Abstract
Reactivation tuberculosis (TB) is a serious problem in immunocompromised individuals, especially those with human immunodeficiency virus (HIV) coinfection. The adaptive immune response mediated by CD4+ and CD8+ T cells is known to confer protection against TB. Hence, vaccines against TB are designed to activate these two components of the immune system. Anti-TB DNA vaccines encoding the immunodominant proteins Ag85A, Ag85B, and PstS-3 from Mycobacterium tuberculosis are ineffective in mice lacking CD4+ T cells (CD4−/− mice). In this study, we demonstrate that reconstitution of the T-cell compartment in CD4−/− mice restores vaccine-specific antibody and gamma interferon (IFN-γ) responses to these DNA vaccines. The magnitude of the immune responses correlated with the extent of reconstitution of the CD4+-T-cell compartment. Reconstituted mice vaccinated with DNA encoding PstS-3, known to encode a dominant Db-restricted CD8+-T-cell epitope, displayed CD8+-T-cell responses not observed in CD4−/− mice. M. tuberculosis challenge in reconstituted mice led to the extravasation of IFN-γ-producing CD4+ and CD8+ T cells into lungs, the primary site of bacterial replication. Importantly, a reconstitution of 12 to 15% of the CD4+-T-cell compartment resulted in Ag85B plasmid DNA-mediated protection against a challenge M. tuberculosis infection. Our findings provide evidence that anti-TB DNA vaccines could be effective in immunodeficient individuals after CD4+-T-lymphocyte reconstitution, as may occur following antiretroviral therapy in HIV+ patients.
Tuberculosis (TB), caused by Mycobacterium tuberculosis, a respiratory pathogen, remains a major global health problem, killing about 2 million individuals each year. Recent studies have indicated that a major proportion of the global TB burden comes from developing countries (13, 14), where the incidence of TB is accelerated by immunocompromise related to human immunodeficiency virus (HIV) infection and poverty (7, 32). The current anti-TB vaccine, BCG (attenuated Mycobacterium bovis), administered annually to over 3 million children worldwide, is effective in infants but fails to protect adults against pulmonary TB and has been reported to be detrimental in some immunodeficient individuals (18). Although effective anti-TB treatment exists, its high cost and long duration makes it virtually inaccessible to patients living in developing countries.
M. tuberculosis is an intracellular bacterium residing primarily in lung macrophages. Cell-mediated responses are known to be involved in the control of this infection. Activation of both CD4+ and CD8+ T cells is seen in primo-infected individuals (19) and in mice after an experimental infection (23). CD8+ and CD4+ T cells are thought to control infection at different stages and sites of infection by their capacity to produce gamma interferon (IFN-γ) in response to infected macrophages presenting mycobacterial antigens (6, 24, 27). IFN-γ in turn activates macrophages to kill the resident bacteria via the induction of reactive nitrogen and oxygen intermediates (5) and by promoting phagolysosome fusion (25).
One of the new approaches that is being studied to develop more effective vaccines against tuberculosis is the use of subunit genetic vaccines, namely, plasmid DNA (pDNA) encoding an immunodominant antigen(s) from M. tuberculosis. In this approach, the immunogenic antigen of interest is made by cells of the vaccinated host, thus bypassing tedious procedures of protein purification. These pDNA vaccines are relatively easy to produce, nonreplicating, and, so far, without side effects. Of the numerous immunodominant proteins of M. tuberculosis, members of the Ag85 complex are well characterized (4, 8). These are a 30- to 32-kDa family of three proteins (Ag85A, Ag85B, and Ag85C), all of which possess a mycolyl transferase activity, required for the formation of the bacterial cell wall. Ag85A induces strong T-cell proliferation and IFN-γ production in most healthy individuals infected with M. tuberculosis or Mycobacterium leprae and in M. bovis BCG-vaccinated mice but not in TB or lepromatous leprosy patients (19).
We have previously reported that pDNA vaccines encoding immunogenic proteins from M. tuberculosis, namely, members of the Ag85 complex and a phosphate transport receptor, PstS-3, when used to immunize mice, elicit CD4+- and CD8+-T-cell responses and protect mice against an experimental challenge infection (21, 34). In this study, we aimed to determine the efficacy of these pDNA vaccines under conditions of immunodeficiency. To address this question, we used mice lacking CD4+ T cells due to a targeted deletion of the CD4 gene (26). These mice were reconstituted with various numbers of syngeneic CD4+ T cells and vaccinated with pDNA coding for Ag85A, Ag85B, or PstS-3. Here, we report on the acquisition of vaccine-mediated immune responses and protection in CD4−/− mice following partial reconstitution of their CD4+-T-cell compartment.
MATERIALS AND METHODS
Mice.
C57BL/6 (B6) CD4 knockout (CD4−/−) (26) mice were purchased from Jackson Laboratories (Bar Harbor, ME) and bred as homozygotes in the animal facilities of the Pasteur Institute of Brussels, Belgium. These mice have been previously shown to develop normally (28). C57BL/6 wild-type (WT) mice were either purchased from Bantin and Kingman (Hull, Great Britain) or bred in our animal facilities. In most experiments female mice, 6 to 8 weeks old, were used.
Adoptive transfer of CD4+ T cells.
Mononuclear cells were isolated from cervical, brachial, and inguinal lymph nodes of naïve mice, and CD4+ T cells were purified by negative selection. Briefly, macrophages were first depleted from the lymph node cell suspension by adhesion to plastic and then the remaining cells were labeled with rat antibodies (BD Biosciences, Belgium) against macrophages (CD11b, clone M1/70), B cells (anti-κ chain), and CD8+ T cells (clone 53-6.7) for 20 min at 4°C. This cell mixture was washed and then incubated with sheep anti-rat antibody-coated magnetic beads (Dynal, France). Cells labeled with the magnetic beads were separated with a magnet (MPC; Dynal), leaving behind a cell suspension enriched in CD4+ T cells (>90%), as determined by flow cytometry. CD4−/− mice were adoptively transferred (AT) with 2.5 × 106, 5 × 106, or 20 × 106 syngeneic CD4+ T cells intravenously and received the DNA vaccine 1 to 2 days later.
DNA vaccines and antigens.
The details of the preparation of Ag85A, Ag85B, and PstS-3 DNA, encoded by the V1Jns.tPA plasmid, have been described previously (34). In brief, the V1Jns.tPA.Ag85A/B or PstS-3 pDNA was transformed in competent Escherichia coli DH5α cells. The recombinant plasmid was amplified in E. coli strain DH5α and purified on two cesium chloride-ethidium bromide gradients followed by 1-butanol and phenol-chloroform extractions and ethanol precipitation. Plasmid DNA concentrations were measured on a spectrophotometer, and aliquots of 1 mg/ml of DNA in saline were kept at −20°C until use. Mice were vaccinated intramuscularly (i.m.) three times, at 3-week intervals, with 100 μg Ag85A, Ag85B, or PstS-3 DNA/mouse, 50 μg in each tibialis muscle. M. bovis BCG (strain GL2) was administered intravenously, 0.5 mg (approximately 2 × 106 CFU)/mouse, 8 to 10 weeks before challenge infection.
