDa, respectively, are given below. The N-terminal amino acid sequence of fragment 4 (faint band) was inconclusive (inc.). Note that Pap31 bound to the α and β forms of both the Hep-1 (fragments 6 and 7, respectively) and Hep-2 (fragments 5 and 3, respectively) domains of Fn. As expected, no binding was observed for the cell-binding (fragment 1) and gelatin-binding (fragment 2) domains of Fn.
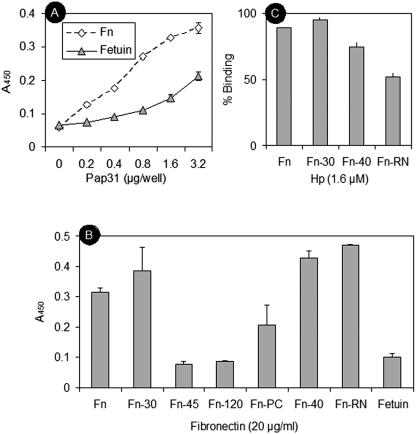
FIG. 5.
Characterization of Pap31 binding to immobilized Fn and Fn fragments. (A) Dose-dependent binding of rPap31 to intact human Fn. Shown is binding of increasing amounts of rPap31 to wells coated with Fn (diamonds) or a fetuin control (triangles). Absorbance values are means from representative experiments performed in triplicate. Error bars show the range of absorbance values. (B) Binding of rPap31 to wells coated with Fn, Fn fragments, or a fetuin control. Absorbance values are means from representative experiments performed in triplicate. Error bars show the range of absorbance values. (C) Binding of purified rPap31 to wells coated with Fn or Fn fragments in the presence of Hep. Binding was expressed as the percentage of binding to wells in the absence of Hep. Bars represent means in triplicate wells ± standard deviations.
Limited digestion of intact human Fn with thermolysin revealed 11 distinct fragments with apparent molecular masses of 200, 160, 120, 43, 40, 34, 30, 29, 24, 22, and 17.5 kDa on an SDS-PAGE gel (Fig. 4B, lane 1). For initial mapping of the Fn domain(s) bound by purified Pap31, transblotted membranes of thermolytic Fn fragments were overlaid with purified Pap31 in ligand blotting. Pap31 specifically bound the ∼40-, 34-, 30-, 29-, and 22-kDa thermolytic fragments but not the 200-, 160-, 120-, 43-, 24-, and 17.5-kDa fragments (Fig. 4C, lane 1). No binding was observed in a blot overlaid with PBSM-T alone (Fig. 4C, lane 2). N-terminal amino acid sequence analysis revealed that the 29- and 22-kDa fragments contain the α and β subunits of the Hep-1 domain of human Fn, respectively (45). Our sequence analysis also indicates that the 30- and 40-kDa thermolytic fragments have identical N-terminal sequences and contain the α and β subunits of the Hep-2 domain of human Fn, respectively (29, 44). These data suggest that Pap31 binds efficiently to both the α and β subunits of the Hep-1 and Hep-2 domains of Fn.
To further explore the binding of Pap31 to the Hep domains of Fn and to extend our investigation to other Fn domains, we tested the 120-kDa, 45-kDa, 40-kDa, and 30-kDa Fn fragments (designated Fn-120, Fn-45, Fn-40, and Fn-30, respectively), as well as Fn-PC and Fn-RN, as target molecules in ELISAs for their abilities to bind Pap31 (Fig. 5B). Purified Pap31 significantly bound intact Fn (P < 0.01), the N-terminal Fn-30 (P < 0.001) and C-terminal Fn-40 (P < 0.001) fragments, and Fn-RN (P < 0.001) (Fig. 5B). The level of adherence to Fn-PC was about half the level of adherence to Fn-30, Fn-40, or Fn-RN but not significantly different (P > 0.05) from that to the fetuin control (Fig. 5B). The levels of binding of Pap31 to the Fn-120 and Fn-45 fragments and to the fetuin negative control were similar and significantly (P < 0.01) lower than those to Fn, Fn-30, Fn-40, and Fn-RN (Fig. 5B). These results suggest that Pap31 bound preferentially the N-terminal and C-terminal Hep-binding domains of Fn and specifically the 12-14FnIII repeat modules.
