Abstract
Streptococcus pneumoniae is the leading cause of otitis media, sinusitis, and pneumonia. Many of these infections result from antecedent influenza virus infections. In this study we sought to determine whether the frequency and character of secondary pneumococcal infections differed depending on the strain of influenza virus that preceded bacterial challenge. In young ferrets infected with influenza virus and then challenged with pneumococcus, influenza viruses of any subtype increased bacterial colonization of the nasopharynx. Nine out of 10 ferrets infected with H3N2 subtype influenza A viruses developed either sinusitis or otitis media, while only 1 out of 11 ferrets infected with either an H1N1 influenza A virus or an influenza B virus did so. These data may partially explain why bacterial complication rates are higher during seasons when H3N2 viruses predominate. This animal model will be useful for further study of the mechanisms that underlie viral-bacterial synergism.
Influenza virus infection predisposes children to secondary bacterial infections such as sinusitis, otitis media, and pneumonia (16, 18, 26, 28). Acute otitis media is the most frequently occurring complication of influenza, and it develops in 40% of children younger than 3 years of age (16). Streptococcus pneumoniae is the most common cause of both community-acquired pneumonia and otitis media (6, 14) and is the bacterial pathogen most commonly associated with secondary infections following influenza (13, 15, 22). Although targeting the pneumococcus with a heptavalent conjugate vaccine has been very successful in reducing invasive disease (1), the impact on otitis media has not been as satisfactory (7). The morbidity in affected children, sequelae of recurrent disease, and economic burden of otitis media remain substantial in both developing and developed countries (17, 24). Sinusitis has classically been considered to be a bacterial disease in adults and children, with pneumococcus as one of the leading etiologic agents (12). However, recovery of viruses from sinus aspirates both alone and in conjunction with bacteria suggests a role for viruses in the pathogenesis of bacterial sinusitis (8, 13). Definitive studies on both the clinical and research levels need to be done.
Influenza viruses are classified into three types, i.e., influenza A, B, and C viruses. Influenza A viruses are further subtyped based on two surface glycoproteins, the hemagglutinin (HA) and neuraminidase (NA). Viruses with an H1 HA and an N1 NA (subtype H1N1 viruses) entered humans in 1918 and circulated within the human population between 1918 and 1957 and from 1976 to the present. A new strain created by reassortment with an avian virus began circulating in 1957 and carried an H2 HA and an N2 NA (subtype H2N2). This strain was itself replaced in 1968 with a strain carrying a new H3 HA but the same N2 NA (subtype H3N2). These H3N2 viruses have circulated in humans since 1968 (for a review, see reference 37). Influenza B viruses are not subtyped and do not have an animal reservoir. Influenza C viruses rarely cause significant disease (23). Epidemiologic studies have shown a difference in the complication rates for different influenza virus types and subtypes. In general, H3N2 strains are more virulent (3, 38) and are associated with more complications than H1N1 or influenza B viruses (34-36).
In this study we sought support for our hypothesis that differences in viral strains account for differences in bacterial complications. Because very few influenza viruses are mouse adapted, we had to use an alternate animal model to investigate secondary bacterial infections following infection with naturally occurring influenza viruses. We had previously demonstrated that pneumococcus could productively colonize adult ferrets and that influenza virus enhanced this colonization (31). Adapting this model to study sinusitis and otitis media, we tested the ability of representative strains of influenza B virus and the H3N2 and H1N1 subtypes of influenza A virus to contribute to secondary infections in weanling ferrets.
MATERIALS AND METHODS
Infectious agenst.
A.166 lux, a type 3 encapsulated strain that had been transformed with the lux operon (9), was used in the ferret studies and was grown on tryptic soy agar plates supplemented with 3% (vol/vol) sheep erythrocytes. Influenza viruses from the St. Jude Children's Research Hospital influenza repository were used in the study. The influenza viruses studied were H1N1 virus A/Taiwan/1/86, H3N2 viruses A/Sydney/5/97 and A/Fujian/411/02, and influenza B virus B/Singapore/222/79. Virus stocks were grown in Madin-Darby canine kidney (MDCK) cells, clarified by centrifugation at 2,000 rpm for 10 min, pelleted by centrifugation at 24,000 × g for 2.5 h, and resuspended in phosphate-buffered saline (PBS).