Native Ag85 protein, purified from M. bovis BCG culture filtrate (8), was used for antibody detection by enzyme-linked immunosorbent assay (ELISA) and for in vitro restimulation of splenocytes from mice vaccinated with pDNA encoding the respective components. CD4+-T-cell responses after reconstitution of CD4−/− mice were analyzed with an I-Ab-restricted peptide from Ag85B (amino acids [aa] 240 to 260) in mice vaccinated with Ag85B pDNA (9). In mice immunized with pDNA encoding the PstS-3 protein, an I-Ab-restricted peptide (aa 191 to 210) and a Db-binding peptide (aa 285 to 293; SGVGNDLVL) were used to detect CD4+- and CD8+-T-cell activity, respectively, as described previously (29), and recombinant PstS-3 protein was used to detect serum antibodies.
Analysis of Ag85- and PstS-3-specific serum antibodies.
Blood samples were taken from mice 3 weeks after the third DNA vaccination, and sera were analyzed in a standard ELISA using native Ag85 protein (3 μg/ml) or recombinant PstS-3 (4 μg/ml) as a coating antigen to detect specific antibodies. Serum titer was converted to antibody concentration for anti-Ag85 antibodies by comparison with a purified monoclonal antibody standard (TD-17) (20). Anti-PstS-3 antibody endpoint titers in the sera of vaccinated and control mice were determined.
ELISPOT assay for the detection of IFN-γ production by splenocytes.
Enzyme-linked immunospot (ELISPOT) plates from Millipore (MAHA S4510; Millipore, Billerica, MA), 96-well format with a nitrocellulose bottom, were incubated overnight at 4°C with 50 μl of purified anti-mouse IFN-γ (15 μg/ml; clone R4-6A2; BD Biosciences) in phosphate-buffered saline (PBS); the next day, empty sites were blocked with RPMI medium containing 10% fetal calf serum. Splenic lymphocytes were added to wells at a known concentration in the presence or absence of peptides (10 μg/106 cells), and plates were incubated for 20 to 24 h in a humidified 37°C incubator with 5% CO2. Plates were then washed with PBS-0.05% Tween 20 and PBS to get rid of cells, incubated overnight at 4°C with 50 μl of biotinylated rat anti-mouse IFN-γ (2 μg/ml, clone XMG 1.2; BD Biosciences), washed as before, and incubated for 45 min at 37°C in 5% CO2 with 0.76 U/ml alkaline phosphate-labeled Extravidin (Sigma-Aldrich). After a wash, spots were revealed with a Bio-Rad (Hercules, CA) alkaline phosphatase conjugate substrate kit, according to the manufacturer's instructions, and plates were analyzed on a Bioreader 3000 LC (BioSys, Germany). Data are reported as spot-forming cells per million splenocytes.
Labeling of cells for flow cytometric analysis.
To check for the presence of transferred syngeneic CD4+ T cells in the CD4−/− hosts, blood samples were obtained from tail bleeds of mice at different time points after transfer and vaccination. Blood lymphocytes were labeled with a fluorescein isothiocyanate (FITC)- conjugated anti-CD4 antibody (clone RM4-4; Pharmingen) (0.5 μg/106 cells) for 20 min on ice and washed twice with PBS-2% fetal calf serum by centrifugation at 1,800 rpm. Erythrocytes were then lysed with FACS Lysing Solution (Pharmingen). Thirty thousand cells in the lymphocyte gate were acquired on a cytofluorometer (FACSCalibur; BD, Mountain View, CA).
For the analysis of intracellular IFN-γ, 2.5 × 106 cells/ml were cultured in 48-well tissue culture plates (Nunclon, Roskilde, Denmark) in the presence of 5 μg of Ag85 protein/ml for 18 to 20 h. Brefeldin A (Sigma, St. Louis, MO) was added to the cultures for the last 5 h to prevent secretion of the intracellular cytokine. One million cells were labeled with the FITC-conjugated anti-CD4 antibody (clone RM4-4; BD Biosciences) for 30 min at 4°C. Cells were then washed, fixed with 4% paraformaldehyde, and permeabilized with PBS containing 0.1% saponin. To label intracellular IFN-γ, cells were incubated with phycoerythrin-conjugated anti-IFN-γ antibody (clone XMG 1.2; BD Biosciences) in the presence of saponin for 30 min at 4°C, washed, and acquired on a cytofluorometer. Lymphocytes were gated by their forward and side light-scattering properties, and 100,000 cells were acquired. Analysis was done with the Cell Quest program.
Assessment of in vivo PstS-3-specific CTL activity.
The method used for assessment of in vivo PstS-3-specific cytotoxic T-lymphocyte (CTL) activity is that described by Aichele et al. (1). Briefly, syngeneic splenocytes used as targets were incubated for 1 h at 37°C-5% CO2 either alone or with 10 μg/ml of a Db-restricted peptide, SGVGNDLVL (aa 285 to 293), of the PstS-3 protein from M. tuberculosis. Subsequently, cells were labeled for 10 min at 37°C in the dark with CFSE (succinimydil ester of carboxyfluorescein diacetate) (Molecular Probes Europe, The Netherlands) either at 1 μM (unpulsed cells; CFSELOW) or 10 μM (peptide-pulsed cells; CFSEHIGH). Twenty million cells at a 1:1 mix of CFSELOW/CFSEHIGH were injected intravenously into PstS-3 DNA-vaccinated WT, CD4−/−, and reconstituted CD4−/− mice. Mice were sacrificed 18 h later, and splenocytes were acquired on a FACSCalibur cytofluorometer. To evaluate the percentage of specific lysis, the ratio of CFSEHIGH/CFSELOW in vaccinated mice was compared to the ratio obtained in naïve or control DNA-vaccinated mice. At least three mice were tested for each experimental group.
Challenge experiments.
Mice were rested for 6 to 8 weeks after vaccination and then challenged intravenously or intratracheally with luminescent M. tuberculosis, a construct of M. tuberculosis H37Rv expressing bacterial luciferase (33). For the intravenous challenge, each mouse received 100 relative light units (RLU), corresponding to approximately 1 × 105 to 2 × 105 CFU. For the intratracheal infection, each mouse received 15 RLU (1 × 104 to 2 × 104 CFU). Mice were sacrificed 4 or 8 weeks later, spleen and lungs were aseptically removed and homogenized in PBS, and bacterial counts in organs were determined on a luminometer (Turner Design; Promega, The Netherlands), as previously described (35).
Statistical analysis.
The Student t test was used to calculate the statistical significances between data obtained from vaccinated versus nonvaccinated mice and data obtained from reconstituted versus nonreconstituted CD4−/− mice.