Pap31 binds to Hep and shares a binding site with Hep on the 13FnIII repeat module.
To investigate whether Pap31 binds specifically to Hep, B. henselae OMPs, purified Pap31, and positive and negative controls (Fn and EGF, respectively) were incubated with Hep Sepharose Fast flow beads, and the eluted Hp-bound protein fractions were analyzed by SDS-PAGE for the presence of Pap31. Both purified Pap31 and the Fn positive control bound Hep (data not shown). A band identified as Pap31 by mass spectrometric analysis was also observed in the OMP eluted fraction (data not shown). As expected, no binding was observed for the EGF negative control (data not shown).
To test the effect of Hep on Pap31 adherence to the Hep-binding domains of Fn, binding assays were performed using wells coated with Fn, Fn-30 (Hep-1), Fn-40 (Hep-2), or Fn-RN (12-14FnIII) in the presence of 1.6 μM of Hep (porcine intestinal mucosa; Sigma, St. Louis, MO) diluted in PBS. Hep had a significant inhibitory effect on the binding of Pap31 to Fn (11%) (P < 0.05), Fn-30 (5%) (P < 0.05), Fn-40 (25%) (P < 0.01), and Fn-RN (48%) (P < 0.01) (Fig. 5C). These results suggest that Pap31 and Hep share the binding site on the Hep-2 binding domain of Fn involving the 12-14FnIII repeat modules. The data also indicate that Pap31 and Hep share significantly more binding sites on the Hep-2 domain of Fn than on the Hep-1 domain.
Binding of purified Pap31 to HUVECs.
The binding of purified Pap31 to intact HUVEC monolayers was assessed by ELISA and Western blot analysis. Purified Pap31, B. henselae whole cells, and OMPs exhibited similar and significant binding to HUVECs (Fig. 6A). Comparatively, the binding of the negative control, fetuin, was negligible (Fig. 6A). Purified Pap31 also bound HUVECs in a dose-dependent manner, whereas binding of the fetuin control remained low (Fig. 6B). Lysates from HUVECs preexposed to purified Pap31 were also investigated by Western blotting for the presence of the protein. A protein band corresponding to Pap31 was detected when the blot was probed with anti-Pap31 polyclonal antibodies (data not shown). No corresponding band was detected on cell lysates derived from HUVEC monolayers exposed to the buffer control only (data not shown).
FIG. 6.
Characterization of purified rPap31 binding to HUVECs by ELISA. (A) Binding of B. henselae whole cells, OMPs, rPap31, and a fetuin control to HUVEC monolayers. (B) Dose-dependent binding of rPap31 to HUVECs. Shown is binding of increasing amounts of rPap31 (diamonds) or a fetuin control (triangles) to HUVEC monolayers. (C and D) Inhibition of B. henselae binding to HUVEC monolayers following pretreatment of bacteria with mouse anti-Pap31 antibodies (C) or a control mouse serum (D). Experiments were performed as described in Materials and Methods. For all experiments, absorbance values are means from representative experiments performed in triplicate. Error bars show the range of absorbance values.
To assess the functionality of anti-Pap31 antibodies in the overall adherence of B. henselae to host cells, biotinylated B. henselae whole cells were first pretreated with the antibodies and then tested for their ability to bind HUVEC monolayers. Pretreatment of biotinylated B. henselae with increasing amounts of anti-Pap31 resulted in a significant (P < 0.01) dose-response inhibition of bacterial adherence (27, 46, and 53% reduction at 1:1,000, 1:100, and 1:10 dilutions, respectively) (Fig. 6C). No significant difference (P > 0.05) was observed in the adherence of normal serum-treated bacteria to HUVECs (Fig. 6D).
Immunoprecipitation.