Ferrets and infection model.
Ferrets serologically negative for influenza virus were obtained from Marshall Farms (North Rose, NY) and bred in the Animal Resource Center at St. Jude Children's Research Hospital. The offspring were weaned at the age of 6 weeks and used in the study at the age of 7 weeks. Infectious agents were administered intranasally in a volume of 400 μl (200 μl/nostril) of PBS to ferrets under general anesthesia with inhaled isoflurane (3.5%). Ferrets were infected with either influenza virus or PBS and then challenged 5 days later with either pneumococcus strain A.166 lux or PBS. Clinical signs of infection were monitored, ferrets were imaged, and nasal wash samples were collected daily after the secondary challenge with bacteria or PBS. For nasal wash samples, 1 ml of PBS was injected into each nostril of ferrets sedated with ketamine intramuscularly at 100 mg/kg of body weight, and expelled samples were collected. Nasal washes were titrated for influenza virus in MDCK cells and for pneumococcal colony counts on tryptic soy agar plates supplemented with 3% (vol/vol) sheep erythrocytes. Ferrets were euthanized 4 days after the pneumococcal or PBS challenge. The lungs and heads of infected animals were removed, fixed overnight in 10% neutral buffered formalin, embedded in paraffin, sectioned at 5 μm, stained with hematoxylin-eosin, and examined microscopically for histopathologic alterations by an experienced veterinary pathologist (K.L.B. or J.E.R.) who was blinded to the composition of the groups. All animal experiments were approved by the St. Jude Children's Research Hospital Animal Care and Use Committee and were performed under biosafety level 2 conditions.
Imaging of live ferrets.
Anesthetized ferrets were imaged for 2 min with an IVIS charge-coupled-device camera (Xenogen Corp.). The total photon emission from selected and defined areas within the images of each animal was quantified with the LivingImage software package version 2.20 (Xenogen Corp.), as described elsewhere (9).
Statistical analysis.
Comparisons between the numbers of bacteria or viruses recovered from nasal washes were done using repeated-measures analysis of variance (Holm-Sidak method). SigmaStat for Windows (SysStat Software, Inc., V 3.11) was utilized for all statistical analyses. A P value of <0.05 was considered significant for these comparisons.
RESULTS
Nasal wash titers from infected ferrets.
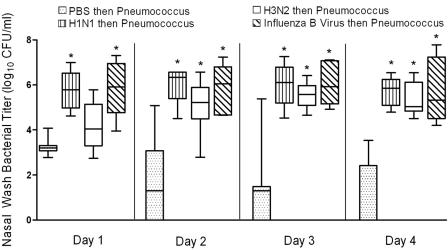
To test the hypothesis that differences in influenza virus strain could correlate with differences in secondary bacterial infections, we used 7-week-old weanling ferrets. Ferrets were infected with influenza virus and then challenged 5 days later with pneumococcus. Four representative viruses were used, i.e., A/Taiwan/1/86 (H1N1), A/Fujian/411/02 (H3N2), A/Sydney/5/97 (H3N2), and B/Singapore/222/79. Experiments with A/Sydney/5/97 followed experiments with the other three viruses and were done to confirm the striking phenotype seen with A/Fujian/411/02 using a second virus of the H3N2 subtype. Because results were similar for these two viruses, the data are considered together. All four viruses increased the nasal wash bacterial titers by several log units within 48 h of secondary challenge compared to results for ferrets mock infected with PBS prior to pneumococcal challenge (Fig. 1). No differences in bacterial titers were seen between groups of virus-infected ferrets. The large variation seen in bacterial titers from mock-infected animals on days 2 to 4 were because two, three, and four animals had no bacteria recovered on days 2, 3, and 4, respectively.