RESULTS
Presence of transferred CD4+ T cells in blood from vaccinated mice.
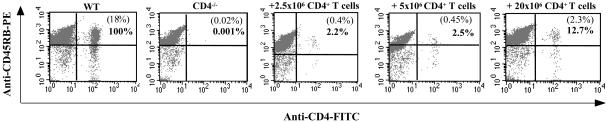
Blood samples were obtained from mice at different time points after transfer of CD4+ T cells and vaccination. Blood lymphocytes were labeled for CD4+ T cells and analyzed on a flow cytometer. Figure 1 shows the presence of CD4+ T cells in blood 2 weeks after three rounds of vaccination (8 weeks after transfer). The transfer of 2.5 ×106 CD4+ T cells reconstituted the CD4+-T-cell compartment to about 2% of that seen in WT mice. The transfer of 5 × 106 CD4+ T cells showed a similar reconstitution (2.5% of WT). However the transfer of 20 × 106 CD4+ T cells restored approximately 13% of this compartment compared to WT mice. An analysis of the presence of CD4+ T cells in the spleen and lymph nodes after transfer of CD4+ T cells showed a percentage of reconstitution similar to that seen in blood (data not shown). A follow-up of mice up to 8 weeks after vaccination (14 weeks after transfer) showed no change in the numbers of CD4+ T cells present in blood and lymphoid organs, indicating that the transferred T cells were persistent and circulating in the host.
FIG. 1.
Detection of CD4+ T cells in blood lymphocytes after adoptive transfer into CD4−/−mice. Purified syngeneic CD4+ T cells (2.5 × 106, 5 × 106, or 20 × 106) were injected intravenously in the tail vein of CD4−/− mice. Beginning 1 to 2 days later, WT, CD4+-T-cell-reconstituted CD4−/−, and nonreconstituted CD4−/− mice received three immunizations of DNA (100 μg of Ag85A or Ag85B DNA/mouse, 50 μg in each tibialis muscle) at 3-week intervals. Blood samples were obtained from mice 2 weeks after the last DNA vaccination, lymphocytes were dually labeled with FITC-conjugated anti-CD4 antibody and phycoerythrin (PE)-conjugated anti-CD45RB antibody, and the numbers of CD4+ T cells present in blood were quantified. The values in parentheses are percentages of CD4+ T cells in blood lymphocytes, and the percentages in bold represent reconstitution compared to WT mice (100%). Data are from one representative experiment of three performed.
Vaccine-specific antibody and cytokine responses in reconstituted mice.
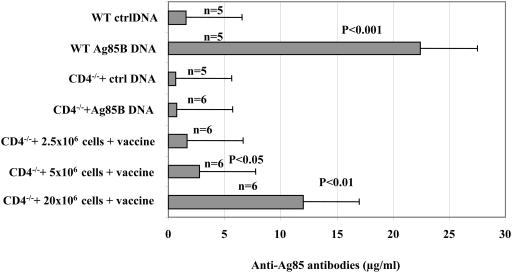
Sera from reconstituted CD4−/− mice vaccinated with Ag85B pDNA were tested for the presence of Ag85-specific antibodies. Figure 2 illustrates that following vaccination, CD4−/− mice did not produce any specific antibodies, compared to WT mice. An antibody response, albeit low, was observed in CD4−/− mice transferred with 2.5 × 106 CD4+ T cells. A higher response was displayed by mice that received a transfer of 5 × 106 CD4+ T cells, which was significant (P < 0.05) compared to vaccinated CD4−/− mice. The transfer of 20 × 106 CD4+ T cells, finally, induced a significantly higher antibody response (P < 0.01). The magnitude of this response was approximately 50% of that seen in WT mice, although the CD4+-T-cell compartment was only 10 to 15% reconstituted. The findings were similar in reconstituted CD4−/− mice vaccinated with the Ag85A pDNA (data not shown).
FIG. 2.
Presence of anti-Ag85-specific antibodies in the sera of CD4+-T-cell-reconstituted CD4−/− mice following vaccination. WT and CD4−/− mice were vaccinated either with Ag85B DNA or control (ctrl) DNA three times at 3-week intervals. In parallel, CD4−/− mice were given an AT with 2.5 × 106, 5 × 106, or 20 × 106 CD4+ T cells and vaccinated with Ag85B DNA. Blood samples were obtained from mice 3 weeks after the third vaccination dose, and sera were tested for the presence of Ag85-specific antibodies by ELISA. Optical density values were converted to concentrations by using a monoclonal antibody (TD-17) of a known concentration as a standard. Five or six mice were tested in each group. Results are shown as means ± standard deviations. Following reconstitution with 5 × 106 and 20 × 106 CD4+ T cells, significant levels (P, <0.05 and <0.01, respectively) of vaccine-specific serum antibodies were detected compared to vaccinated CD4−/− mice.
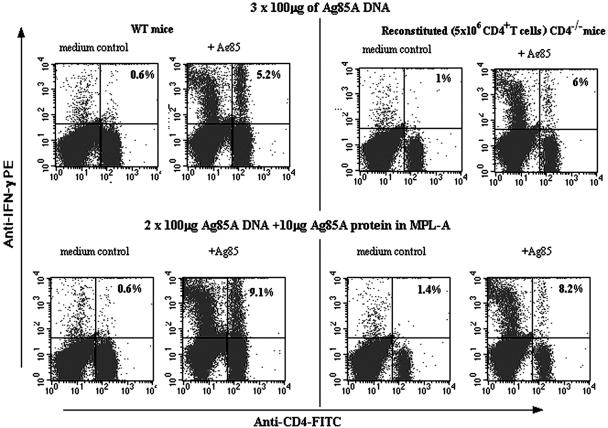
The capacity of these reconstituted mice to produce IFN-γ in response to recall antigen was also studied. After three rounds of vaccination, 6% of splenic CD4+ T cells from mice reconstituted with 5 × 106 CD4+ T cells produced IFN-γ when stimulated in vitro with Ag85 protein. This result was similar to that seen with WT mice. A vaccination protocol constituted of two doses of Ag85A DNA followed by a dose of Ag85A protein in MPL-A adjuvant increased the percentage of IFN-γ-producing CD4+ T cells in reconstituted CD4−/− mice, as in WT mice (Fig. 3).
FIG. 3.
CD4+-T-cell-reconstituted CD4−/− mice are primed by the vaccine to produce cytokine responses. WT and reconstituted (5 × 106 CD4+ T cells) CD4−/− mice were either vaccinated with three doses of 100 μg of Ag85A DNA i.m. or received two doses of 100 μg of Ag85A DNA followed by a third immunization with 10 μg of Ag85A native protein in MPL-A adjuvant. Three weeks later, mice were sacrificed and isolated spleen cells were cultured for 24 h in the presence of medium or Ag85A protein (5 μg/ml). Splenic T cells were thereafter labeled for surface CD4 molecules and intracellular IFN-γ, respectively. Percentages of IFN-γ-producing cells within the CD4+-T-cell population are indicated. PE, phycoerythrin.
