Abstract
Important to malaria vaccine design is the phenomenon of “strain-specific” immunity. Using an accurate and sensitive assay of parasite genotype, real-time quantitative PCR, we have investigated protective immunity against mixed infections of genetically distinct cloned “strains” of the rodent malaria parasite Plasmodium chabaudi chabaudi in mice. Four strains of P. c. chabaudi, AS, AJ, AQ, and CB, were studied. One round of blood infection and drug cure with a single strain resulted in a partial reduction in parasitemia, compared with levels for naïve mice, in challenge infections with mixed inocula of the immunizing (homologous) strain and a heterologous strain. In all cases, the numbers of blood-stage parasites of each genotype were reduced to similar degrees. After a second, homologous round of infection and drug cure followed by challenge with homologous and heterologous strains, the parasitemias were reduced even further. In these circumstances, moreover, the homologous strain was reduced much faster than the heterologous strain in all of the combinations tested. That the immunity induced by a single infection did not show “strain specificity,” while the immunity following a second, homologous infection did, suggests that the “strain-specific” component of protective immunity in malaria may be dependent upon immune memory. The results show that strong, protective immunity induced by and effective against malaria parasites from a single parasite species has a significant “strain-specific” component and that this immunity operates differentially against genetically distinct parasites within the same infection.
The asexual blood stages of malaria parasites are exclusively responsible for the pathology and disease symptoms of malarial infections in human and other vertebrate host species. Protective immunity against these stages is the objective of much malaria vaccine research and development. Blood-stage protective immunity can be acquired by exposed individuals living in areas where malaria is endemic. However, its extent is highly variable and is dependent, among other things, upon the degree and duration of exposure of an individual to infection with malaria. By contrast, immunity to a challenge infection is readily induced in humans and other animals by clinically induced or laboratory-induced blood infections followed by drug cure when the challenge infection is with parasites of the same stock as the strain used for immunization. However, when challenge infections are with parasites of a different genetic origin, albeit of the same species, the degree of protection against the blood parasites and the disease symptoms that they cause is generally significantly less (2, 5, 12, 15).
These observations have led to the concept of “strain-specific” protective immunity in malaria, according to which each naturally experienced inoculum of malaria parasites usually contains a characteristic and genetically determined set of target antigens of protective immunity. Unless containing antigen types previously experienced, each new infection will be poorly protected against until all antigen types in the prevailing parasite population have been experienced. This has become the most commonly cited hypothesis to account for the length of time taken to achieve effective protective immunity against malaria in humans exposed to malaria transmission in areas of endemicity.
The literature around this concept has identified several proteins of malaria parasites that have the properties required by the above hypothesis. Prominent are two merozoite surface proteins, MSP-1 and MSP-2, and the apical membrane antigen 1 (AMA-1), which is located in the micronemes of the apical complex of merozoites. All three proteins are products of highly polymorphic genes of the malaria parasite, and there is evidence that each is a target of protective immunity and of “strain-specific” protective immunity in particular against blood-stage malarial infection (6, 9, 16, 17).
Here we have investigated the strain specificity of immunity to four genetically distinct cloned lines (“strains”) of the rodent malaria parasite Plasmodium chabaudi chabaudi from isolates collected from a single location in central Africa where rodent malaria is endemic. The effects of the immunity, induced by infection and drug cure, against each of the parasite cloned lines have been followed using highly accurate and sensitive gene sequence-specific real-time quantitative PCR (RTQ-PCR) (4).
MATERIALS AND METHODS
Origins of the cloned lines of P. c. chabaudi used in this study.