FIG. 1.
Nasal wash bacterial titers. Groups of 5 to 10 ferrets mock infected with PBS or infected with influenza virus A/Fujian/411/02 (H3N2), A/Sydney/5/97 (H3N2), A/Taiwan/1/86 (H1N1), or B/Singapore/222/79 were challenged with pneumococcus strain A.166 5 days later, and daily nasal washes were taken. The 25th to 75th percentiles of measurements collected on four consecutive days are represented by the shaded box plots, with the horizontal bar indicating the median value. Error bars indicate the standard deviation of the measurements. An asterisk indicates a significant difference (P < 0.05) compared to mock-infected animals.
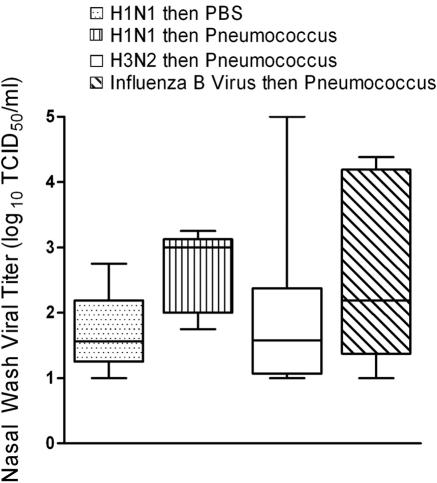
Nasal wash viral titers were not significantly higher 24 h after bacterial challenge in dually infected animals than in ferrets infected with influenza virus but mock challenged with PBS at day 5 (Fig. 2). Viral titers decreased in all groups by 48 h after challenge and were negative in all animals by 96 h after pneumococcal infection (data not shown). Bacterial lung titers were positive in 3/5 ferrets infected with A/Taiwan/1/86 (H1N1), in 5/5 infected with A/Fujian/411/02 (H3N2), in 5/6 infected with B/Singapore/222/79, and in 0/6 mock infected with PBS before bacterial challenge. All viral lung titers were negative at necropsy (9 days after initial infection with influenza virus). Lung titers were not determined for A/Sydney/5/97-infected animals.
FIG. 2.
Nasal wash viral titers. Groups of 5 to 10 ferrets mock infected with PBS or infected with influenza virus A/Fujian/411/02 (H3N2), A/Sydney/5/97 (H3N2), A/Taiwan/1/86 (H1N1), or B/Singapore/222/79 were challenged with pneumococcus strain A.166 5 days later, and daily nasal washes were taken. The 25th to 75th percentiles of measurements taken the first day after bacterial challenge are represented by the shaded box plots, with the horizontal bar indicating the median value. Error bars indicate the standard deviation of the measurements. TCID50, 50% tissue culture infective dose.
Secondary bacterial infections in young ferrets.
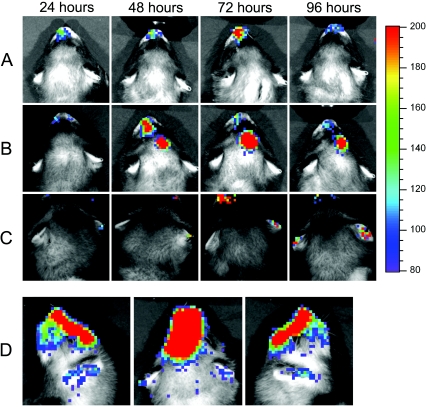
Ferrets were imaged daily for detection of bioluminescence indicative of a focal bacterial infection. All ferrets given pneumococcus developed evidence of nasal colonization by imaging (Fig. 3A). Four of five ferrets infected with A/Fujian/411/02 (H3N2) developed secondary infections, one sinusitis (Fig. 3B), and three otitis media (Fig. 3C). By comparison, only one of five ferrets infected with A/Taiwan/1/86 (H1N1) developed sinusitis, and none of the six ferrets infected with B/Singapore/222/79 developed secondary infections. In the second experiment with another H3N2 subtype virus, A/Sydney/5/97, five out of five animals had evidence of secondary bacterial infections (all five had sinusitis, and two also had otitis media). Bioluminescence was not detected from the thorax of any animal. It is unclear whether this is due to the thickness of the chest wall preventing detection or because no animals had a significant concentration of bacteria in their lungs.