Reconstitution of the CD4+-T-cell compartment in CD4−/− mice restores CD8+-T-cell mediated in vivo CTL activity and IFN-γ production to a PstS-3 protein-derived Db peptide.
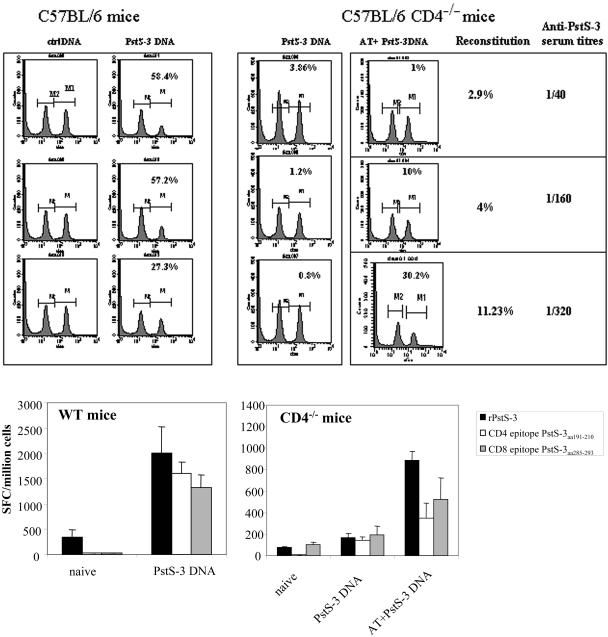
In order to obtain a complete picture of the restoration of immune functions in reconstituted CD4−/− mice, we also studied CD8+-T-cell responses. We have previously demonstrated that B6 (H-2b) mice are not able to generate Db or Kb epitopes from Ag85A and Ag85B proteins (11). Therefore, we investigated the capacity of CD4−/− mice before and after reconstitution to generate CD8+-T-cell responses following vaccination with PstS-3 pDNA, known to encode a dominant Db-restricted CD8+-T-cell epitope (aa 285 to 293) (29). PstS-3 pDNA-vaccinated WT B6 mice showed on average 45% specific in vivo lysis of peptide-pulsed targets and a serum antibody titer of 1/3,560. In contrast, PstS-3 DNA-vaccinated CD4−/− mice displayed negligible CTL activity. The recovery of CTL activity observed following adoptive transfer of CD4+ T cells into CD4−/− recipients correlated with the percentage of reconstitution of the CD4+-T-cell compartment. Hence, with less than 5% reconstitution of the CD4+-T-cell compartment, 10% in vivo CTL activity and a serum antibody titer of 1/160 was restored. On the other hand, in vivo CTL activity to the PstS-3 CD8+-T-cell epitope was significantly increased, to 30.2%, with 11% reconstitution; in parallel, an antibody titer of 1/320 was observed in these mice (Fig. 4, top).
FIG. 4.
Analysis of in vivo CTL activity, antibody production, and in vitro IFN-γ production by CD8+ T cells in WT, CD4−/−, and reconstituted CD4−/− mice vaccinated with PstS-3 pDNA. (Top) CD4−/− mice, CD4−/− mice adoptively transferred with 2.5 × 106, 5 × 106, or 20 × 106 CD4+ T cells, and WT mice were vaccinated with 100 μg of PstS-3 DNA three times at 3-week intervals. One week after the third immunization, mice were analyzed for in vivo CTL activity. Syngeneic splenic T cells (20 × 106/mouse) were used as targets; of these, half were pulsed with the PstS-3 peptide (aa 285 to 293, SGVGNDLVL, 10 μg/ml) and labeled with CFSEHIGH and the other half were labeled with CFSELOW, as described in Materials and Methods, and a 1:1 mix was injected into the vaccinated hosts. After 20 h, mice were sacrificed, spleens were removed and made into single-cell suspensions, and cell counts were acquired on a cytofluorometer. A decrease in the peak of cells corresponding to peptide-pulsed and CFSEHIGH-labeled cells is indicative of specific in vivo CTL activity. Percentages represent CTL activity in each mouse compared to control DNA-vaccinated mice. In vivo CTL activity and anti-PstS-3 antibody titers in sera correlated with reconstitution of the CD4-T-cell compartment, as illustrated. (Bottom) Splenic cells from WT and CD4−/− mice before and after vaccination with PstS-3 pDNA, and after AT (20 × 106 CD4+ T cells) and vaccination in the case of CD4−/− mice, were tested for the capacity to produce IFN-γ in response to recombinant PstS-3 protein and its CD4+- and CD8+-T-cell peptides, aa 191 to 210 and aa 285 to 293, respectively. Data are from one representative experiment of three performed. SFC, spot-forming cells.
Likewise, the recovery of PstS-3 DNA vaccine-induced IFN-γ production by CD8+ T cells was also observed in CD4−/− mice following an adoptive transfer of 20 × 106 CD4+ T cells. Splenic cells from these mice, unlike those from nonreconstituted mice, produced IFN-γ when stimulated by the PstS-3-restricted Db peptide (aa 285 to 293) (Fig. 4, bottom). In parallel, the adoptively transferred CD4+ T cells could also be induced to produce IFN-γ in response to the I-Ab-restricted peptide from PstS-3 (aa 191 to 210) and to recombinant PstS-3 protein. These responses were two- to threefold less than those seen in WT counterparts.
Presence of IFN-γ-producing CD4+ and CD8+ T cells in lungs of reconstituted mice following vaccination and challenge.
Eight weeks after the last vaccination dose, WT mice and reconstituted CD4−/− mice were challenged with M. tuberculosis. Mice were sacrificed 4 weeks later, spleen and lungs were removed and homogenized, and mononuclear cells were isolated. These cells were stimulated in vitro with Ag85A protein for 24 h, after which they were labeled for surface CD4 expression on T cells and for IFN-γ produced within them; subsequently, the percentage of CD4+ T cells producing IFN-γ was evaluated by flow cytometry. CD4+ T cells were observed in lungs of reconstituted CD4−/− mice following M. tuberculosis infection. In vitro culturing of these lung-derived cells with Ag85A protein demonstrated that the adoptively transferred CD4+ T cells could be elicited to produce IFN-γ; however, the percentage of positive cells was lower than in spleens (Fig. 5A).
FIG. 5.
