Abstract
BBA68 (BbCRASP-1) of the Lyme disease spirochetes binds human factor H (FH) and FH-like protein 1 (FHL-1). Here we assess transcription of the BBA68 gene and production of BBA68 in infected mice and humans using real-time reverse transcriptase PCR and immunoblotting. The species specificity of FH binding to BBA68 was also tested. The data suggest that BBA68 does not play an important role in immune evasion in animals.
The Lyme disease spirochete, Borrelia burgdorferi, can bind the human complement regulatory proteins, factor H (FH) and FH-like protein 1 (FHL-1) (1, 8, 11, 16). FH and FHL-1 serve as cofactors in the factor I-mediated cleavage of C3b (18). C3b is an important opsonin and central component of the complement system. The binding of FH and FHL-1 to the bacterial surface is thought to locally increase the efficiency of C3b cleavage, locally downregulate the alternative complement cascade, and inhibit C3b-mediated opsonophagocytosis (30). The FH/FHL-1 binding proteins (FHBPs) identified in B. burgdorferi B31MI include the OspE proteins (BBL39, BBN38, and BBP38) and BBA68 (also referred to as BbCRASP-1) (1, 8, 10, 11, 15, 16). Analyses of in vitro-cultivated bacteria indicate that BBA68 is the dominant FHBP and is the only paralog of the 14-member protein family 54 (The Institute for Genome Research designation) to exhibit human FH/FHL-1 binding activity (10, 15). Inactivation of BBA68 increases sensitivity to complement in vitro, and the introduction of BBA68 into Borrelia garinii strains that lack the gene decreases their serum sensitivity (3). While it is widely held that BBA68 plays an important role in immune evasion, earlier studies provided evidence that it may not be expressed during infection (2, 17, 23, 25). In fact, Tokarz et al. demonstrated that, when human blood was added to actively growing cultures, BBA68 and BBA69 gene transcription was downregulated 1.8-fold. In addition, Brooks et al. demonstrated a sevenfold reduction in BBA68 transcript levels in spirochetes cultivated in dialysis membrane chambers implanted into the peritoneal cavities of rats (2, 23). The goals of this study were to further investigate the putative contribution of BBA68 in immune evasion in humans and other animals. In summary, the data indicate that the BBA68 gene is not transcribed during infection in mice and does not elicit an antibody response in mice and humans. In addition, BBA68 does not bind to FH produced by animals other than humans, and hence it is not likely to be involved in FH-mediated immune evasion in its natural mammalian reservoirs. Collectively, the data suggest that BBA68 does not carry out an important function directly in infected humans and animals.
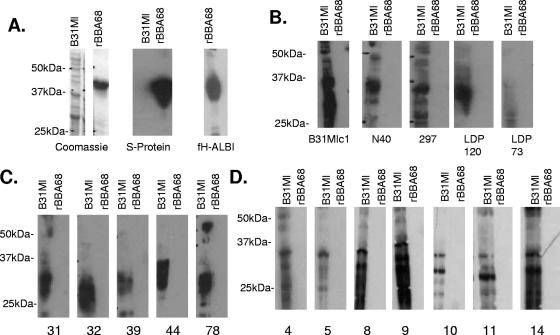
As one approach to assessing production of BBA68 during infection we screened for an antibody response to BBA68 in infected mice and humans. Since an understanding of the potential heterogeneity of BBA68 is an essential first step in interpreting immunoblot analyses and identifying the appropriate test antigen to be employed in the screening, we first assessed the genetic conservation of BBA68 among strains. Using previously described primers (13) BBA68 was amplified by PCR (standard methods) from a diverse panel of previously described isolates (n = 15) (21), the amplicons were cloned into the TOPO vector (Invitrogen), and the sequences of individual clones were determined (MWG Biotech). The sequence analyses revealed that BBA68 is conserved (Fig. 1) among North American isolates, with amino acid identity values among orthologs ranging from 96.5% to 100%. The conservation of BBA68 among isolates indicates that a single test antigen (from B. burgdorferi B31MI) is sufficient to assess the potential antibody response to BBA68 in mice and North American human Lyme disease patients. r-BBA68 was then generated using ligase-independent cloning methodologies as previously described (13), and the expression and integrity of the r protein were demonstrated by Coomassie staining and screening of an immunoblot strip with horseradish peroxidase-conjugated