If Pap31 binds to Fn, then it should coprecipitate with exogenous Fn as well as with endogenous host cell Fn. Western blotting of a mixture of exogenous Fn and Pap31 immunoprecipitated with anti-Fn antibodies showed that purified Pap31 coprecipitated with exogenous Fn (Fig. 7A, lane 1) when probed with anti-Pap31 antibodies. Precipitation with normal rabbit serum (negative control) did not show any band corresponding to purified Pap31 (Fig. 7A, lane 2). Similarly, anti-Fn polyclonal antibody immunoprecipitates of lysates from HUVECs preexposed to Pap31 revealed a protein band corresponding to Pap31 when probed with anti-Pap31 in Western blots (Fig. 7B, lane 1). No corresponding band was detected in similar normal rabbit serum immunoprecipitates (Fig. 7 B, lane 2).
FIG. 7.
(A) Purified Pap31 was incubated with Fn for 1 h at 37°C, and the complex was immunoprecipitated with anti-Fn (lane 1) or normal rabbit serum (lane 2) as described in Materials and Methods. (B) Similarly, lysates from HUVECs preexposed to purified Pap31were immunoprecipitated with anti-Fn (lane 1) or normal rabbit serum (lane 2). Immunoprecipitates were subjected to Western blot analysis using mouse anti-Pap31 antibodies.
DISCUSSION
B. henselae infection initiates after trauma to the skin, suggesting that adherence to host cells may be mediated by specific interactions between B. henselae surface proteins such as Pap31 and components of the host ECM such as Fn and Hep. Pap31 was previously shown to be involved in acquisition of heme and thus may be an important virulence factor for B. henselae (8, 53). Because heme receptor molecules are surface exposed, we undertook to determine if Pap31 had another virulence function as an adhesin to ECM. We have expressed and purified B. henselae Pap31 with high yield and purity and demonstrated its immunogenicity. We also demonstrated that Pap31 acts as a potential ligand for Fn and Hep, indicating its broad-range binding ability. Recognition of anti-Pap31 antibodies in rabbits or mice vaccinated with live B. henselae or purified Pap31, respectively, indicated that the protein is expressed in vivo and contributes to the humoral immune response in the host defense against B. henselae. Taken together, these data indicate that Pap31 is an adhesin with broad binding ability and may be important in the interaction of B. henselae with hosts.
The role of the humoral immune response in Bartonella infections in cats is not clear (11). Differences in B. henselae antigen-specific antibody responses have been reported in cats and may be dependent on the strain or the individual cat's immune system (18, 36). Recently, Chenoweth and al. reported virtually no Ab stimulation in cats by members of the heme-binding protein (Hbp) family of proteins, which includes B. henselae Pap31 (9), perhaps due to antigenic variation among Hbp homologs (34). In the same study, no anti-lipopolysaccharide (LPS) antibody was found in sera from B. henselae-infected cats (9), and this lack of immunogenicity was attributed to the composition of lipid A and attached long-chain fatty acids of B. henselae LPS (51). The authors concluded that the immunoevasive characteristics of LPS and members of the Hbp family may be responsible for the pathogen's ability to establish long-term survival within its natural host (i.e., cats) (9). Our present study provides clear and convincing evidence that Pap31 is recognized by rabbit and mouse humoral systems. Recognition of purified Pap31 by cat immune systems is under investigation.