The malaria parasites used in this study are derived from four different isolates of the parasites from tree rats (Thamnomys rutilans) collected from a single locality of malaria endemicity at La Maboke, near Bangui in the Central African Republic, during 1969 to 1970 (3). The four isolates, designated AJ, AQ, AS, and CB, were each obtained by subinoculation into laboratory mice of P. c. chabaudi-infected blood from a single captured specimen of T. rutilans. A line of P. c. chabaudi was subsequently obtained by cloning by dilution from each of the three isolates AJ, AS, and CB (D. Walliker and R. Carter, unpublished data) and of AQ (M. Mackinnon, unpublished data). The original cloned lines in these records are designated 42AS37(Pyr1) (line cloned after selection for resistance to pyrimethamine), 96AJ, 50CB, and 31AQ4. For convenience, these are referred to in the present text as strains AS, AJ, CB, and AQ, respectively. Following their cloning, the parasite lines were maintained in the laboratory by serial blood passage in mice, with occasional transmission through mosquitoes and as stabilates in liquid nitrogen, as recorded in the laboratory records of D. Walliker, R. Carter, and M. Mackinnon.
Each of the strains has been characterized using electrophoretic variants of metabolic enzymes of P. c. chabaudi in the blood stages (3). These variants are randomly assorted among the individual parasites isolated for this population and indicate that the parasites within this population are highly outbred (3, 13). MSP-1 sequence analysis of each of these strains (AJ, AQ, AS, and CB) supports this conclusion (S. Cheesman and R. Carter, unpublished data).
Infection of naïve mice with mixed strain infections of P. c. chabaudi.
Groups of two 6- to 8-week-old inbred female CBA/Ca mice were infected by intraperitoneal inoculation with 5 × 106 parasitized erythrocytes composed of approximately equal numbers of strains AS and CB, AJ and CB, or AJ and AQ. DNA was later extracted from a sample of each inoculum for RTQ-PCR analysis to accurately measure the proportions of each strain used in the mixed strain challenge infection; the proportions were calculated at 40:60% (AS and CB), 32:68% (AJ and CB), and 57:43% (AJ and AQ). The mixed infections were allowed to progress until day 6 postinfection, at which point the animals were humanely killed. Tail blood samples were collected daily into physiological citrate saline from the third day after the challenge inoculation. All experiments conducted in this study conform to the British Home Office Animals (Scientific Procedures) Act 1986.
P. c. chabaudi strain-specific immunization of mice.
Groups of female CBA/Ca mice, 6 to 8 weeks old, were infected by intraperitoneal injection with 1 × 106 parasitized erythrocytes of one of the following P. c. chabaudi cloned lines: AS, AJ, AQ, or CB. Infections were allowed to proceed for 5 days postinoculation, at which point each mouse was treated with a daily dose, by oral gavage, of 20 mg/kg mouse body weight of mefloquine in 0.1 ml dimethyl sulfoxide for 5 days. Blood parasitemias were followed daily until the day after the last drug dosing and thereafter weekly. All mice remained blood smear negative at the point of observation following the drug treatment. Mice infected and treated in this way are referred to as 1× immunized. Mefloquine treatment was included in the protocol to ensure complete clearance of the parasites from the experimental mice before the mixed strain challenge infections were conducted. Mefloquine has a reported half-life of around 18 h in mice (7), and we have not found any evidence to indicate that administration of the drug alters the timing or course of a subsequent infection in mice that had experienced two rounds of mefloquine treatment administered at 14-day intervals prior to infection 41 days after mefloquine treatment (R. Culleton and R. Carter, unpublished data).
One month to 6 weeks after their first infection, some mice were reinfected by intraperitoneal injection with 1 × 106 parasitized erythrocytes of the same (homologous) strain of P. c. chabaudi with which they had first been infected. Infections were treated with mefloquine as described above, and the mice were monitored for the presence of the blood-stage parasites. All such twice-infected mice became parasitemic on their second infection but at lower parasitemias than those following their first infection. All mice remained blood smear negative at the point of observation following the completion of the mefloquine treatment. These reinfected and drug-cured mice are referred to as 2× immunized.
