Abstract
Mice infected by Candida albicans and treated with monoclonal antibody C7 survived longer than saline-treated animals. A prozone-like effect was observed. The in vitro candidacidal activity of macrophages was strongly enhanced when C. albicans was opsonized by C7 and complete murine serum was present.
The incidence of candidiasis has increased dramatically in the last few decades, and bloodstream infections due to Candida spp. are becoming an important cause of morbidity and mortality in different types of immunocompromised patients (6). Historically, the therapy of serious fungal infections has been dominated by monotherapy with the polyene antibiotic amphotericin B or alternative therapies with fluconazole, voriconazole, and caspofungin (2). However, the toxicity of and emergence of resistance to these antifungal agents are potential problems. Often treatment with antifungal drugs is not very effective because of impaired immunity in patients. Thus, there is an increasing interest in novel, immune-based prophylactic and therapeutic approaches to treat invasive candidiasis.
Cell-mediated immunity and innate immunity are considered to be the most important lines of defense against candidiasis. However, recent evidence demonstrates that antibodies with defined specificities show different degrees of protection against systemic and mucosal candidiasis (4, 5, 9, 10). In a previous report we have described a mouse immunoglobulin M (IgM) monoclonal antibody (MAb), designated C7, which displays three different biological anti-Candida albicans activities, i.e., inhibition of adherence of C. albicans to HEp2 and oral epithelial cells, inhibition of C. albicans germination, and direct candidacidal activity (13). In this work we have studied the protection exerted by MAb C7 in a murine model of invasive candidiasis.
Female BALB/c mice, 6 to 8 weeks old, were infected intravenously with 5 × 105 C. albicans yeast cells (C. albicans 3153 from the National Collection of Pathogenic Fungi, Bristol, United Kingdom) suspended in 0.1 ml saline. The experimental protocols were approved by the Institutional Review Board of the School of Medicine and Odontology at the University of the Basque Country.
MAb C7 was produced as previously described (13). Treated animals received 200 μg of MAb C7 intraperitoneally at 4 h before infection and either two or six successive 100-μg doses at 1 and 2 days or at 1, 2, 3, 4, 6, and 9 days after infection, for a total 400 or 800 μg, respectively. Controls were injected with saline. Protection was evaluated by monitoring animal survival for 20 days. Groups of at least eight animals were used for each experiment. The mean survival time and numbers of CFU of C. albicans in infected tissues were calculated as reported previously (17). The Kaplan-Meier and log rank tests were applied to survival data. Data on CFU in infected tissues were analyzed by the Mann-Whitney test. P values of ≤0.05 were considered significant.
For the candidacidal assays, C. albicans opsonization with MAb C7 was accomplished by incubating C. albicans yeast cells (5 × 105 cells ml−1) in complete RPMI containing 50 μg ml−1 of MAb C7 for 30 min at 4°C. A mixture of 2 × 105 macrophages (ANA-1, kindly provided by E. Blasi, University of Modena, Italy) and 5 × 104 C. albicans cells in 100 μl of complete RPMI was incubated at 37°C in 5% CO2 for 90 min in 96-well plates. The medium was then replaced by 0.2% Triton X-100, and the wells were washed with sterile water. RPMI in these assays was completed with murine serum obtained from healthy mice. The viability of the remaining C. albicans cells and germ tubes was determined by the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide reduction method (14). Differences in the candidacidal assays were analyzed by Student's t test. P values of ≤0.05 were considered significant.
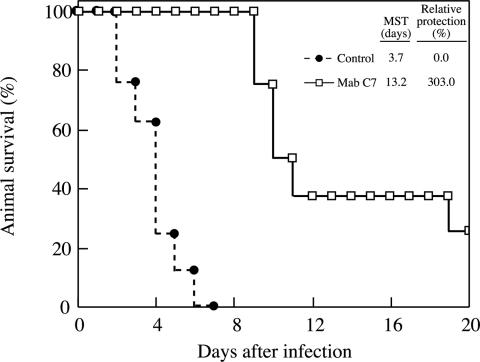
MAb C7 conferred protection against invasive candidiasis, as was demonstrated by counts of CFU in infected tissues and by survival curve analysis. Treatment of infected animals with 400 μg of MAb C7 had a protective effect revealed not only by a longer mean survival time but also by a higher percentage of final survival (Fig. 1). These results are comparable to those reported by other groups using different anti-C. albicans MAbs (1, 7, 12). Higher doses of the MAb did not improve the protective effect. On the contrary, mean survival time and final survival were lower when mice were treated with 800 μg of MAb C7 (11.4 days and 0%, respectively). Similar prozone-like effects have been described for IgG and IgM antibodies against C. albicans in vivo (8) and in vitro (11) and for Cryptococcus neoformans (15, 16). Inhibition of complement binding on the yeast cell surface by the high concentration of monoclonal antibody has been suggested as a reason for the lower protection observed with the higher doses of MAb (11), as activation of the complement is the proposed mechanism of protection in some cases (8).
FIG. 1.
Effect of MAb C7 on the survival curve for mice infected intravenously with 5 × 105 yeast cells of C. albicans. MAb C7, mice treated with 400 μg of the MAb; control, mice treated with saline. The survival curve for MAb C7-treated animals was significantly different (P < 0.001) from that for control mice. MST, mean survival time.