FIG. 3.
Secondary bacterial infections in ferrets. Ferrets were infected with influenza virus, followed 5 days later by a bioluminescent variant of pneumococcus strain A.166. Daily imaging of live, anesthetized ferrets allowed visualization of secondary bacterial infections. Representative examples from single animals of A) nasopharyngeal colonization, B) sinusitis, and C) otitis media are pictured. The color bar on the right indicates the number of relative light units per pixel measured over 2 min. A posterio-anterior view and two lateral views of a ferret with concomitant sinusitis and otitis media are shown in D).
Histopathology.
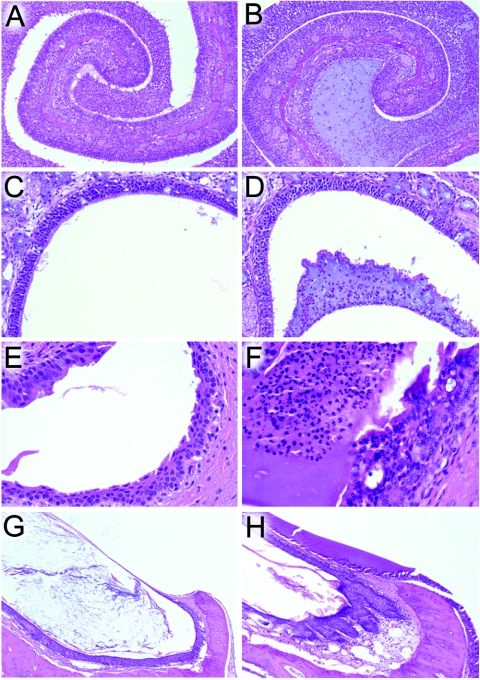
On examination after necropsy, mucopurulent rhinitis was present in all animals infected with both influenza virus and pneumococcus (Fig. 4A and B). Other histopathologic abnormalities of the upper respiratory tract were found only in animals with evidence of secondary bacterial infections on imaging. In animals with visual evidence of sinusitis, an exudate composed of mucus admixed with viable and degenerate neutrophils and a few macrophages filled the frontal sinus (Fig. 4D). Transmigration of neutrophils across the sinus mucosa was observed, and there was an absence of mucosal necrosis or erosion. Bilaterally, the eustachian tubes were filled with copious amounts of the same mucopurulent material found in the sinuses, but with focal erosion of the respiratory mucosa (Fig. 4F). Additionally, there was bilateral extension of the exudate into the bony labyrinth of the middle ear (Fig. 4H). The tympanic membranes were thickened, and the inner surfaces were coated by a film of neutrophils, macrophages, and proteinaceous fluid. The sinuses, eustachian tubes, and middle ears of animals which did not develop visual evidence of secondary infections were essentially normal (Fig. 4C, E, and G).
FIG. 4.
Histopathology of upper respiratory tract complications. Representative views of the nasal cavity (A and B) (magnification, ×10), frontal sinus (C and D) (magnification, ×20), eustachian tube (E and F) (magnification, ×40), and middle ear (G and H) (magnification, ×10) are shown for a ferret infected with A/Taiwan/1/86 (H1N1) which did not develop secondary bacterial complications (A, C, E, and G) and for a ferret infected with A/Sydney/5/97 (H3N2) which had both otitis media and sinusitis on imaging (B, D, F, H).
The lungs of the groups infected with virus and bacteria were histologically similar and undistinguishable from those of the viral and bacterial group controls mock infected with PBS. Some ferrets in each group had a patchy distribution of mononuclear cells within the alveoli and a mild increase in cellularity within the interstitial septae, but no frank pneumonic process was evident in any animal.