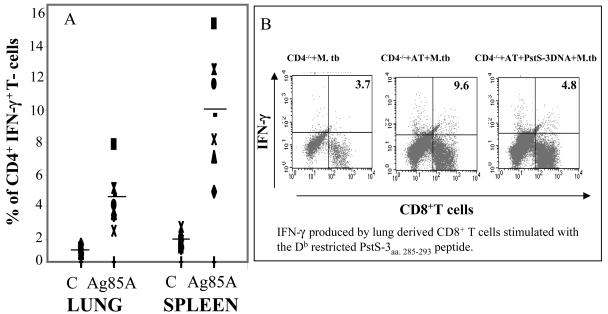
Induction of IFN-γ production by CD4+ T cells in lungs and spleens of reconstituted CD4−/− mice after vaccination and intravenous M. tuberculosis challenge. (A) Reconstituted (20 × 106 CD4+ T cells) CD4−/− mice and WT mice that received three doses of Ag85A DNA, as described previously, were rested for 8 weeks and then administered an M. tuberculosis challenge (5 log10 mRLU/mouse, intravenously). Four weeks later, mice were sacrificed and lungs and spleens were homogenized to obtain single-cell suspensions. Homogenized lung tissue was passed on a nylon-wool column to get rid of cell debris, and 2.5 × 106 cells were cultured overnight in the presence of Ag85A protein (5 μg/ml). Lung- and spleen-derived T cells were then doubly labeled for surface CD4 molecules and intracellular IFN-γ, respectively. (B) Four weeks after intratracheal infection, cells were isolated from lungs of infected CD4−/− mice that had either received the PstS-3 DNA vaccine, an AT (20 × 106 CD4+ T cells) followed by control DNA, or PstS-3 DNA. Lung-derived cells from four mice/group were pooled and stimulated in vitro for 48 h in the presence of the Db peptide from PstS-3 (aa 285 to 293, SGVGNDLVL, 10 μg/ml). Intracellular IFN-γ produced by CD8+ T cells was analyzed by flow cytometry. The numbers in the right-hand quadrants indicate the percentages of CD8+ T cells producing IFN-γ.
Mononuclear cells isolated from lungs of nonreconstituted, infected CD4−/− mice contained CD8+ T cells. However, they could not be stimulated by the immunodominant PstS-3 CD8+-T-cell peptide (aa 285 to 293) to produce IFN-γ except in mice reconstituted with CD4+ T cells. In reconstituted CD4−/− mice, following Psts-3 DNA vaccination and intratracheal challenge, fewer IFN-γ-producing CD8+ T cells specific to peptide aa 285 to 293 were observed (Fig. 5B).
A DNA vaccine encoding Ag85B partially protects CD4+-T-cell-reconstituted CD4−/− mice against an M. tuberculosis challenge infection.
Mice were sacrificed 4 or 8 weeks post-intravenous infection, and viable bacterial counts in spleen and lungs were determined. We observed that 4 weeks postinfection, M. tuberculosis replication was already controlled in spleens of naïve B6 WT mice and that bacterial counts were therefore similar to those seen in vaccinated mice (Table 1). However, in lungs, the bacterial load in Ag85B DNA-vaccinated WT mice was significantly lower than the load in the lungs of naïve mice (Δ = 0.6 log10 protection; P < 0.03). In contrast, vaccinated CD4−/− mice were incapable of controlling bacterial infection in either lungs or spleen, and they harbored bacterial loads similar to naïve CD4−/− mice. However, CD4−/− mice adoptively transferred with 20 × 106 CD4+ T cells displayed vaccine-mediated control of infection in spleens (P < 0.05), whereas mice that received 5 × 106 CD4+ T cells were not protected (Table 1). The protection seen in spleens of reconstituted mice was sustained over time. Partial protection was apparent at the level of lungs in reconstituted CD4−/− mice at 8 weeks postinfection.
TABLE 1.
Protection against a challenge M. tuberculosis infection in CD4−/− mice reconstituted with CD4+ T cells and vaccinated with Ag85B DNAa
| Mouse treatment parameter | Log10 mRLU (SD)b
|
|||||
|---|---|---|---|---|---|---|
| Spleen
|
Lungs
|
|||||
| WT (4 wk) | CD4−/− (4 wk) | CD4−/− (8 wk) | WT (4 wk) | CD4−/− (4 wk) | CD4−/− (8 wk) | |
| Naive (n = 4) | 3.57 (0.13) | 4.72 (0.29) | 4.74 (0.47) | 4.80 (0.17) | 5.08 (0.40) | 5.45 (0.07) |
| Ag85B DNA (n = 4) | 3.42 (0.03) | 4.96 (0.39) | 5.33 (0.14) | 4.20 (0.34)* | 4.75 (0.40) | 5.25 (0.30) |
| BCG (n = 4) | 3.43 (0.09) | 3.73 (0.26)** | ND | 3.71 (0.13)** | 3.80 (0.30)** | ND |
| AT (20 × 106 CD4+ T cells) (n = 3) | 4.37 (0.14) | 4.49 (0.14) | 5.24 (0.16) | 5.40 (0.10) | ||
| AT (20 × 106 CD4+ T cells) + control DNA (n = 4) | ND | 4.58 (0.3) | ND | 5.63 (0.22) | ||
| AT (5 × 106 CD4+ T cells) + Ag85B DNA (n = 4) | 4.57 (0.15) | ND | 5.17 (0.33) | ND | ||
| AT (20 × 106 CD4+ T cells) + Ag85B DNAc | 4.28 (0.38)* | 4.1 (0.24)** | 5.22 (0.29) | 5.05 (0.50) | ||
Eight weeks after the third dose of pDNA vaccination and 8 weeks after BCG vaccination, mice received a luminescent M. tuberculosis challenge (2 × 105 CFU = 5 log10 mRLU/mouse, intravenously). Four and eight weeks later, viable bacterial counts in spleen and lungs were determined by luminescence.
*, P < 0.05; **, P <0.01 (compared to Ag85B DNA-vaccinated CD4−/− mice for reconstituted CD4−/− mice and compared to naïve mice for the other groups); ND, not done.
n = 5 at 4 weeks; n = 4 at 8 weeks.
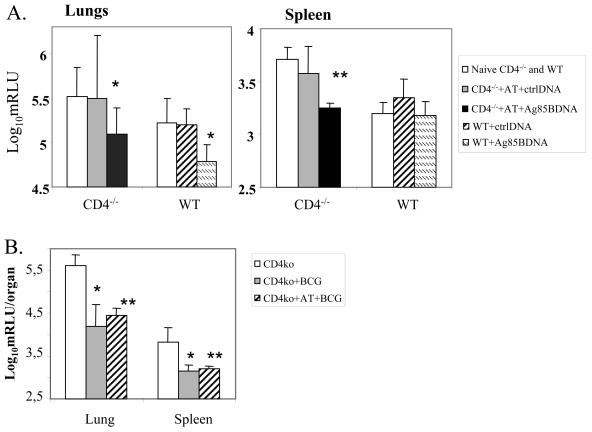
An intratracheal challenge infection with 104 CFU of luminescent M. tuberculosis resulted in enhanced protection in reconstituted CD4−/− mice (Fig. 6A). An adoptive transfer of 20 × 106 CD4+ T cells into CD4−/− mice followed by Ag85B pDNA led to protection 4 weeks after a challenge infection, as indicated by the significant decrease in bacterial load in both lungs (Δ = 0.43 log10 protection; P < 0.05) and in spleen (Δ = 0.46 log10 protection; P < 0.001) compared to nonreconstituted animals or reconstituted animals vaccinated with control pDNA. The decrease in bacterial load in lungs of reconstituted CD4−/− mice postvaccination was similar to that seen in vaccinated WT mice. A significant decrease in M. tuberculosis load was seen in lungs (Δ = 1.41 log10 protection) and spleen (Δ = 0.68 log10 protection) of BCG-vaccinated CD4−/− mice that received an intratracheal infection compared to nonvaccinated mice. CD4+-T-cell reconstitution of CD4−/− mice followed by BCG vaccination also showed a high degree of protection against a challenge infection (lungs, Δ = 1.14 log10 protection; spleen, Δ = 0.63 log10 protection) compared to nonvaccinated mice. However, there was no significant difference in protection induced by BCG vaccination before and after reconstitution of the CD4+-T-cell compartment in these mice (Fig. 6B).