S protein (diluted 1:5,000; Invitrogen) (Fig. 2A). In these and all subsequent blots 1 μg of r-BBA68 was loaded per lane. To further confirm that the properties of r-BBA68 had not been altered by expression in Escherichia coli, the protein was tested for its ability to bind FH/FHL-1 using the affinity ligand binding immunoblot assay (14). r-BBA68 readily bound FH (Fig. 2A). To determine if BBA68 elicits an antibody response during infection in mice, mice were infected with nine different BBA68-expressing strains of B. burgdorferi and serum was collected out to 12 weeks postinfection as previously described (5). The sera (diluted 1:1,000) were used to screen immunoblot strips containing a lane of r-BBA68 and a lane of B. burgdorferi B31MI cell lysate. The specific strains that were used to generate the infection serum are indicated below each blot in Fig. 2B. None of the serum samples collected from mice displayed immunoreactivity with r-BBA68, but all were immunoreactive with the B. burgdorferi B31MI cell lysate (Fig. 2B). Even after prolonged exposure, antibody to BBA68 was not detected. As verification of the sensitivity of the assay, the same serum samples reacted strongly with the same amount of r-OspC and r-OspE (data not shown), which are proteins known to be expressed during infection in mice and humans (5, 7, 16, 20, 22, 27). Based on the conservation of BBA68 it is clear that failure to detect an antibody response in mice infected with different strains is not due to antigenic differences in the protein.
FIG. 1.
Demonstration of the conservation of BBA68 among diverse isolates of the Lyme disease spirochetes. The BBA68 gene was amplified using primers designed to amplify the entire reading frame minus the leader peptide. The amplicons were cloned into the TOPO vector and sequenced. The deduced amino acid sequences are presented. The isolate of origin is indicated to the left. Identical sequences from different strains are presented together. Periods in the alignment indicate residues identical to that of BBA68 from B. burgdorferi B31MI.
FIG. 2.
Demonstration that BBA68 does not elicit an antibody response in mice or humans infected with the Lyme disease spirochetes. (A) The loading and integrity of r-BBA68 (1 μg per lane) and cell lysates of B. burgdorferi B31MI were assessed by Coomassie staining and by screening with horseradish peroxidase-conjugated S protein. r-BBA68 was also tested for factor H binding ability using the affinity ligand binding immunoblot assay. (B) Immunoblot strips containing B31MI cell lysate and r-BBA68 were screened with serum from mice infected with different strains of B. burgdorferi (indicated below each blot). Identical immunoblots were screened with serum from human Lyme disease patients with either early (C)- or late (D)-stage infections, respectively. All methods are described in the text.
To determine if an antibody response to BBA68 develops during human infection, immunoblot strips of r-BBA68 were screened with serum collected from patients with early (erythema migrans; n = 11)- or late-stage (arthritis; n = 16) (19) Lyme disease. Serum samples were provided by Allen Steere (Harvard Medical School) and were collected from patients that met the Centers for Disease Control and Prevention criteria for Lyme disease. The serum samples from patients with early-stage infection were assayed for an immunoglobulin G (IgG) response to BBA68 and to whole-cell lysates of B. burgdorferi B31MI. All human serum samples were tested at a 1:400 dilution with the appropriate secondary antibody (Pierce) at 1:10,000. None of the serum samples were positive for a response to BBA68 but were strongly reactive with cell lysates of B. burgdorferi B31MI (Fig. 2C and D). Collectively, the immunoblot data indicate that BBA68 is not produced during infection. However, an alternative possibility is that BBA68 is nonantigenic. In light of the surface exposure of BBA68, its biochemical properties, and the ability of r-BBA68 to elicit a strong antibody response in mice (13), the possibility that it is nonantigenic seems unlikely.