DISCUSSION
Influenza viruses are not natural pathogens of mice. Therefore, we sought to develop a nonmouse model to study secondary bacterial superinfections using unmanipulated influenza viruses. We had previously demonstrated that pneumococcus could colonize the nasopharynxes of adult ferrets, a model animal frequently used for influenza studies (31). In this study we used young ferrets and a bioluminescent pneumococcus in an attempt to characterize potential secondary bacterial infections. Young ferrets infected with influenza virus had higher titers of bacteria in the nasal wash than mock-infected animals and had a distinct rhinitis, but no differences were seen in the effect of different viruses on bacterial titers or on the histopathology of the nose, indicating that bacterial colonization and pathology at that site did not differ. When less accessible sites were examined using the live imaging system, H3N2 viruses supported development of sinusitis or otitis media in 90% of the animals, compared to in only 17% following an H1N1 virus infection and 0% following an influenza B virus infection. The model shares characteristics with the chinchilla model of viral-bacterial coinfection (11, 25) and should be a useful tool in further exploring the mechanisms that underlie the development of otitis media and sinusitis.
Upon introduction of H1N1 viruses into humans in 1918, exceptionally high mortality was seen during the first 2 years that this subtype circulated, primarily due to bacterial pneumonia (27, 32). Thereafter, the overall mortality rate declined, but excess mortality continued to be seen (4, 5). Since H1N1 viruses reentered the population in 1976, however, very little excess mortality has occurred during years where H1N1 viruses were the predominant circulating strain, and the relative contribution of bacterial superinfections to excess mortality has declined (33, 36). Similarly, influenza B viruses, which caused notable excess mortality early in the 20th century (4), have contributed very little to excess mortality in the past several decades (33, 36). Since their introduction in 1968, H3N2 viruses have typically caused more excess mortality than either H1N1 or influenza B viruses. The differences in secondary complications reported here in the young ferret model, comparing H3N2 viruses to H1N1 or influenza B viruses, support the concept that these observed differences in epidemiology have a virologic basis.
Although the mechanisms underlying the synergistic interaction between influenza viruses and pneumococcus are poorly understood, it is clearly a multifactorial process (21). It is likely that multiple viral virulence factors contribute by affecting the host in ways that benefit the bacteria. Differences in these virulence factors may account for differences in bacterial complication rates seen between different strains. We have characterized one such virulence factor, the NA (29). NA activity of the virus enhanced adherence of pneumococcus to epithelial cells in vitro and predisposed to fatal pneumonia in a mouse model (20). The secondary bacterial pneumonia could be prevented or treated using an inhibitor of the influenza virus NA, independent of the effect on viral lung load (19, 20). Pairs of otherwise isogenic viruses engineered by reverse genetics to express different N2 NAs differentially contributed to both adherence and pneumonia at a rate proportional to their NA activity (30). N2 subtype viruses in general have higher NA activities than recently circulating N1 or influenza B virus strains, which may account for some of the differences in excess mortality seen when comparing H2N2 or H3N2 seasons to seasons where H1N1 or influenza B viruses predominate. The two seasons with the highest excess mortality in the last 50 years are the 1957 to 1958 and 1997 to 1998 seasons (33, 36), during which the two viruses with the highest NA activities circulated (30).
Another intriguing possibility is that the newly recognized PB1-F2 protein plays a role in priming the host for secondary bacterial infections. PB1-F2 is a short protein encoded in the +1 reading frame of the PB1 gene and containing an amphipathic helical domain and a mitochondrial targeting sequence that are involved in induction of cell death (2, 10). Influenza B viruses do not express this protein, and H1N1 viruses circulating after 1956 have a predicted truncation in the protein which would eliminate the C-terminal region encoding the mitochondrial targeting sequence and helical domain. One could speculate that any effects on virulence mediated by PB1-F2 might aid in priming the host for secondary bacterial infections, and the presence of the full-length protein in H3N2 viruses would enhance their support for such infections relative to H1N1 or influenza B viruses. In addition to the possibilities discussed here, it is likely that as-yet-unappreciated strain-specific differences in other viral virulence factors also contribute to the differences in the rates of complications observed with different strains of influenza virus.