Fn, a multifunctional glycoprotein, consists of two similar subunits (α and β) held together near the C terminus by two disulfide bonds (Fig. 1). Each subunit comprises six distinct domains, and the domains in turn are made up of amino acid repeat modules known as FnI, FnII, and/or FnIII (Fig. 1) (35). The five domains are referred to as Hep-1/Fib-1, Gel, Cell/DNA, Hep-2, and Fib-2 because of their affinity for heparin (Hep), gelatin (Gel), cell surface (Cell), or fibrin (Fib) and are aligned from the NH2 to the COOH terminus in the above order. Fn-30 (Hep-1) is the preferred binding site of most bacteria and bacterial adhesins, while binding to Fn-40 (Hep-2) by bacteria is uncommon (27). Recent studies mapped the target region of the Hep-1 domain to the 4-5FnI repeat modules (25, 43). We have shown in this study that Pap31 bound both thermolytic Fn-30 and Fn-40 fragments (containing the α and β subunits of the Hep-2 domain of human Fn, respectively) in ligand blotting. We also mapped the binding site of the purified protein to the 13FnIII repeat module on the Hep-2 domain of Fn. Pap31 bound significantly to the commercially purchased Fn-30 (Hep-1 domain), Fn-40 (Hep-2 domain), and Fn-RN (containing the central cell-binding domain, the Hep-2 12-14FnIII repeat modules, and the CS-1 region). The binding of Pap31 to the synthetic peptide Fn-PC (located at the COOH-terminal end of the 14FnIII repeat module) (14) was comparatively low. Pap31 did not bind Fn-120 (cell-binding domain) or Fn-45 (gelatin-binding domain), and there is no report in the literature of involvement of the CS-1 region (part of RetroNectin) in bacterial adherence to Fn. The above results provide clear evidence that the observed efficient binding of Pap31 to the Hep-2 domain or RetroNectin was predominantly mediated by the 13FnIII repeat module on the Hep-2 domain of Fn.
The binding sites and/or mode of interactions of Hep with the Hep-1 and Hep-2 domains of Fn have been reported for several pathogens and adhesins (29, 38). Studies also indicate that the cationic cradle of the 13FnIII repeat module of Fn accounts for the major binding activity of Hep on the Hep-2 domain of Fn (7, 28, 41). Recent studies identified the 13FnIII repeat module of Fn as a primary binding site for ShdA, a nonfimbrial adhesin from Salmonella enterica (29). In addition, the binding of ShdA to 13FnIII was found to be sensitive to the osmotic strength of the buffer, suggesting that the binding involved ionic interactions. The significant binding (P < 0.001) of Pap31 to Fn-40 and Fn-RN was inhibited (∼25% and 48%, respectively) by 1.6 μM Hep, suggesting that Pap31 and Hep share a similar binding site(s) involving the 13FnIII repeat module on the Hep-2 domain. Comparatively, Hep had a reduced but significant (P < 0.05) inhibitory effect (5%) on the binding of Pap31 to Fn-30, suggesting few common binding sites for Pap31 and Hep on Fn-30 (the Hep-1 domain of Fn).
The ability of B. henselae to colonize host tissues may be facilitated by bacterial surface proteins with high affinity for ECM molecules, and the outcome of such colonization depends largely on the receptor/ligand interactions between the host cells and the bacterium. Fn-binding proteins such as those of Neisseria meningitidis promote the process of infection of host cells, possibly via Fn bridging to α5β1 integrins (47). Binding of Bartonella to different host cell types likely involves adhesins that are variably expressed. A family of variably expressed and multifunctional B. quintana OMPs (Vomps) has been shown to be involved in the binding of collagen (VompC) and in autoaggregation (VompA) (52). Riess et al. identified a B. henselae adhesin A (BadA) that mediates the binding of the bacterium to ECM proteins and demonstrated that β1 integrins are crucial for cell adhesion of B. henselae, possibly via Fn bridging (39). This is in line with previous reports that show that β1 integrins are required for YadA binding to host cells (5). We demonstrated in this study the dose-dependent binding of purified Pap31 to HUVECs and coimmunoprecipitation with Fn and anti-Fn. Consistent with these results, we detected Fn on the surfaces of HUVEC monolayers when they were probed with an anti-Fn monoclonal antibody (data not shown). Our results also demonstrated that pretreatment of B. henselae with anti-Pap31 inhibited the adherence of the bacterium to HUVECs. Together these data suggest a role for Pap31 in the adherence of the bacterium to HUVECs involving host cell surface Fn.
Although GAGs such as Hep and HS have a prominent role in cell-cell interactions and in cell growth, they are also increasingly implicated as strong targets for bacterial adhesion during early infection (1, 22, 23, 32, 49). GAGs have been shown to participate in the GAG-mediated recruitment of host ECM proteins, a novel strategy in bacterial adherence and invasion (15). According to this strategy, soluble HS, exposed during wound healing on the cell surface, binds directly to bacterial surface molecules and serves as a universal recruiting site for a wide range of the host Hep-binding proteins, such as Fn, without producing separate receptors for each protein. We have shown here that Pap31 also bound Hep, suggesting that Hep could act as a bridge between Pap31 and other ECM molecules such as Fn, further enhancing B. henselae adhesion.