P. c. chabaudi mixed strain challenge infections in immunized mice.
Mice previously immunized by blood infection and drug cure within each group (AS and CB, AJ and CB, or AJ and AQ) were challenged with an intraperitoneal inoculum of 5 × 106 parasitized erythrocytes. Within each group, mice were challenged at the same time point with a mixture of the homologous (immunizing) strain and a heterologous strain of P. c. chabaudi in the following combinations and proportions: AS and CB (40:60%), AJ and CB (32:68%), and AJ and AQ (57:43%). Infections were monitored by blood smear. Tail blood samples were collected daily into physiological citrate saline from the third day after the challenge inoculation.
DNA extraction from infected mouse blood.
A 10-μl sample of tail blood collected daily, as described above, was centrifuged briefly, and the red blood cell pellet was stored at −70°C prior to DNA extraction using a High Pure PCR template preparation kit (Roche Diagnostics).
Quantitative PCR.
Quantitative PCR was performed on blood samples taken from mice whose parasitemias were ≥0.05%, which is the limit of detection of the RTQ-PCR assay. Quantitative PCRs were performed on a LightCycler instrument (Roche Diagnostics) as described by Cheesman et al. (4) by use of an assay that discriminates quantitatively between each strain of P. c. chabaudi used in this study. Briefly, oligonucleotide primers were designed to amplify a strain-specific region of the MSP-1 gene of P. c. chabaudi by using reaction and thermocycling conditions very similar to those described previously for AJ and AQ strains (4), with the following modifications. Assays designed to discriminate between AS and CB strains utilized the following primer and cycling conditions. The primers used were AS forward primer, 5′ CCACAACACCTGAAACCAC 3′, and reverse primer, 5′ CTGGTGCTGCTGGGACA 3′, and CB forward primer, 5′ CTGTTACAACCCAAACC 3′, and reverse primer, 5′ AGTTGTTCCTGTGGCAG 3′. The primer annealing step was performed at 64°C with a 6-s hold for AS or at 58°C with a 6-s hold for CB. Quantitative data were acquired after the polymerization step, which was performed at 72°C with a 6-s hold for AS or a 4-s hold for CB. Assays designed to discriminate between AJ and CB strains utilized the AJ-specific forward primer, 5′ ACTGAAGCAACAACACCAGC 3′, and reverse primer, 5′ GTTGTTGATGCACTTGCGGGTTC 3′, and the CB-specific forward primer, 5′ ATCGGCACAAGCACCAGAAG 3′, and reverse primer, 5′ CTTGTGCTTTTGGTGCTGCT 3′. The primer annealing step was performed at 63°C for 6 s for AJ or at 62°C for 6 s for CB, and quantitative data were acquired at 72°C for 6 or 7 s, respectively. Data obtained from each LightCycler run were checked to ensure that the correct allele-specific melting peak and no other was produced.
RESULTS
Quantitative analyses of mixed strain infections in nonimmune mice.
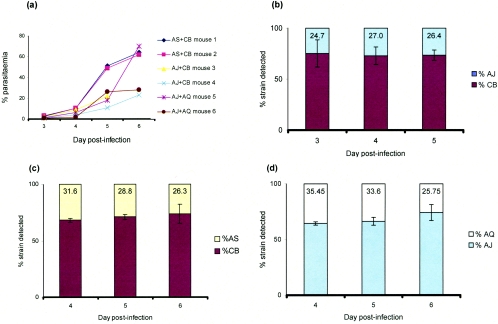
A total of 5 × 106 blood-stage parasites containing approximately equal numbers of parasites of each of two cloned strains of P. c. chabaudi were inoculated intraperitoneally into 6- to 8-week-old female CBA mice. The mixed infections contained the following combinations of strains: AJ and CB, AS and CB, and AJ and AQ. The infections were monitored microscopically up to day 6 following inoculation of the parasites (Fig. 1a). Measurements by RTQ-PCR of the proportions of individual strains in the mixed infections were conducted from day 3 (AJ and CB) (Fig. 1b) and from day 4 (AS and CB and AJ and AQ) (Fig. 1c and d).