FIG. 6.
Ag85B pDNA vaccine protects reconstituted CD4−/− mice against an intratracheal challenge infection. (A) All mice received four doses of Ag85B DNA (100 μg/dose, i.m.) at 3-week intervals. Six weeks later, adoptively transferred (20 × 106 CD4+ T cells) CD4−/− mice that received the control pDNA or Ag85B pDNA, as well as naïve CD4−/− mice, were given an intratracheal challenge with luminescent M. tuberculosis (104 CFU/mouse = 15 RLU) in parallel with WT vaccinated mice. (B) B6 CD4−/− (ko) mice before and after reconstitution with 20 × 106 CD4+ T cells were vaccinated with BCG (intravenously) and, 8 weeks later, infected intratracheally with luminescent M. tuberculosis, as described above. Four weeks later, mice were sacrificed and the load of bacilli in lungs and spleen was measured on a luminometer as RLU. *, P < 0.05 compared to naïve CD4−/− mice or naïve WT mice; **, P < 0.001 compared to naïve CD4−/− mice.
DISCUSSION
Reactivation TB contributes significantly to the global burden of TB both in Western and in Eastern societies due to immunodeficiencies mainly related to HIV infection. Therefore, M. tuberculosis infection and fatal TB disease are very frequently associated with a drop in CD4+-T-cell counts. It is estimated that more than one-third of HIV-positive individuals are coinfected with M. tuberculosis and approximately 12% of AIDS deaths are due to TB (7, 13, 14). This illustrates the urgent need to develop an effective anti-TB vaccine for this large group of individuals at risk.
In mice, DNA vaccination encoding single immunogenic proteins of M. tuberculosis is very effective in activating the immune system and conferring protection against a challenge infection (21, 22). The activation of CD8+ T cells was initially thought to be the hallmark of DNA vaccines; however, several reports have also demonstrated CD4+-T-cell responses following pDNA vaccination (12, 34, 36). There is evidence that following i.m. vaccination with pDNA, the encoded protein is produced by the transfected myocytes, but the in vivo presentation of protein-derived peptides to T cells appears to require professional antigen-presenting cells (APCs) (15). Thus, the uptake of pDNA-encoded protein, either free or in apoptotic vesicles, by professional APCs via the exogenous route is thought to be the major pathway involved in the priming of CD4+ and CD8+ T cells after DNA vaccination.
It was previously shown that the Ag85A and Ag85B DNA vaccines stimulate CD8+ T cells to mediate cytolytic activity and produce IFN-γ in BALB/c mice (9) and in HLA-A2 transgenic mice (16); however, CD8+-T-cell epitopes could not be processed from these antigens in B6 (H-2b) mice (11). Nevertheless, the Ag85A DNA vaccine can protect B6 mice against a challenge infection. Furthermore, the vaccine efficacy was comparable in B6 β2-microglobulin knockout mice that lack CD8+ T cells and in their WT counterparts, suggesting that the vaccine efficacy could be mediated primarily by CD4+ T cells. Confirming this hypothesis, we demonstrated that the vaccine was ineffective in B6 mice lacking CD4+ T cells (11).
The very encouraging efficacy of anti-TB DNA vaccines in mice led us to test these vaccines in a murine model of immunocompromise. To achieve this, we reconstituted the CD4+-T-cell compartment in CD4−/− mice to different extents, in an attempt to mimic the immune system of immunocompromised individuals. Interestingly, vaccine-specific antibody responses were readily seen and restored to more than 50% with only 12 to 15% reconstitution of the CD4+-T-cell compartment. This may suggest that maximum CD4+-T-cell help for antibody production can be obtained with <100% reconstitution of this compartment. Vaccine-specific cytokine responses were seen in mice with 2.5% (and above) CD4+-T-cell reconstitution, compared to their WT counterparts. The percentages of Ag85-specific splenic CD4+ T cells induced to produce IFN-γ were similar in WT and reconstituted CD4−/− mice, probably indicating that the transferred T cells represent a complete T-cell repertoire.
There is no doubt that activation of both CD8+ and CD4+ T cells would be required for the optimal efficacy of a DNA vaccine. Therefore, it was important to investigate the capacity of anti-TB DNA vaccines to activate CD8+ T cells in our murine model of immunocompromise, especially since these cells are not affected by HIV and could be the main effectors in these individuals. However, CD8+ T cells from PstS-3 DNA-vaccinated CD4−/− mice did not display IFN-γ production or CTL activity when stimulated with the dominant PstS-3-derived CD8+-T-cell epitope. Recovery of the CD8+-T-cell responses was clearly dependent on CD4+ T cells and hence was observed only after reconstitution. In this context, it has been demonstrated that CD4+ T cells are required for the generation of functional CD8+-T-cell memory (31). Protective immunity against TB has also been shown to be dependent on CD4+ T cells for effective CD8+-T-cytotoxic function in the lungs of M. tuberculosis-infected mice (30). To our knowledge, this is the first study demonstrating the requirement of CD4+ T cells in inducing CD8+-T-cell responses to anti-TB DNA vaccines. It is probable that the generation of CD8+-T-cell responses to DNA vaccines is mediated through “cross-priming” by host APCs, which has been previously shown to be dependent on cognate CD4+-T-cell help (3).