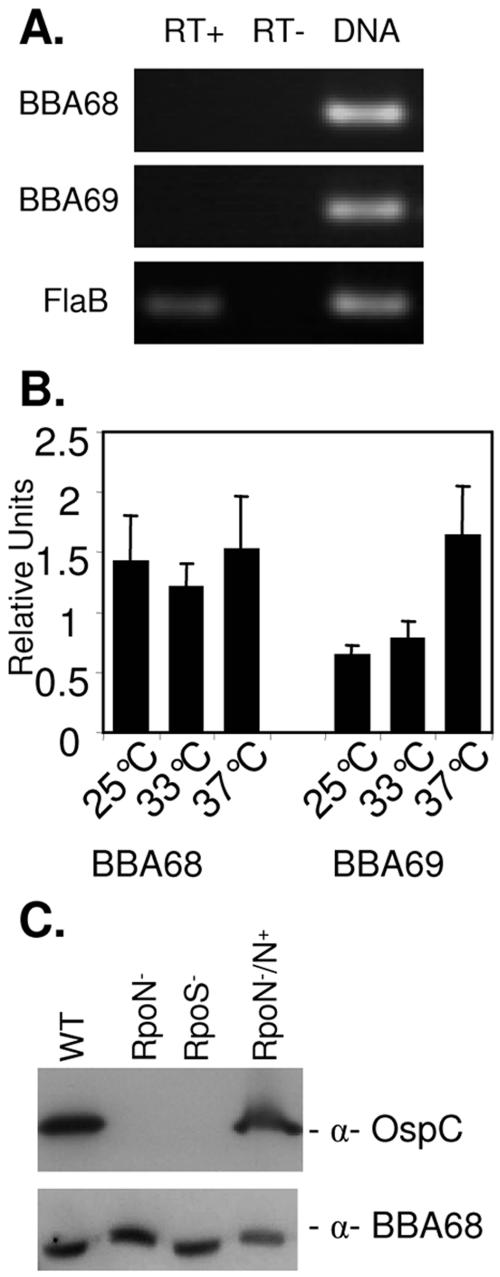
To address potential questions surrounding the antigenicity of BBA68, we directly tested for BBA68 expression during infection using an antibody-independent approach, real-time reverse transcriptase PCR (RT-PCR). All procedures were as previously described (29). The template RNA was extracted from ear punch biopsy samples collected from B. burgdorferi B31MI-infected mice (n = 3; time frame of infection = 4 weeks). The possible expression of BBA69, a family 54 member whose gene maps immediately downstream of the BBA68 gene, was also assessed. The BBA68 and BBA69 gene primer sequences (5′ to 3′) were as follows: A68RT74F, GCGCACCTTTTAGCAAAATC; A68RT226R, GCTCTGAAGCGATAGTTTCCAT; A69RT90F, AATCAATCCCAAGGCAAATG; A69RT205R, CGCCAAGGTCTCCAGATTTA. The flaB primers, which target the constitutively expressed flaB gene, have been previously described (29). While the flaB transcript was readily detected by real-time RT-PCR, the transcripts from the BBA68 and BBA69 genes were not (Fig. 3A). To determine if the elevated temperature encountered by the spirochetes in mammals might in and of itself downregulate BBA68 expression and to verify that the primers employed were functional, real-time RT-PCR was performed using RNA extracted from B. burgdorferi B31MI temperature shifted to either 25, 33, or 37°C (Fig. 3B). After normalization against flaB transcript levels (as described previously [29]), BBA68 gene transcription was determined to be slightly elevated at 37 versus 25°C. In contrast, BBA69 gene transcription showed a clear increase at 37°C. Consistent with the apparent absence of BBA68 gene transcription in tissue, Tokarz et al. previously demonstrated that BBA68 is downregulated in vitro when human blood is added to Borrelia cultures (23). Brooks et al. also demonstrated that both BBA68 and BBA69 are downregulated approximately sevenfold in spirochetes propagated in dialysis membrane chambers implanted in rats (2), and Lederer et al. reported that transcription of the gene encoding the BBA68 ortholog of B. burgdorferi ZS7 could not be detected in infected mouse tissue (12). Collectively these analyses indicate that host-specific factors, and not temperature alone, are responsible for the downregulation of BBA68.
FIG. 3.