The expression of Opa proteins in N. gonorrhoeae has been shown to promote the adherence of the bacterium to epithelial cells (4, 50). Furthermore, one such Opa protein, OpaA, has been shown to bind Fn and Hep and to be involved in bacterial entry into host cells using a concerted action of Hep, Fn, and integrin receptors (48). It is likely that expression of Pap31 during B. henselae infection may contribute similarly to bacterial colonization and invasion, and as such, Pap31 may be a potential target antigen for vaccine development and induction of protective immunity using anti-adhesion strategies. Future studies will seek to further elucidate the mode, site(s), and mechanism(s) of Pap31 binding to Hep-binding domains of Fn, map the region(s) on Pap31 involved in this binding, and assess whether these interactions lead to bacterial colonization and invasion or influence subsequent cellular functions in host cells.
Acknowledgments
This work was partially funded by a grant from the Noble Foundation, Ardmore, Okla. We are grateful for the support of the Oklahoma State University Center for Veterinary Health Sciences.
We thank Betty Handlin for helping with the artwork.
Editor: V. J. DiRita
REFERENCES
- 1.Alvarez-Dominguez, C., J. A. Vazquez-Boland, E. Carrasco-Marin, P. Lopez-Mato, and F. Leyva-Cobian. 1997. Host cell heparan sulfate proteoglycans mediate attachment and entry of Listeria monocytogenes, and the listerial surface protein ActA is involved in heparan sulfate receptor recognition. Infect. Immun. 65:78-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, B. E., and M. A. Neuman. 1997. Bartonella spp. as emerging human pathogens. Clin. Microbiol. Rev. 10:203-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauer, M. E., and S. M. Spinola. 1999. Binding of Haemophilus ducreyi to extracellular matrix proteins. Infect. Immun. 67:2649-2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bessen, D., and E. C. Gotschlich. 1986. Interactions of gonococci with HeLa cells: attachment, detachment, replication, penetration, and the role of protein II. Infect. Immun. 54:154-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bliska, J. B., M. C. Copass, and S. Falkow. 1993. The Yersinia pseudotuberculosis adhesin YadA mediates intimate bacterial attachment to and entry into HEp-2 cells. Infect. Immun. 61:3914-3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowers, T. J., D. Sweger, D. Jue, and B. Anderson. 1998. Isolation, sequencing and expression of the gene encoding a major protein from the bacteriophage associated with Bartonella henselae. Gene 206:49-52. [DOI] [PubMed] [Google Scholar]
- 7.Busby, T., W. Argraves, S. Brew, I. Pechik, G. Gilliland, and K. Ingham. 1995. Heparin binding by fibronectin module III-13 involves six discontinuous basic residues brought together to form a cationic cradle. J. Biol. Chem. 270:18558-18562. [DOI] [PubMed] [Google Scholar]
- 8.Carroll, J., S. Coleman, L. Smitherman, and M. Minnick. 2000. Hemin-binding surface protein from Bartonella quintana. Infect. Immun. 68:6750-6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chenoweth, M., C. Greene, D. Krause, and F. Gherardini. 2004. Predominant outer membrane antigens of Bartonella henselae. Infect. Immun. 72:3097-3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chomel, B., A. Wey, R. Kasten, B. Stacy, and P. Labelle. 2003. Fatal case of endocarditis associated with Bartonella henselae type I infection in a domestic cat. J. Clin. Microbiol. 