Most importantly, in this reconstitution model the DNA vaccine encoding Ag85B from M. tuberculosis conferred protection against a challenge infection. Mice that received the challenge infection via the intratracheal route were better protected than those that were infected intravenously. This is most probably due to the presence of M. tuberculosis mainly in lungs after intratracheal infection, resulting in the extravasation of IFN-γ-producing CD4+ and CD8+ T cells to this site. The lower numbers of IFN-γ-producing CD8+ T cells detected in lungs post-PstS-3 vaccination and infection, compared to reconstituted mice postinfection, could be due to the earlier activation and loss of vaccine-induced effectors at this primary site of infection, whereas after intravenous infection of partially reconstituted mice, the bacilli accumulated in the spleens and therefore may have attracted the IFN-γ-producing immune cells to this site rather than to the lungs, at least at the early time points postinfection. It should be noted, however, that in contrast to our findings, a recent study has demonstrated anti-TB protective responses induced by vaccination with Sindbis virus replicon-based Ag85B and Ag85B DNAs in CD4−/− mice (10). These disparate findings could be related to the differences in the immunization and challenge infection schedule, the mode of challenge, and the M. tuberculosis strain used (Erdman versus H37Rv). Importantly, although the above study suggested that CD8+ T cells may have mediated protective immunity, direct evidence for the activation of CD8+ T cells was not provided. It is noteworthy that the BCG vaccine was protective in CD4−/− mice against intravenous and intratracheal challenges. However, the protective efficacy of the BCG vaccine in these mice was not augmented by the adoptive transfer of CD4+ T cells. While BCG-vaccinated CD4−/− mice did not lose weight, neither did activation of CD8+ T cells occur (data not shown), suggesting that the protective effects of BCG may be related to its potential to activate innate immune responses, as demonstrated in other studies (17).
In humans, a 65 to 75% drop in CD4+-T-cell counts, for various reasons, in particular HIV infection, is often associated with M. tuberculosis reactivation. The BCG vaccine is not recommended for immunocompromised individuals, since it has shown deleterious effects in some cases. In this regard, DNA vaccines that are nonintegrating and nonreplicating may be safe for these patients. Here, we demonstrate for the first time that anti-TB DNA vaccines are functional and protective in mice reconstituted to 12 to 15% of their CD4+-T-cell compartment compared to their WT counterparts. CD4+-T-cell counts are known to increase under effective, highly active antiretroviral therapy in HIV-infected patients (2). Collectively, our findings provide evidence to support the possibility that anti-TB DNA vaccines may be effective for these HIV+ patients.
Acknowledgments
This work was partially funded by a European Community FP5 Tuberculosis Vaccine-Cluster grant (QLK2-CT-1999-01093) to Kris Huygen. We thank also the Damiaan Foundation, the Brussels Capital Region, and FWO-Vlaanderen (G.0376.05) for financial support.
We are grateful for the technical assistance of Nathalie Castiglioni, Rachid Laali, Marie-Thérèse Denis, and her team at the Pasteur Institute animal facility.
Editor: J. B. Bliska
REFERENCES
- 1.Aichele, P., K. Brduscha-Riem, S. Oehen, B. Odermatt, R. M. Zinkernagel, H. Hengartner, and H. Pircher. 1997. Peptide antigen treatment of naïve and virus-immune mice: antigen-specific tolerance versus immunopathology. Immunity 6:519-529. [DOI] [PubMed] [Google Scholar]
- 2.Autran, B. 1999. Effects of antiretroviral therapy on immune reconstitution. Antivir. Ther. 4(Suppl. 3):3-6. [PubMed] [Google Scholar]
- 3.Bennett, S. R., F. R. Carbone, F. Karamalis, J. F. Miller, and W. R. Heath. 1997. Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help. J. Exp. Med. 186:65-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borremans, M., L. de Wit, G. Volkaert, J. Ooms, J. de Bruyn, K. Huygen, J. P. Van Vooren, M. Stelandre, R. Verhofstadt, and J. Content. 1989. Cloning, sequence determination, and expression of a 32-kilodalton-protein gene of Mycobacterium tuberculosis. Infect. Immun. 57:3123-3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan, J., and J. Flynn. 2004. The immunological aspects of latency of tuberculosis. Clin. Immunol. 110:2-12. [DOI] [PubMed] [Google Scholar]
- 6.Cooper, A. M., D. K. Dalton, T. A. Stewart, J. P. Griffin, D. G. Russell, and I. M. Orme. 1993. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 178:2243-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corbett, E. L., C. J. Watt, N. Walker, D. Maher, B. G. Williams, M. C. Raviglione, and C. Dye. 2003. The global burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch. Intern. Med. 163:1009-1021. [DOI] [PubMed] [Google Scholar]
- 8.De Bruyn, J., K. Huygen, R. Bosmans, M. Fauville, R. Lippens, J. P. Van Vooren, P. Falmagne, M. Weckx, H. G. Wiker, M. Harboe, et al. 1987. Purification, characterization and identification of a 32 kDa protein antigen of Mycobacterium bovis BCG. Microb. Pathog. 2:351-366. [DOI] [PubMed] [Google Scholar]
- 9.Denis, O., A. Tanghe, K. Palfliet, F. Jurion, T. P. van den Berg, A. Vanonckelen, J. Ooms, E. Saman, J. B. Ulmer, J. Content, and K. Huygen. 1998. Vaccination with plasmid DNA encoding mycobacterial antigen 85A stimulates a CD4+ and CD8+ T-cell epitopic repertoire broader than that stimulated by Mycobacterium tuberculosis H37Rv infection. Infect. Immun. 66:1527-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Derrick, S. C., A. Li Yang, and S. L. Morris. 2005. Vaccination with a Sindbis virus-based DNA vaccine expressing antigen 85B induces protective immunity against Mycobacterium tuberculosis. Infect. Immun. 73:7727-7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Souza, S., O. Denis, T. Scorza, F. Nzabintwali, H. Verschueren, and K. Huygen. 2000. CD4+ T cells contain Mycobacterium tuberculosis infection in the absence of CD8+ T cells in mice vaccinated with DNA encoding Ag85A. Eur. J. Immunol. 30:2455-2459. [DOI] [PubMed] [Google Scholar]