In summary, we have developed a young ferret model of secondary bacterial otitis and sinusitis to address the economically important question of how antecedent respiratory virus infections prime the host for pyogenic infections with pneumococcus at normally sterile sites. Using this model, we are able to reproduce experimentally the epidemiologic observation that H3N2 subtype viruses are better partners with pneumococcus in the induction of these superinfections than are other influenza viruses. We speculate that strain-specific differences in viral virulence factors account for this finding, and we discuss some of the virulence factors that may be involved. A corollary of this hypothesis is that strain-specific differences in S. pneumoniae may account for differences in the invasiveness of different pneumococcal strains. These differences may achieve prominence in the setting of antecedent viral infection.
Acknowledgments
This work was supported by NIH grants AI-49178 and AI-54802 and by the American Lebanese Syrian Associated Charities (ALSAC). V.T.P. was supported by the Academy of Finland and the Pediatric Research Foundation of Finland.
Editor: J. N. Weiser
REFERENCES
- 1.Black, S., H. Shinefield, B. Fireman, E. Lewis, P. Ray, J. R. Hansen, L. Elvin, K. M. Ensor, J. Hackell, G. Siber, F. Malinoski, D. Madore, I. Chang, R. Kohberger, W. Watson, R. Austrian, K. Edwards, et al. 2000. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr. Infect. Dis. J. 19:187-195. [DOI] [PubMed] [Google Scholar]
- 2.Chen, W., P. A. Calvo, D. Malide, J. Gibbs, U. Schubert, I. Bacik, S. Basta, R. O'Neill, J. Schickli, P. Palese, P. Henklein, J. R. Bennink, and J. W. Yewdell. 2001. A novel influenza A virus mitochondrial protein that induces cell death. Nat. Med. 7:1306-1312. [DOI] [PubMed] [Google Scholar]
- 3.Coates, D. M., C. Sweet, and H. Smith. 1986. Severity of fever in influenza: differential pyrogenicity in ferrets exhibited by H1N1 and H3N2 strains of differing virulence. J. Gen. Virol. 67:419-425. [DOI] [PubMed] [Google Scholar]
- 4.Collins, S. D. 1945. Influenza and pneumonia excess mortality at specific ages in the epidemic of 1943-44, with comparative data for preceding epidemics. Public Health Rep. 60:821-835. [Google Scholar]
- 5.Collins, S. D., and J. Lehmann. 1951. Trends and epidemics of influenza and pneumonia: 1918-1951. Public Health Rep. 66:1487-1516. [PMC free article] [PubMed] [Google Scholar]
- 6.Dowell, S. F., J. C. Butler, G. S. Giebink, M. R. Jacobs, D. Jernigan, D. M. Musher, A. Rakowsky, and B. Schwartz. 1999. Acute otitis media: management and surveillance in an era of pneumococcal resistance—a report from the Drug-Resistant Streptococcus pneumoniae Therapeutic Working Group. Pediatr. Infect. Dis. J. 18:1-9. [PubMed] [Google Scholar]
- 7.Eskola, J., T. Kilpi, A. Palmu, J. Jokinen, J. Haapakoski, E. Herva, A. Takala, H. Kayhty, P. Karma, R. Kohberger, G. Siber, and P. H. Makela. 2001. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N. Engl. J. Med. 344:403-409. [DOI] [PubMed] [Google Scholar]
- 8.Evans, F. O., Jr., J. B. Sydnor, W. E. Moore, G. R. Moore, J. L. Manwaring, A. H. Brill, R. T. Jackson, S. Hanna, J. S. Skaar, L. V. Holdeman, S. Fitz-Hugh, M. A. Sande, and J. M. Gwaltney, Jr. 1975. Sinusitis of the maxillary antrum. N. Engl. J. Med. 293:735-739. [DOI] [PubMed] [Google Scholar]