FIG. 1.
Quantitative PCR analysis of genetically mixed strain infections of P. c. chabaudi in nonimmune mice. (a) Percentages of parasitemia recorded on a log scale over the period that blood samples were collected for DNA extraction and quantitative PCR analyses. (b, c, and d) Proportions of different strains of P. c. chabaudi measured by RTQ-PCR in two nonimmune mice infected with approximately equal proportions of parasites of each strain (see Materials and Methods) in combinations of (b) AJ and CB, (c) AS and CB, and (d) AJ and AQ. Numbers in columns represent the mean value obtained for the minority strain. Error bars represent the standard deviations between the two mice in each group (AS and CB, AJ and CB, and AJ and AQ).
Consistent with our previous findings from comparisons between single-strain infections (data not shown), CB achieved the highest parasite numbers in each of the mixed infections with either AJ or AS (Fig. 1b and c). In the mixed infections of AJ with AQ, parasites of strain AJ achieved higher numbers than those of AQ (Fig. 1d). Our data also show that within each of the three mixed strain infections, the proportions of parasites of each strain remained constant, i.e., they multiplied at the same rate, during the three days (days 3 to 5 or 4 to 6) (Fig. 1) that each could be measured by the RTQ-PCR method.
Quantitative analysis of mixed strain infections in strain-specific immunized mice.
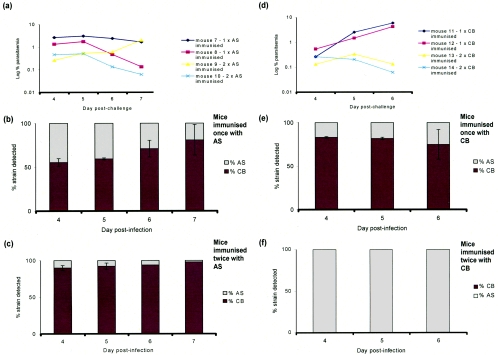
Two groups of mice were infected with 1 × 106 parasites of cloned P. c. chabaudi strain AS or CB and drug cured prior to the peak parasitemia, as described in Materials and Methods. In one group of mice, a single round of infection and cure was given (1×-immunized mice). In the other, a second round of infection and cure was given (2×-immunized mice). Following their last infection and drug cure, the mice were challenged with an inoculum of 5 × 106 parasites containing approximately equal numbers of parasites of homologous (immunizing) and heterologous cloned strains. The infections were monitored by microscopic examination of stained blood smears and by allele-specific RTQ-PCR (4) (Fig. 2).
FIG. 2.
Quantitative PCR analysis of genetically mixed strain infections of P. c. chabaudi in mice and the effect of different levels of infection-acquired immunity on strain survival. (a and d) Percentages of parasitemia in mixed strain infections of AS and CB in 1×-immunized or 2×-immunized mice, recorded on a log scale during the period when mice had patent infections. (b, c, e, and f) Proportions of each strain measured in mixed infections of mice made immune by one (b and e) or two (c and f) successive drug-treated bouts of parasitemia with the homologous strain (AS or CB). Each subsequent mixed strain challenge comprised a total of 5 × 106 strains, approximately half of which were AS and half CB (see Materials and Methods). (b and e) Effect of partial host immunity on the outcome of strain survival, where mice have experienced only one drug-terminated infection with the homologous strain administered 36 days before challenge with the homologous/heterologous mixed strain inoculum comprising approximately equal proportions of AS and CB strains totaling 5 × 106 parasites (see Materials and Methods). (c and f) Results for mice that were treated with one further drug-terminated infection administered 80 days prior to the mixed strain challenge as described above by use of the same protocol and numbers of each strain in the postdrug treatment challenge. Error bars represent the standard deviations between two mice. No error bars are shown in panel f because no DNA from the homologous strain was found in any of the samples.