41:5337-5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chomel, B. B., R. W. Kasten, K. Floyd-Hawkins, B. Chi, K. Yamamoto, J. Roberts-Wilson, A. N. Gurfield, R. C. Abbott, N. C. Pedersen, and J. E. Koehler. 1996. Experimental transmission of Bartonella henselae by the cat flea. J. Clin. Microbiol. 34:1952-1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dabo, S., A. Confer, and R. Quijano-Blas. 2003. Molecular and immunological characterization of Pasteurella multocida serotype A:3 OmpA: evidence of its role in P. multocida interaction with extracellular matrix molecules. Microb. Pathog. 35:147-157. [DOI] [PubMed] [Google Scholar]
- 13.Dabo, S. M., A. W. Confer, and G. L. Murphy. 1997. Outer membrane proteins of bovine Pasteurella multocida serogroup A isolates. Vet. Microbiol. 54:167-183. [DOI] [PubMed] [Google Scholar]
- 14.Drake, S., J. Varnum, K. Mayo, P. Letourneau, L. Furcht, and J. McCarthy. 1993. Structural features of fibronectin synthetic peptide FN-C/H II, responsible for cell adhesion, neurite extension, and heparin sulfate binding. J. Biol. Chem. 268:15859-15867. [PubMed] [Google Scholar]
- 15.Duensing, T. D., J. S. Wing, and J. P. van Putten. 1999. Sulfated polysaccharide-directed recruitment of mammalian host proteins: a novel strategy in microbial pathogenesis. Infect. Immun. 67:4463-4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dziewanowska, K., J. M. Patti, C. F. Deobald, K. W. Bayles, W. R. Trumble, and G. A. Bohach. 1999. Fibronectin binding protein and host cell tyrosine kinase are required for internalization of Staphylococcus aureus by epithelial cells. Infect. Immun. 67:4673-4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flock, J. I., S. A. Hienz, A. Heimdahl, and T. Schennings. 1996. Reconsideration of the role of fibronectin binding in endocarditis caused by Staphylococcus aureus. Infect. Immun. 64:1876-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freeland, R. L., D. T. Scholl, K. R. Rohde, L. J. Shelton, and K. L. O'Reilly. 1999. Identification of Bartonella-specific immunodominant antigens recognized by the feline humoral immune system. Clin. Diagn. Lab. Immunol. 6:558-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gatto, N., S. Dabo, R. Hancock, and A. Confer. 2002. Characterization of, and immune responses of mice to, the purified OmpA-equivalent outer membrane protein of Pasteurella multocida serotype A:3 (Omp28). Vet. Microbiol. 87:221-235. [DOI] [PubMed] [Google Scholar]
- 20.Griffiss, J. M., C. J. Lammel, J. Wang, N. P. Dekker, and G. F. Brooks. 1999. Neisseria gonorrhoeae coordinately uses pili and Opa to activate HEC-1-B cell microvilli, which causes engulfment of the gonococci. Infect. Immun. 67:3469-3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamill, R. 1987. Role of fibronectin in infective endocarditis. Rev. Infect. Dis. 9(Suppl. 4):S360-S371. [DOI] [PubMed] [Google Scholar]
- 22.Henry-Stanley, M., D. Hess, E. Erickson, R. Garni, and C. Wells. 2003. Role of heparan sulfate in interactions of Listeria monocytogenes with enterocytes. Med. Microbiol. Immunol. (Berlin) 192:107-115. [DOI] [PubMed] [Google Scholar]
- 23.Hileman, R., J. Fromm, J. Weiler, and R. Linhardt. 1998. Glycosaminoglycan-protein interactions: definition of consensus sites in glycosaminoglycan binding proteins. Bioessays 20:156-167. [DOI] [PubMed] [Google Scholar]
- 24.Jerris, R. C., and R. L. Regnery. 1996. Will the real agent of cat-scratch disease please stand up? Annu. Rev. Microbiol. 50:707-725. [DOI] [PubMed] [Google Scholar]