- 9.Francis, K. P., J. Yu, C. Bellinger-Kawahara, D. Joh, M. J. Hawkinson, G. Xiao, T. F. Purchio, M. G. Caparon, M. Lipsitch, and P. R. Contag. 2001. Visualizing pneumococcal infections in the lungs of live mice using bioluminescent Streptococcus pneumoniae transformed with a novel gram-positive lux transposon. Infect. Immun. 69:3350-3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibbs, J. S., D. Malide, F. Hornung, J. R. Bennink, and J. W. Yewdell. 2003. The influenza A virus PB1-F2 protein targets the inner mitochondrial membrane via a predicted basic amphipathic helix that disrupts mitochondrial function. J. Virol. 77:7214-7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giebink, G. S. 1999. Otitis media: the chinchilla model. Microb. Drug Resist. 5:57-72. [DOI] [PubMed] [Google Scholar]
- 12.Gwaltney, J. M. 1995. Sinusitis, p. 585-590. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Mandell, Douglas and Bennett's principles and practices of infectious diseases. Churchill Livingstone, Inc., New York, N.Y.
- 13.Hamory, B. H., M. A. Sande, A. Sydnor, Jr., D. L. Seale, and J. M. Gwaltney, Jr. 1979. Etiology and antimicrobial therapy of acute maxillary sinusitis. J. Infect. Dis. 139:197-202. [DOI] [PubMed] [Google Scholar]
- 14.Heffelfinger, J. D., S. F. Dowell, J. H. Jorgensen, K. P. Klugman, L. R. Mabry, D. M. Musher, J. F. Plouffe, A. Rakowsky, A. Schuchat, and C. G. Whitney. 2000. Management of community-acquired pneumonia in the era of pneumococcal resistance: a report from the Drug-Resistant Streptococcus pneumoniae Therapeutic Working Group. Arch. Intern. Med. 160:1399-1408. [DOI] [PubMed] [Google Scholar]
- 15.Heikkinen, T., and T. Chonmaitree. 2003. Importance of respiratory viruses in acute otitis media. Clin. Microbiol. Rev. 16:230-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heikkinen, T., H. Silvennoinen, V. Peltola, T. Ziegler, R. Vainionpaa, T. Vuorinen, L. Kainulainen, T. Puhakka, T. Jartti, P. Toikka, P. Lehtinen, T. Routi, and T. Juven. 2004. Burden of influenza in children in the community. J. Infect. Dis. 190:1369-1373. [DOI] [PubMed] [Google Scholar]
- 17.Klein, J. O. 2000. The burden of otitis media. Vaccine 19(Suppl. 1):S2-S8. [DOI] [PubMed] [Google Scholar]
- 18.Madhi, S. A., and K. P. Klugman. 2004. A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat. Med. 10:811-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCullers, J. A. 2004. Effect of antiviral treatment on the outcome of secondary bacterial pneumonia after influenza. J. Infect. Dis. 190:519-526. [DOI] [PubMed] [Google Scholar]
- 20.McCullers, J. A., and K. C. Bartmess. 2003. Role of neuraminidase in lethal synergism between influenza virus and Streptococcus pneumoniae. J. Infect. Dis. 187:1000-1009. [DOI] [PubMed] [Google Scholar]
- 21.McCullers, J. A., and J. E. Rehg. 2002. Lethal synergism between influenza virus and Streptococcus pneumoniae: characterization of a mouse model and the role of platelet-activating factor receptor. J. Infect. Dis. 186:341-350. [DOI] [PubMed] [Google Scholar]
- 22.Michelow, I. C., K. Olsen, J. Lozano, N. K. Rollins, L. B. Duffy, T. Ziegler, J. Kauppila, M. Leinonen, and G. H. McCracken, Jr. 2004. Epidemiology and clinical characteristics of community-acquired pneumonia in hospitalized children. Pediatrics 113:701-707. [DOI] [PubMed] [Google Scholar]