A single round of infection with either AS or CB and cure (1×-immunized mice) induced immunity which greatly reduced the absolute parasitemias in challenge blood infections with a mixture of AS and CB relative to those in nonimmune mice (cf. Fig. 1a with Fig. 2a and d). The second round of infection and cure (2×-immunized mice) reduced the parasitemias in the mixed challenge infections even further (Fig. 2a and d). In the 1×-immunized mice, over the 4 days in which the parasite strains were measured by RTQ-PCR, the levels of immunity were effective to similar degrees against both strains in the infection and, therefore, no indication of strain-specific immunity was observed (cf. Fig. 1c with Fig. 2b and e). By contrast, in the 2×-immunized mice, the strain homologous to the immunizing strain was preferentially removed or eliminated in the mixed strain challenge infections (cf. Fig. 1c with Fig. 2c and f).
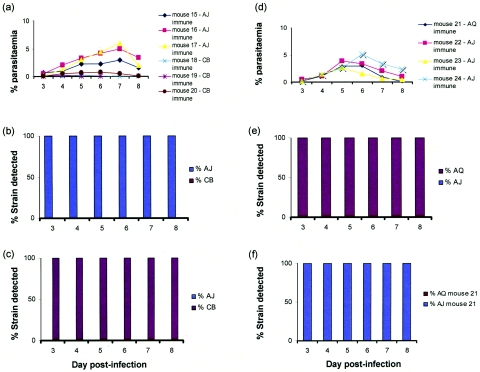
Equivalent experiments were conducted with 2×-immunized mice by using the combinations of strains AJ and CB and strains AJ and AQ (Fig. 3). Similarly to the experiment with AS and CB, the strain homologous to the immunizing strain was preferentially eliminated in the mixed strain challenge infections (cf. Fig. 1b and d with Fig. 3b, c, e, and f).
FIG. 3.
Quantitative PCR analysis of genetically mixed strain infections of P. c. chabaudi in immune mice. (a) Percentages of parasitemia on a log scale for groups of AJ- or CB-immunized mice. The infection in mouse 18 resolved to subpatent levels by day 7 and could not be used in the analyses after that day. (b and c) Proportions of each strain of P. c. chabaudi, measured by RTQ-PCR, in three mice immunized with (b) CB or (c) AJ by two successive drug-treated bouts of parasitemia with the homologous strain. The mixed strain challenge was administered 43 days later for AJ-immunized mice or 45 days later for CB-immunized mice and comprised approximately equal proportions of homologous and heterologous strains amounting to a total of 5 × 106 parasites per inoculum (see Materials and Methods). No error bars are shown for the RTQ-PCR measurements as no DNA derived from the homologous strain was found in any of the samples analyzed over the 6 days where the parasitemia of the mice was high enough to be able to extract sufficient genomic DNA for quantitative analysis. The data shown in panel b comprise results from three mice for days 3 to 6 (mice 18, 19, and 20) and from two mice for days 7 and 8 (mice 18 and 20). (e and f) Proportions of each strain were measured by RTQ-PCR (another experiment) for (e) three mice made immune to AJ or (f) one mouse made immune to AQ (mouse number 21) by two successive drug-treated bouts of parasitemia with the homologous strain administered 43 days later for AJ or 45 days later for AQ. The mixed strain challenge comprised 5 × 106 parasites, approximately half of which were the homologous strain and half the heterologous strain (see Materials and Methods). (d) Parasitemias for the mice described for panels e and f, monitored over the period when the mice were parasitemic.
We had previously tested for strain-specific protective immunity the combinations of P. c. chabaudi strains AS and AJ and CB and AJ in AJ-immune mice and AS and AQ and CB and AQ in AQ-immune mice by using a strain-specific electrophoretic mobility enzyme marker (lactate dehydrogenase) (3) to detect the presence of each strain in the mixed infections (data not shown). In each of these combinations, the strain in the mixed challenge infection which was homologous to the immunizing strain was eliminated while the heterologous strain survived.