Real-time RT-PCR analysis of BBA68 and BBA69 gene transcription and analysis of the effect of RpoN and RpoS knockouts on protein production. RNA was isolated from infected mouse tissue and tested by real-time RT-PCR for BBA68 and BBA69 gene and flaB transcripts. Detection of flaB served as the positive control, and reactions run without reverse transcriptase served as the negative controls. As an additional positive control, the primers were also assessed for their ability to PCR amplify their corresponding target genes using genomic DNA as the template. After 40 cycles, an aliquot of each reaction mixture was analyzed by agarose gel electrophoresis and ethidium bromide staining (A). (B) Data from real-time RT-PCR analysis of BBA68 and BBA69 gene and flaB transcription in B. burgdorferi B31MI grown at either 25, 33, or 37°C in BSK-H complete media. Reactions were run in triplicate with two replicates, and the variance is indicated. RNA isolation and all associated methods were exactly as previously described (29). (C) Immunoblots of B. burgdorferi strain 297 in which RpoS or RpoN had been inactivated were screened with anti-BBA68 (α-BBA68) and anti-OspC antiserum (positive control). As controls, the wild type (WT) and the RpoN (RpoN−/N+) complemented strain were included on each blot.
To determine if BBA68 gene transcription might be controlled via the RpoN and RpoS regulatory network, immunoblot analyses were conducted using cell lysates of B. burgdorferi isolate 297 RpoN and RpoS knockout mutants (and the complemented strain) (28) (kindly provided by Michael Norgard, University of Texas Southwestern). The RpoN/RpoS regulatory network controls in part the expression of some important Borrelia virulence factors including OspC (4, 6, 28). BBA68 production was not affected by inactivation of either RpoN or RpoS (Fig. 3C). As a control, an immunoblot was screened with anti-OspC antiserum, and as expected OspC production was eliminated in the RpoN and RpoS knockout mutants, with production fully restored in the complemented strain. The molecular mechanisms associated with the transcriptional control of the BBA68 gene and other genes that are turned on/off in the mammalian environment are yet to be determined.
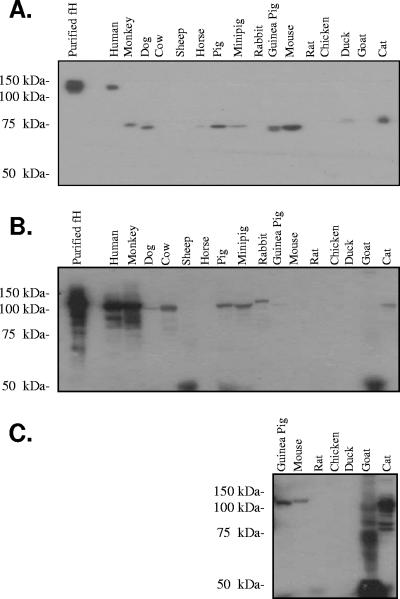
Even though BBA68 is not expressed in humans and mice, the possibility remained that it could be expressed and function during infection in other animals. In that the only known ligands bound by BBA68 are human FH and FHL-1, we sought to determine if BBA68 can bind to FH/FHL-1 from other animals. To test this, serum from several different animals (Valley Biomedical) was fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using Criterion precast gels (Bio-Rad) under nonreducing conditions. Hovis et al. have demonstrated that electrophoresis under nonreducing conditions is required to allow for the binding of FH binding proteins to membrane-immobilized FH (9). Nonreducing conditions maintain conserved disulfide bonds within the short consensus repeats of FH that are the basis for its beaded globular structure and presumably important in the formation of its interaction domain (30). BBA68 bound a protein of a size consistent with FH only in the human serum sample, indicating a strictly species-specific interaction (Fig. 4A). Hence, BBA68 differs significantly from the FH binding OspE protein, which has recently been demonstrated to bind FH and other serum proteins from a wide range of animals using the exact same experimental protocol and serum samples as those employed here (9). As an additional control to demonstrate the presence of FH in serum from each animal, the serum samples were separated by SDS-PAGE under reducing conditions, immunoblotted, and screened with goat anti-human FH antiserum (Calbiochem; diluted 1;800) followed by rabbit anti-goat IgG antiserum (Calbiochem; diluted 1:40,000). As expected human and monkey FH reacted strongly with the antiserum (Fig. 4B). With longer exposure, FH was detected in all other animals tested except ducks, chickens, rats, and horses (Fig. 4C). Weaker or no immunoreactivity is not surprising in light of sequence variation in FH among animals. In any event, this control and the earlier study by Hovis et al. (9) demonstrate that FH is available for binding in the serum samples tested. Interestingly, BBA68 also bound to unidentified serum proteins of approximately 75 kDa in several serum samples. These proteins were not immunoreactive with anti-human FH antiserum and were not bound by other control S-tagged proteins including BBO39 and BBR42 (data not shown). The identities of these serum proteins are unknown, and the significance of the interaction remains to be determined. It is interesting that BBA68 did not bind to FH from mice, which are important natural reservoirs for the Lyme disease spirochetes. While BBA68 has been demonstrated to be expressed in ticks (25) and the protein has been demonstrated on the surface of spirochetes that have just entered mouse skin (24), its lack of expression in animals and its inability to bind mouse FH with high affinity suggest that it does not function to protect against mouse complement in an FH-dependent manner during the blood meal. When one considers that humans are only accidental hosts of the Lyme disease spirochetes, the biological rationale for the evolution of species-specific binding of human FH by BBA68 is puzzling and somewhat of a paradox.