- 23.Moriuchi, H., N. Katsushima, H. Nishimura, K. Nakamura, and Y. Numazaki. 1991. Community-acquired influenza C virus infection in children. J. Pediatr. 118:235-238. [DOI] [PubMed] [Google Scholar]
- 24.Murphy, T. F., L. O. Bakaletz, J. M. Kyd, B. Watson, and D. L. Klein. 2005. Vaccines for otitis media: proposals for overcoming obstacles to progress. Vaccine 23:2696-2702. [DOI] [PubMed] [Google Scholar]
- 25.Novotny, L. A., K. M. Mason, and L. O. Bakaletz. 2005. Development of a chinchilla model to allow direct, continuous, biophotonic imaging of bioluminescent nontypeable Haemophilus influenzae during experimental otitis media. Infect. Immun. 73:609-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Brien, K. L., M. I. Walters, J. Sellman, P. Quinlisk, H. Regnery, B. Schwartz, and S. F. Dowell. 2000. Severe pneumococcal pneumonia in previously healthy children: the role of preceding influenza infection. Clin. Infect. Dis. 30:784-789. [DOI] [PubMed] [Google Scholar]
- 27.Olson, D. R., L. Simonsen, P. J. Edelson, and S. S. Morse. 2005. Epidemiological evidence of an early wave of the 1918 influenza pandemic in New York City. Proc. Natl. Acad. Sci. USA 102:11059-11063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peltola, V., T. Ziegler, and O. Ruuskanen. 2003. Influenza A and B virus infections in children. Clin. Infect. Dis. 36:299-305. [DOI] [PubMed] [Google Scholar]
- 29.Peltola, V. T., and J. A. McCullers. 2004. Respiratory viruses predisposing to bacterial infections: role of neuraminidase. Pediatr. Infect. Dis. J. 23:S87-S97. [DOI] [PubMed] [Google Scholar]
- 30.Peltola, V. T., K. G. Murti, and J. A. McCullers. 2005. Influenza virus neuraminidase contributes to secondary bacterial pneumonia. J. Infect. Dis. 192:249-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peltola, V. T., J. E. Rehg, and J. A. McCullers. 2004. A ferret model of synergism between influenza virus and Streptococcus pneumoniae. Int. Congr. Ser. 1263C:486-490. [Google Scholar]
- 32.Reid, A. H., J. K. Taubenberger, and T. G. Fanning. 2001. The 1918 Spanish influenza: integrating history and biology. Microbes Infect. 3:81-87. [DOI] [PubMed] [Google Scholar]
- 33.Simonsen, L. 1999. The global impact of influenza on morbidity and mortality. Vaccine 17(Suppl. 1):S3-S10. [DOI] [PubMed] [Google Scholar]
- 34.Simonsen, L., M. J. Clarke, G. D. Williamson, D. F. Stroup, N. H. Arden, and L. B. Schonberger. 1997. The impact of influenza epidemics on mortality: introducing a severity index. Am. J. Public Health 87:1944-1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simonsen, L., K. Fukuda, L. B. Schonberger, and N. J. Cox. 2000. The impact of influenza epidemics on hospitalizations. J. Infect. Dis. 181:831-837. [DOI] [PubMed] [Google Scholar]
- 36.Thompson, W. W., D. K. Shay, E. Weintraub, L. Brammer, N. Cox, L. J. Anderson, and K. Fukuda. 2003. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 289:179-186. [DOI] [PubMed] [Google Scholar]
- 37.Webster, R. G., W. J. Bean, O. T. Gorman, T. M. Chambers, and Y. Kawaoka. 1992. Evolution and ecology of influenza A viruses. Microbiol. Rev. 56:152-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wright, P. F., J. Thompson, and D. T. Karzon. 1980. Differing virulence of H1N1 and H3N2 influenza strains. Am. J. Epidemiol. 112:814-819. [DOI] [PubMed] [Google Scholar]