The results of all of the experiments on strain-specific immunity in mixed infections reported here are summarized in Table 1.
TABLE 1.
Summary of P. c. chabaudi strain-specific immunity experiments with mice
| Heterologous strain in mixed challenge infectiona | Test(s)b for immunizing strain:
|
|||
|---|---|---|---|---|
| AS | AJ | AQ | CB | |
| AS | EE | EE | RTQ-PCR, EE | |
| AJ | NT | RTQ-PCR | RTQ-PCR | |
| AQ | NT | RTQ-PCR | NT | |
| CB | RTQ-PCR, EE | RTQ-PCR, EE | EE | |
Combinations of genetically distinct cloned lines of P. c. chabaudi which have been tested for evidence of strain-specific immunity in mixed infections of two strains, one of which is the same as (homologous) and the other different from (heterologous) the strain used to induce immunity in female CBA mice.
Differential elimination of homologous (immunizing) strain from mixed challenge infection in strain-specifically-immunized mice, as measured by RTQ-PCR or by enzyme electrophoresis (EE). NT, combination not tested.
DISCUSSION
The experiments described here were designed to study how cloned lines of P. c. chabaudi from different isolation events from the wild and known to be genetically distinct from each other (3) interact in mixed strain infections (i) in naïve host animals, i.e., those that have not previously experienced a malarial infection and (ii) in animals that had acquired by natural infection(s) various levels of immunity to a single strain of parasite. We found that in nonimmune mice coinfected with two genetically distinct strains of parasite, the proportions of the two strains remained approximately constant during the period of measurement within the acute primary infection. This behavior was observed to occur in all three groups of mice infected with the following strains: AJ and CB, AJ and AQ, and AS and CB. The parasite strain which has previously been shown to have a particularly high growth rate and virulence, CB (data not shown), retains this high-growth-rate characteristic in coinfections with either strain AS or strain AJ (Fig. 1).
We also investigated the effects in mice of P. c. chabaudi infection-induced immunity against blood-stage challenge infections containing a mixture of the immunizing strain and a genetically heterologous strain of the parasites. In mice immunized by a single round of infection of either strain AS or strain CB of P. c. chabaudi, the immunity against a blood-stage challenge infection reduced the numbers of parasites of both homologous and heterologous strains in the mixed infections to similar degrees (Fig. 2). We found no evidence, therefore, that the immunity induced by a single immunizing infection was strain specific.
However, following two rounds of infection and drug cure with AS or CB, the parasites of the immunizing strain in the mixed strain challenge infections were reduced to a much greater extent than were the parasites of the heterologous (nonimmunizing) strain. Similar results were obtained for mice immunized by two rounds of infection and drug cure by use of the combinations of P. c. chabaudi strains AJ and CB and strains AJ and AQ (Fig. 3). Moreover, previously unpublished data from this laboratory, obtained using a strain-specific semiquantitative isozyme marker, showed that the same schedule of immunization induced homologous strain-specific preferential elimination of parasites in the combinations of strains AS and AJ, AS and AQ, and CB and AQ (data not shown), in addition to the combination AS and CB as shown in the present study. Thus, in every combination of these parasites of African rodents from a single locality where malaria is endemic which we have tested, a total of six such combinations, clone- or strain-specific protective immunity has been shown to occur between the parasites.
It is noteworthy that in addition to inducing strong strain-specific immunity, two rounds of immunization and drug cure also increased protection against both homologous and heterologous strains. Thus, strain-transcending immunity is induced and boosted by the first and second infections, respectively, whereas the strain-specific element of this immunity appears only after two infections. It would be of great interest to uncover the mechanisms involved in the induction of both types of immunity.