- 25.Joh, D., P. Speziale, S. Gurusiddappa, J. Manor, and M. Hook. 1998. Multiple specificities of the staphylococcal and streptococcal fibronectin-binding microbial surface components recognizing adhesive matrix molecules. Eur. J. Biochem. 258:897-905. [DOI] [PubMed] [Google Scholar]
- 26.Joh, D., E. R. Wann, B. Kreikemeyer, P. Speziale, and M. Hook. 1999. Role of fibronectin-binding MSCRAMMs in bacterial adherence and entry into mammalian cells. Matrix Biol. 18:211-223. [DOI] [PubMed] [Google Scholar]
- 27.Joh, H., K. House-Pompeo, J. Patti, S. C. Gurusiddappa, and M. Hook. 1994. Fibronectin receptors from Gram-positive bacteria: comparison of active sites. Biochemistry 33:6086-6092. [DOI] [PubMed] [Google Scholar]
- 28.Kapila, Y., D. Doan, E. Tafolla, and R. Fletterick. 2001. Three-dimensional structural analysis of fibronectin heparin-binding domain mutations. J. Cell. Biochem. 81:156-161. [DOI] [PubMed] [Google Scholar]
- 29.Kingsley, R., A. Keestra, M. de Zoete, and A. Baumler. 2004. The ShdA adhesin binds to the cationic cradle of the fibronectin 13FnIII repeat module: evidence for molecular mimicry of heparin binding. Mol. Microbiol. 52:345-355. [DOI] [PubMed] [Google Scholar]
- 30.Lowrance, J., L. Baddour, and W. Simpson. 1990. The role of fibronectin binding in the rat model of experimental endocarditis caused by Streptococcus sanguis. J. Clin. Investig. 86:7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maurin, M., R. Birtles, and D. Raoult. 1997. Current knowledge of Bartonella species. Eur. J. Clin. Microbiol. Infect. Dis. 16:487-506. [DOI] [PubMed] [Google Scholar]
- 32.Menozzi, F. D., R. Mutombo, G. Renauld, C. Gantiez, J. H. Hannah, E. Leininger, M. J. Brennan, and C. Locht. 1994. Heparin-inhibitable lectin activity of the filamentous hemagglutinin adhesin of Bordetella pertussis. Infect. Immun. 62:769-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Middleton, A., M. Chadwick, A. Nicholson, A. Dewar, R. Groger, E. Brown, and R. Wilson. 2000. The role of Mycobacterium avium complex fibronectin attachment protein in adherence to the human respiratory mucosa. Mol. Microbiol. 38:381-391. [DOI] [PubMed] [Google Scholar]
- 34.Minnick, M. F., K. N. Sappington, L. S. Smitherman, S. G. Andersson, O. Karlberg, and J. A. Carroll. 2003. Five-member gene family of Bartonella quintana. Infect. Immun. 71:814-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mosher, D. F. 1989. Fibronectin. Academic Press, New York, N.Y.
- 36.O'Reilly, K. L., R. W. Bauer, R. L. Freeland, L. D. Foil, K. J. Hughes, K. R. Rohde, A. F. Roy, R. W. Stout, and P. C. Triche. 1999. Acute clinical disease in cats following infection with a pathogenic strain of Bartonella henselae (LSU16). Infect. Immun. 67:3066-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Powderly, W. G., S. L. Stanley, and G. Medoff. 1986. Pneumococcal endocarditis: report of a series and review of the literature. Rev. Infect. Dis. 8:786-791. [DOI] [PubMed] [Google Scholar]
- 38.Rabenstein, D. 2002. Heparin and heparan sulfate: structure and function. Nat. Prod. Rep. 19:312-331. [DOI] [PubMed] [Google Scholar]
- 39.Riess, T., S. G. E. Andersson, A. Lupas, M. Schaller, A. Schäfer, P. Kyme, J. Martin, J. Wälzlein, U. Ehehalt, H. Lindroos, M. Schirle, A. Nordheim, I. B. Autenrieth, and V. A. J. Kempf. 2004. Bartonella adhesin A mediates a proangiogenic host cell response. J. Exp. Med. 200:1267-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodrick, D., B. Dillon, M. Dexter, I. Nicholson, S. Marcel, D. Dickeson, and J. Iredell. 2004. Culture-negative endocarditis due to Houston complex Bartonella henselae acquired in Noumea, New Caledonia. J. Clin. Microbiol. 42:1846-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sachchidanand, O. Lequin, D. Staunton, B. Mulloy, M. Forster, K. Yoshida, and I. Campbell. 2002. Mapping the heparin-binding site on the 13-14F3 fragment of fibronectin. J. Biol. Chem. 277:50629-50635. [DOI] [PubMed] [Google Scholar]