FIG. 4.
Demonstration of the species specificity of BBA68 binding to FH and detection of additional BBA68 binding serum proteins. Serum from several different animals (as indicated above each lane) was diluted 1:4 with phosphate-buffered saline and prepared in nonreducing SDS-PAGE sample buffer. The samples were incubated at 37°C for 30 min, and then the proteins were separated in 7.5% Criterion gels (Bio-Rad). The proteins were transferred to polyvinylidene difluoride membranes, blocked with 5% milk, and screened with S-tagged recombinant BBA68 (100 ng μl−1) (A). After a washing, bound BBA68 was detected with horseradish peroxidase-conjugated S protein (diluted 1:40,000). Serum samples were also fractionated by SDS-PAGE under reducing conditions, immunoblotted, and screened with goat anti-human FH antiserum (diluted 1:800; Calbiochem) (B). Rabbit anti-goat IgG antiserum served as the secondary antibody. In panel C, a longer exposure of the several lanes of the immunoblot presented in panel B is shown. Molecular mass standards are indicated.
In closing, based on the observation that BBA68 is the most dominant FHBP produced by in vitro-cultivated Lyme disease spirochetes, we and others have postulated that BBA68 likely plays a critical role in immune evasion and persistence in animals (3, 10, 13, 15, 26). In fact, Brooks et al. demonstrated that the inactivation of BBA68 led to increased susceptibility to human serum and that introduction of BBA68 into a B. garinii strain lacking the BBA68 gene resulted in increased serum resistance (3). However, it is important to note that the serum sensitivity assays were done in vitro using in vitro-cultivated bacteria. The data presented here indicate that BBA68 is not expressed during infection in humans and mice and that it is not able to bind FH from other potential mammalian reservoirs. Hence the specific role of BBA68 in immune evasion in spirochetes residing within animals remains to be determined. Analyses are under way to further investigate the possible role of this highly conserved protein in spirochetes residing within ticks, the only natural environment in which BBA68 gene transcription has been demonstrated.
Acknowledgments
This work was supported in part by grants from the NIAID, NIH, to R. T. Marconi and an F31 award from NINDS to K. M. Hovis. J. V. McDowell was supported in part by a NIAID training grant in molecular pathogenesis to the Department of Microbiology and Immunology at VCU.