Recently, Elliott et al. (8) showed that subpatent infections with P. c. chabaudi induced an immunity against the blood stages that was not parasite strain specific. In this study, we show that protective strain-specific immunity against the blood stages develops after two rounds of immunization and drug cure of patent infections. In all strain combinations tested, the strain-specific component of the protective immunity almost completely eliminates, within the limits of detection of the PCR assay that was used (>0.05% parasitemia), the homologous but not the heterologous parasites in a challenge infection by the third day after challenge. The speed with which the host cleared the homologous parasites in these previously twice-infected mice could indicate the possible involvement of an antibody-dependent immune response; to elucidate the immune effector mechanisms involved in the specificity of this response is now a priority for future studies.
In these investigations, some cloned strains of P. c. chabaudi were more effective than others in inducing strong cross-strain protective immunity. Thus, of the mice twice infected with strain AQ (Fig. 3f), only one of eight produced parasites detectable on blood smears following mixed challenge with homologous and heterologous cloned strains.
We also noted that infection-induced immunity, even after two rounds of infection and drug cure, against the AS strain was not sufficient to make the host completely refractory to reinfection with that strain (Fig. 2c). This result contrasts with those for CB-, AJ-, and AQ-immunized mice, which become highly immune to these strains following two rounds of infection-induced immunity (Fig. 2f and Fig. 3b, c, e, and f). Several possibilities exist that might account for this. AS is a relatively slow-growing strain compared to the other strains used in this study (unpublished data). It is possible that the immune system of the host might respond differently to infection with slow- or fast-growing strains of parasite. Another possibility is that clonal antigenic switching of variant surface antigens may have occurred, as reported previously (1, 14). Antigenic switching, possibly mediated by P. chabaudi cir genes (10), would enable a small number of parasites to survive strong immunity targeted against other antigens that do not switch. If such a mechanism occurred in the AS-immune mice tested here, the RTQ-PCR assay would not detect this, as it is based on the measurement of a strain-specific marker, the polymorphic gene msp-1.
The phenomenon of “strain-specific” immunity between P. c. chabaudi lines AS and CB was originally demonstrated by Jarra and Brown (11) in a protocol in which parasites of one or the other strains were used for challenge. Only recently, by the use of quantitative PCR, has it been possible to monitor how immunity operates in mixed strain infections. By use of this method, our present experiments demonstrate that the strain specificity of an immune response also operates when parasites of different genetic types are simultaneously present in the blood of an immune animal. Our results have also shown that such immune strain specificity exists widely, perhaps almost universally, between malaria parasites of the same species from a single local population in areas where malaria is endemic.
Acknowledgments
We thank Les Steven for technical assistance and Jacobus de Roode for supplying calibrated parasite mixtures. We also thank Richard Culleton and Katrina Grech for critical reading of the manuscript and Sittiporn Pattaradilokrat for help with the figures.
This work was funded by the Wellcome Trust (grant 005743) and the Medical Research Council (grant G9603694PB).