- 42.Scheld, W. M., R. W. Strunk, G. Balian, and R. A. Calderone. 1985. Microbial adhesion to fibronectin in vitro correlates with production of endocarditis in rabbits. Proc. Soc. Exp. Biol. Med. 180:474-482. [DOI] [PubMed] [Google Scholar]
- 43.Schwarz-Linek, U., J. Werner, A. Pickford, S. Gurusiddappa, J. Kim, E. Pilka, J. Briggs, T. Gough, M. Hook, I. Campbell, and J. Potts. 2003. Pathogenic bacteria attach to human fibronectin through a tandem beta-zipper. Nature 423:177-181. [DOI] [PubMed] [Google Scholar]
- 44.Sekiguchi, K., and S. Hakomori. 1983. Domain structure of human plasma fibronectin. Differences and similarities between human and hamster fibronectins. J. Biol. Chem. 258:3967-3973. [PubMed] [Google Scholar]
- 45.Skorstengaard, K., M. Jensen, P. Sahl, T. Petersen, and S. Magnusson. 1986. Complete primary structure of bovine plasma fibronectin. Eur. J. Biochem. 161:441-453. [DOI] [PubMed] [Google Scholar]
- 46.Steel, R., and J. Torrie. 1960. Principles and procedures of statistics. McGraw-Hill Book Co., Inc., New York, N.Y.
- 46a.Takara Bio, Inc. 2006. RetroNectin chimeric peptide structure. [Online.] www.takara-bio.com/products/pro4-1.htm.
- 47.Unkmeir, A., K. Latsch, G. Dietrich, E. Wintermeyer, B. Schinke, S. Schwender, K. S. Kim, M. Eigenthaler, and M. Frosch 2002. Fibronectin mediates Opc-dependent internalization of Neisseria meningitidis in human brain microvascular endothelial cells. Mol. Microbiol. 46:933-946. [DOI] [PubMed] [Google Scholar]
- 48.van Putten, J., T. Duensing, and R. Cole. 1998. Entry of OpaA+ gonococci into HEp-2 cells requires concerted action of glycosaminoglycans, fibronectin, and integrin receptors. Mol. Microbiol. 29:369-379. [DOI] [PubMed] [Google Scholar]
- 49.Wadstrom, T., and A. Ljungh. 1999. Glycosaminoglycan-binding microbial proteins in tissue adhesion and invasion: key events in microbial pathogenicity. J. Med. Microbiol. 48:223-233. [DOI] [PubMed] [Google Scholar]
- 50.Weel, J. F., C. T. Hopman, and J. P. van Putten. 1991. In situ expression and localization of Neisseria gonorrhoeae opacity proteins in infected epithelial cells: apparent role of Opa proteins in cellular invasion. J. Exp. Med. 173:1395-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zahringer, U., B. Lindner, Y. Knirel, W. van den Akker, R. Hiestand, H. Heine, and C. Dehio. 2004. Structure and biological activity of the short-chain lipopolysaccharide from Bartonella henselae ATCC 49882T. J. Biol. Chem. 279:21046-21054. [DOI] [PubMed] [Google Scholar]
- 52.Zhang, P., B. B. Chomel, M. K. Schau, J. S. Goo, S. Droz, K. L. Kelminson, S. S. George, N. W. Lerche, and J. E. Koehler. 2004. A family of variably expressed outer-membrane proteins (Vomp) mediates adhesion and autoaggregation in Bartonella quintana. Proc. Natl. Acad. Sci. USA 101:13630-13635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zimmermann, R., V. Kempf, E. Schiltz, K. Oberle, and A. Sander. 2003. Hemin binding, functional expression, and complementation analysis of Pap 31 from Bartonella henselae. J. Bacteriol. 185:1739-1744. [DOI] [PMC free article] [PubMed] [Google Scholar]