Editor: J. B. Bliska
REFERENCES
- 1.Alitalo, A., T. Meri, H. Lankinen, I. Seppala, P. Lahdenne, P. S. Hefty, D. Akins, and S. Meri. 2002. Complement inhibitor factor H binding to Lyme disease spirochetes is mediated by inducible expression of multiple plasmid-encoded outer surface protein E paralogs. J. Immunol. 169:3847-3853. [DOI] [PubMed] [Google Scholar]
- 2.Brooks, C. S., P. S. Hefty, S. E. Joliff, and D. R. Akins. 2003. Global analysis of Borrelia burgdorferi genes regulated by mammalian host-specific signals. Infect. Immun. 71:3371-3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brooks, C. S., S. R. Vuppala, A. M. Jett, A. Alitalo, S. Meri, and D. R. Akins. 2005. Complement regulator-acquiring surface protein 1 imparts resistance to human serum in Borrelia burgdorferi. J. Immunol. 175:3299-3308. [DOI] [PubMed] [Google Scholar]
- 4.Caimano, M. J., C. H. Eggers, K. R. O. Hazlett, and J. D. Radolf. 2004. RpoS is not central to the general stress response in Borrelia burgdorferi but does control expression of one or more essential virulence determinants. Infect. Immun. 72:6433-6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Earnhart, C. G., E. L. Buckles, J. S. Dumler, and R. T. Marconi. 2005. Demonstration of OspC type diversity in invasive human Lyme disease isolates and identification of previously uncharacterized epitopes that define the specificity of the OspC antibody response. Infect. Immun. 73:7869-7877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eggers, C. H., M. J. Caimano, and J. D. Radolf. 2004. Analysis of promoter elements involved in the transcriptional initiation of RpoS-dependent Borrelia burgdorferi genes. J. Bacteriol. 186:7390-7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hefty, P. S., S. E. Jolliff, M. J. Caimano, S. K. Wikel, J. D. Radolf, and D. R. Akins. 2001. Regulation of the OspE-related, OspF-related, and Elp lipoproteins of Borrelia burgdorferi strain 297 by mammalian host-specific signals. Infect. Immun. 69:3618-3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hellwage, J., T. Meri, T. Heikkila, A. Alitalo, J. Panelius, P. Lahdenne, I. J. T. Seppala, and S. Meri. 2001. The complement regulator factor H binds to the surface protein OspE of Borrelia burgdorferi. J. Biol. Chem. 276:8427-8435. [DOI] [PubMed] [Google Scholar]
- 9.Hovis, K. M., J. V. McDowell, and R. T. Marconi. 2006. Selective binding of Borrelia burgdorferi OspE paralogs to factor H and serum proteins from diverse animals: possible expansion of the role of OspE in Lyme disease. Infect. Immun. 74:1967-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kraiczy, P., J. Hellwage, C. Skerka, H. Becker, M. Kirschfink, M. S. Simon, V. Brade, P. F. Zipfel, and R. Wallich. 2004. Complement resistance of Borrelia burgdorferi correlates with the expression of BbCRASP-1, a novel linear plasmid-encoded surface protein that interacts with human factor H and FHL-1 and is unrelated to Erp proteins. J. Biol. Chem. 279:2421-2429. [DOI] [PubMed] [Google Scholar]
- 11.Kraiczy, P., C. Skerka, M. Kirschfink, V. Brade, and P. F. Zipfel. 2001. Immune evasion of Borrelia burgdorferi by acquisition of human complement regulators FHL-1/reconectin and factor H. Eur. J. Immunol. 31:1674-1684. [DOI] [PubMed] [Google Scholar]
- 12.Lederer, S., C. Brenner, T. Stehle, L. Gern, R. Wallich, and M. M. Simon. 2005. Quantitative analysis of Borrelia burgdorferi gene expression in naturally (tick) infected mouse strains. Med. Microbiol. Immunol. 194:81-90. [DOI] [PubMed] [Google Scholar]
- 13.McDowell, J. V., M. E. Harlin, E. Rogers, and R. T. Marconi. 2005. Putative coiled-coil structural elements of the BBA68 protein of the Lyme disease spirochetes are required for formation of its factor H binding site. J. Bacteriol. 187:1317-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDowell, J. V., J. Lankford, L. Stamm, T. Sadlon, D. L. Gordon, and R. T. Marconi. 2005. Demonstration of factor H-like protein 1 binding to Treponema denticola, a pathogen associated with periodontal disease in humans. Infect. Immun. 73:7126-7132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDowell, J. V., J. Wolfgang, E. Tran, M. S. Metts, D. Hamilton, and R. T. Marconi. 2003. Comprehensive analysis of the factor H binding capabilities of Borrelia species associated with Lyme disease: delineation of two distinct classes of factor H binding proteins. Infect. Immun. 