- 12.D'Souza, S., V. Rosseels, M. Romano, O. Denis, F. Jurion, N. Castiglioni, A. Vanonckelen, K. Palfliet, and K. Huygen. 2003. Mapping of murine Th1 helper T-cell epitopes of mycolyl transferases Ag85A, Ag85B, and Ag85C from Mycobacterium tuberculosis. Infect. Immun. 71:483-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dye, C., S. Scheels, P. Dolin, V. Pathania, and M. C. Raviglione. 1999. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA 282:6776-6786. [DOI] [PubMed] [Google Scholar]
- 14.Dye, C., C. J. Watt, D. M. Bleed, and B. G. Williams. 2003. What is the limit to case detection under the DOTS strategy for tuberculosis control? Tuberculosis (Edinburgh) 83:35-43. [DOI] [PubMed] [Google Scholar]
- 15.Fu, T. M., J. B. Ulmer, M. J. Caulfield, R. R. Deck, A. Friedman, S. Wang, X. Liu, J. J. Donnelly, and M. A. Liu. 1997. Priming of cytotoxic T lymphocytes by DNA vaccines: requirement for professional antigen presenting cells and evidence for antigen transfer from myocytes. Mol. Med. 3:362-371. [PMC free article] [PubMed] [Google Scholar]
- 16.Geluk, A., K. E. van Meijgaarden, K. L. Franken, J. W. Drijfhout, S. D'Souza, A. Necker, K. Huygen, and T. H. Ottenhoff. 2000. Identification of major epitopes of Mycobacterium tuberculosis AG85B that are recognized by HLA-A*0201-restricted CD8+ T cells in HLA-transgenic mice and humans. J. Immunol. 165:6463-6471. [DOI] [PubMed] [Google Scholar]
- 17.Gillerons, M., V. F. J. Quesniaux, and G. Puzo. 2003. Acylation state of the phosphatidylinositol hexamannosides from Mycobacterium bovis bacillus Calmette Guérin and Mycobacterium tuberculosis H37Rv and its implication in Toll-like receptor response. J. Biol. Chem. 278:29880-29889. [DOI] [PubMed] [Google Scholar]
- 18.Hesseling, A. C., H. S. Schaaf, W. A. Hanekom, N. Beyers, M. F. Cotton, R. P. Gie, B. J. Marais, P. van Helden, and R. M. Warren. 2003. Danish bacille Calmette-Guerin vaccine-induced disease in human immunodeficiency virus-infected children. Clin. Infect. Dis. 37:1226-1233. [DOI] [PubMed] [Google Scholar]
- 19.Huygen, K., J. P. Van Vooren, M. Turneer, R. Bosmans, P. Dierckx, and J. De Bruyn. 1988. Specific lymphoproliferation, gamma interferon production, and serum immunoglobulin G directed against a purified 32 kDa mycobacterial protein antigen (P32) in patients with active tuberculosis. Scand. J. Immunol. 27:187-194. [DOI] [PubMed] [Google Scholar]
- 20.Huygen, K., D. Abramowicz, P. Vandenbussche, F. Jacobs, J. De Bruyn, A. Kentos, A. Drowart, J. P. Van Vooren, and M. Goldman. 1992. Spleen cell cytokine secretion in Mycobacterium bovis BCG-infected mice. Infect. Immun. 60:2880-2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huygen, K., J. Content, O. Denis, D. L. Montgomery, A. M. Yawman, R. R. Deck, C. M. DeWitt, I. M. Orme, S. Baldwin, C. D'Souza, A. Drowart, E. Lozes, P. Vandenbussche, J. P. Van Vooren, M. A. Liu, and J. B. Ulmer. 1996. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat. Med. 2:893-898. [DOI] [PubMed] [Google Scholar]
- 22.Huygen, K. 2005. Plasmid DNA vaccination. Microb. Infect. 7:932-938. [DOI] [PubMed] [Google Scholar]
- 23.Kaufmann, S. H. E. 2001. How can immunology contribute to the control of tuberculosis? Nat. Rev. Immunol. 1:20-30. [DOI] [PubMed] [Google Scholar]
- 24.Lazarevic, V., and J. Flynn. 2002. CD8+ T cells in tuberculosis. Am. J. Respir. Crit. Care Med. 166:1116-1121. [DOI] [PubMed] [Google Scholar]
- 25.MacMicking, J. D., G. A. Taylor, and J. D. McKinney. 2003. Immune control of tuberculosis by IFN-gamma-inducible LRG-47. Science 302:654-659. [DOI] [PubMed] [Google Scholar]
- 26.Mak, T. W., A. Rahemtulla, M. Schilham, D. R. Kohl, and W. P. Fung-Leung. 1992. Generation of mutant mice lacking surface expression of CD4 or CD8 by gene targeting. J. Autoimmun. 5(Suppl. A):55-59. [DOI] [PubMed] [Google Scholar]
- 27.Orme, I. M., A. D. Roberts, J. P. Griffin, and J. S. Abrams. 1993. Cytokine secretion by CD4 T lymphocytes acquired in response to Mycobacterium tuberculosis infection. J. Immunol. 151:518-525. [PubMed] [Google Scholar]
- 28.Rahemtulla, A., W. P. Fung-Leung, M. W. Schilham, T. M. Kündig, S. R. Sambhara, A. Narendran, A. Arabian, A. Wakeham, C. J. Paige, R. M. Zinkernagel, R. G. Miller, and T. W. Mak. 1991. Normal development and function of CD8+ cells but markedly decreased helper activity in mice lacking CD4. Nature 353:180-184. [DOI] [PubMed] [Google Scholar]
- 29.Romano, M., O. Denis, S. D'Souza, X.-M. Wang, T. H. M. Ottenhoff, J.-M. Brulet, and K. Huygen. 2004. Induction of in vivo functional Db-restricted cytolytic T cell activity against a putative phosphate transport receptor of Mycobacterium tuberculosis. J. Immunol. 172:6913-6921. [DOI] [PubMed] [Google Scholar]
- 30.Serbina, N. V., V. Lazarevic, and J. L. Flynn. 2001. CD4(+) T cells are required for the development of cytotoxic CD8(+) T cells during Mycobacterium tuberculosis infection. J. Immunol. 167:6991-7000. [DOI] [PubMed] [Google Scholar]
- 31.Shedlock, D. J., and H. Shen. 2003. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science 300:337-342. [DOI] [PubMed] [Google Scholar]
- 32.Small, P. M., R. W. Shafer, P. C. Hopewell, S. P. Singh, M. J. Murphy, E. Desmond, M. F. Sierra, and G. K. Schoolnik. 1993. Exogenous reinfection with multidrug-resistant Mycobacterium tuberculosis in patients with advanced HIV infection. N. Engl. J. Med. 328:1137-1144. [DOI] [PubMed] [Google Scholar]
- 33.Snewin, V. A., M. P. Gares, P. O. Gaora, Z. Hasan, I. N. Brown, and D. B. Young. 1999. Assessment of immunity to mycobacterial infection with luciferase reporter constructs. Infect. Immun. 67:4586-4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanghe, A., P. Lefevre, O. Denis, S. D'Souza, M. Braibant, E. Lozes, M. Singh, D. Montgomery, J. Content, and K. Huygen. 1999. Immunogenicity and protective efficacy of tuberculosis DNA vaccines encoding putative phosphate transport receptors. J. Immunol. 162:1113-1119. [PubMed] [Google Scholar]
- 35.Tanghe, A., S. D'Souza, V. Rosseels, O. Denis, T. H. M. Ottenhoff, W. Dalemans, C. Wheeler, and K. Huygen. 2001. Improved immunogenicity and protective efficacy of a tuberculosis DNA vaccine encoding Ag85 by protein boosting. Infect. Immun. 69:3041-3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ulmer, J. B., T. M. Fu, R. R. Deck, A. Friedman, L. Guan, C. DeWitt, X. Liu, S. Wang, M. A. Liu, J. J. Donnelly, and M. J. Caulfield. 1998. Protective CD4+ and CD8+ T cells against influenza virus induced by vaccination with nucleoprotein DNA. J. Virol. 72:5648-5653. [DOI] [PMC free article] [PubMed] [Google Scholar]