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Brannan, L. R., C. M. Turner, and R. S. Phillips. 1994. Malaria parasites undergo antigenic variation at high rates in vivo. Proc. R. Soc. Lond. B 256:71-75. [DOI] [PubMed] [Google Scholar]
- 2.Cadigan, F. C., and V. Chiacumpa. 1969. Plasmodium falciparum in the white-handed gibbon: protection afforded by previous infection with homologous and heterologous strains obtained in Thailand. Mil. Med. 134:1135-1139. [PubMed] [Google Scholar]
- 3.Carter, R. 1978. Studies on enzyme variation in the murine malaria parasites Plasmodium berghei, P. yoelii, P. vinkei and P. chabaudi by starch gel electrophoresis. Parasitology 76:241-267. [DOI] [PubMed] [Google Scholar]
- 4.Cheesman, S., J. de Roode, A. Read, and R. Carter. 2003. Real-time quantitative PCR for analysis of genetically mixed infections in malaria parasites: technique validation and applications. Mol. Biol. Parasitol. 131:83-91. [DOI] [PubMed] [Google Scholar]
- 5.Ciuca, M., L. Ballif, and M. Chelarescu-Vieru. 1934. Immunity in malaria. Trans. R. Soc. Trop. Med. Hyg. 27:619-622. [Google Scholar]
- 6.Crewther, P. E., M. L. Matthew, R. H. Flegg, and R. F. Anders. 1996. Protective immune responses to apical membrane antigen 1 of Plasmodium chabaudi involve recognition of strain-specific epitopes. Infect. Immun. 64:3310-3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desjardins, R. E., C. L. Pamplin, J. von Bredow, K. G. Barry, and C. J. Canfield. 1987. Kinetics of a new antimalarial, mefloquine, p. 262-263. In W. Peters (ed.), Chemotherapy and drug resistance in malaria, 2nd ed., vol. 1. Academic Press, Oxford, United Kingdom. [Google Scholar]
- 8.Elliott, S. R., R. D. Kuns, and M. F. Good. 2005. Heterologous immunity in the absence of variant-specific antibodies after exposure to subpatent infection with blood-stage malaria. Infect. Immun. 73:2478-2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Genton, B., I. Betuela, I. Felger, F. Al-Yaman, R. F. Anders, A. Saul, L. Rare, M. Baisor, K. Lorry, G. V. Brown, D. Pye, D. O. Irving, T. A. Smith, H.-P. Beck, and M. P. Alpers. 2002. A recombinant blood-stage malaria vaccine reduces Plasmodium falciparum density and exerts selective pressure on parasite populations in a phase 1-2b trial in Papua New Guinea. J. Infect. Dis. 185:820-827. [DOI] [PubMed] [Google Scholar]
- 10.Janssen, C. S., M. P. Barrett, C. M. Turner, and R. S. Phillips. 2002. A large family for putative variant antigens shared by human and rodent malaria parasites. Proc. R. Soc. Lond. B 269:431-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jarra, W., and K. Brown. 1985. Protective immunity to malaria: studies with cloned lines of Plasmodium chabaudi and P. berghei in CBA/Ca mice. I. The effectiveness and inter- and intra-species specificity of immunity induced by infection. Parasite Immunol. 7:595-606. [DOI] [PubMed] [Google Scholar]
- 12.Jeffrey, G. M. 1966. Epidemiological significance of repeated infections with homologous and heterologous strains and species of Plasmodium. Bull. W. H. O. 35:873-882. [PMC free article] [PubMed] [Google Scholar]
- 13.Killick-Kendrick, R., and W. Peters (ed.). 1978. Rodent malaria, p. 218-219. Academic Press, London, United Kingdom.
- 14.Phillips, R., L. Brannan, P. Balmer, and P. Neuville. 1997. Antigenic variation during malaria infection—the contribution from the murine parasite Plasmodium chabaudi. Parasite Immunol. 9:427-434. [DOI] [PubMed] [Google Scholar]
- 15.Powell, R. D., J. V. McNamara, and K. H. Rieckman. 1972. Clinical aspects of acquisition of immunity to Falciparum malaria. Proc. Helminthol. Soc. Wash. 39:51-68. [Google Scholar]
- 16.Renia, L., I. T. Ling, M. Marussig, F. Miltgen, A. A. Holder, and D. Mazier. 1997. Immunization with a recombinant C-terminal fragment of Plasmodium yoelii merozoite surface protein 1 protects mice against homologous but not heterologous P. yoelii sporozoite challenge. Infect. Immun. 65:4419-4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rotman, H. L., T. M. Daly, and C. A. Long. 1999. Plasmodium: immunization with carboxyl-terminal regions of MSP-1 protects against homologous but not heterologous blood-stage parasite challenge. Exp. Parasitol. 91:78-85. [DOI] [PubMed] [Google Scholar]