71:3597-3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Metts, S., J. V. McDowell, M. Theisen, P. R. Hansen, and R. T. Marconi. 2003. Analysis of the OspE determinants involved in the binding of factor H and OspE targeting antibodies elicited during infection in mice. Infect. Immun. 71:3587-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ojaimi, C., C. Brooks, S. Casjens, P. Rosa, A. Elias, A. G. Barbour, A. Jasinskas, J. Benach, L. Katona, J. Radolf, M. Caimano, J. Skare, K. Swingle, D. Akins, and I. Schwartz. 2003. Profiling of temperature-induced changes in Borrelia burgdorferi gene expression by using whole genome arrays. Infect. Immun. 71:1689-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruddy, S., and K. F. Austen. 1971. C3b inactivator of man. II. Fragments produced by C3b inactivator cleavage of cell-bound or fluid phase C3b. J. Immunol. 107:742-750. [PubMed] [Google Scholar]
- 19.Salazar, C. A., M. Rothemich, E. E. Drouin, L. Glickstein, and A. C. Steere. 2005. Human Lyme arthritis and the immunoglobulin G antibody response to the 37-kilodalton arthritis-related protein of Borrelia burgdorferi. Infect. Immun. 73:2951-2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suk, K., S. Das, W. Sun, B. Jwang, S. W. Barthold, R. A. Flavell, and E. Fikrig. 1995. Borrelia burgdorferi genes selectively expressed in the infected host. Proc. Natl. Acad. Sci. USA 92:4269-4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sung, S.-Y., C. LaVoie, J. A. Carlyon, and R. T. Marconi. 1998. Genetic divergence and evolutionary instability in ospE-related members of the upstream homology box gene family in Borrelia burgdorferi sensu lato complex isolates. Infect. Immun. 66:4656-4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sung, S. Y., J. McDowell, J. A. Carlyon, and R. T. Marconi. 2000. Mutation and recombination in the upstream homology box-flanked ospE-related genes of the Lyme disease spirochetes results in the development of new antigenic variants during infection. Infect. Immun. 68:1319-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tokarz, R., J. Anderton, L. Katona, and J. Benach. 2004. Combined effects of blood and temperature shift on Borrelia burgdorferi gene expression as determined by whole genome DNA array. Infect. Immun. 72:5419-5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Lackum, K., J. C. Miller, T. Bykowski, S. P. Riley, M. E. Woodman, V. Brade, P. Kraiczy, B. Stevenson, and R. Wallach. 2005. Borrelia burgdorferi regulates expression of complement regulator-acquiring surface protein 1 during the mammal-tick infection cycle. Infect. Immun. 73:7398-7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallich, R., O. Jahraus, T. Stehle, T. T. T. Tran, C. Brenner, H. Hofmann, L. Gern, and M. M. Simon. 2003. Artificial-infection protocols allow immunodetection of novel Borrelia burgdorferi antigens suitable as vaccine candidates against Lyme disease. Eur. J. Immunol. 33:708-719. [DOI] [PubMed] [Google Scholar]
- 26.Wallich, R., J. Pattathu, V. Kitiratschky, C. Brenner, P. F. Zipfel, V. Brade, M. M. Simon, and P. Kraiczy. 2005. Identification and functional characterization of complement regulator acquiring surface protein 1 of the Lyme disease spirochetes Borrelia afzelii and Borrelia garinii. Infect. Immun. 73:2351-2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilske, B., V. Preac-Mursic, S. Jauris, A. Hofmann, I. Pradel, E. Soutschek, E. Schwab, G. Will, and G. Wanner. 1993. Immunological and molecular polymorphisms of OspC, an immunodominant major outer surface protein of Borrelia burgdorferi. Infect. Immun. 61:2182-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang, X. F., S. M. Alani, and M. V. Norgard. 2003. The response regulator Rrp2 is essential for the expression of major membrane lipoproteins in Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA 100:11001-11006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang, H., and R. T. Marconi. 2005. Demonstration of cotranscription and 1-methyl-3-nitroso-nitroguanidine induction of a 30-gene operon of Borrelia burgdorferi: evidence that the 32-kilobase circular plasmids are prophages. J. Bacteriol. 187:7985-7995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zipfel, P. F., C. Skerka, J. Hellwage, S. T. Jokiranta, S. Meri, V. Brade, P. Kraiczy, M. Noris, and G. Remuzzi. 2002. Structure-function studies of the complement system. Biochem. Soc. Trans. 30:971-978. [DOI] [PubMed] [Google Scholar]