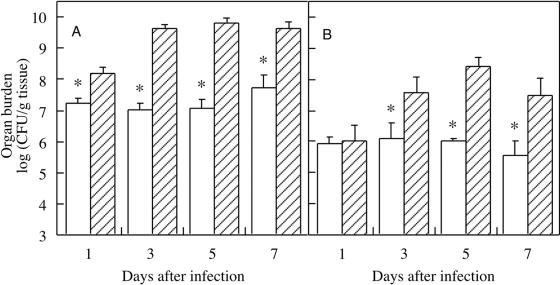
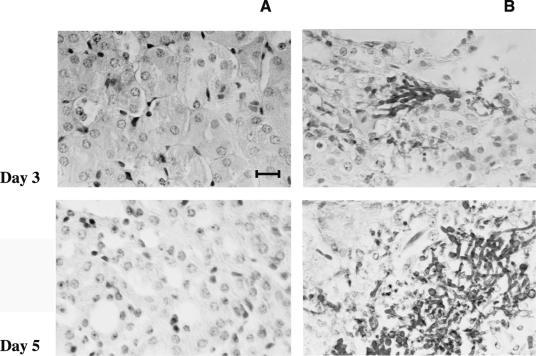
In the survival experiments, 25% of mice treated with MAb C7 survived the entire observation period, whereas all controls died by day 7 (Fig. 1). The survival curves correlated well with differences in fungal burden in kidneys and brain. Mice treated with 400 μg of MAb C7 contained significantly fewer organisms than did control mice (Fig. 2). Differences in number of C. albicans cells between MAb C7-treated animals and controls were confirmed by microscopic observation of the tissues (Fig. 3).
FIG. 2.
Fungal burdens of (A) left kidney and (B) brain of mice infected intravenously with 5 × 105 yeast cells of C. albicans. Open bars, mice treated with MAb C7 (400 μg); hatched bars, control mice treated with saline. On days marked with an asterisk, mice that received MAb C7 had significantly fewer CFU than control mice (P < 0.05). Error bars show standard deviations.
FIG. 3.
Photomicrographs of periodic acid-Schiff-stained kidney tissue of mice infected intravenously with 5 × 105 yeast cells of C. albicans. (A) Mice treated with MAb C7 (400 μg); (B) mice treated with saline. Bar, 5 μm. Only tissues from animals not treated with MAb C7 showed fungal cells.
The mechanisms by which antibodies protect against candidiasis are unclear. The in vitro activities of MAb C7, such as direct candidacidal activity, inhibition of Candida adherence to host cells, and inhibition of germination, must be, at least in part, responsible for the in vivo effect. On the other hand, Caesar and Cutler (3) showed that the protective antibody B6.1 enhanced the candidacidal activity of murine neutrophils. Another IgM has been also shown to be opsonic for peritoneal murine macrophages phagocytosing C. neoformans (15). Opsonization of C. albicans with MAb C7 did not significantly improve the candidacidal activity of macrophages in medium supplemented with decomplemented serum (data not shown). Only when complete murine serum was present was a synergic effect on the candidacidal effect of MAb C7 and macrophages observed (Fig. 4).
FIG. 4.
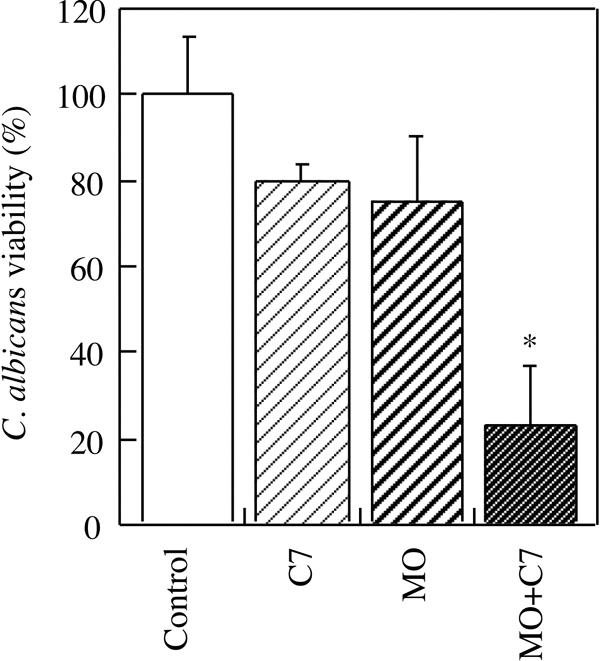
Viability of C. albicans in the presence of MAb C7 (C7), macrophages (MO), or macrophages plus MAb C7 (MO + C7). Control, C. albicans in RPMI. The assay medium was supplemented with complete murine serum. Statistically significant differences (P < 0.05) with respect to the control are marked with an asterisk.
The results presented in this study indicate that, in addition to previously reported activities such as inhibition of Candida adherence to host cells, inhibition of germination, and direct candidacidal activity (13), complement activation might contribute to the protection by MAb C7. In conclusion, treatment with MAb C7 extends the survival of mice with invasive candidiasis. This result extends the concept of protective antibodies and may provide a new drug for the future treatment of this mycosis.
Acknowledgments
This investigation was supported by grants PB98-0248 and PM99-0033 from the Dirección General de Enseñanza Superior e Investigación Científica of the Spanish Ministerio de Educación y Cultura and grant 9/UPV 0093.327-13550/2001 from the Universidad del País Vasco.
We thank Elisabetta Blasi, University of Modena, Italy, for her kind supply of the macrophage cell line ANA-1. We thank José Manuel Aguirre and María José Rodríguez for their help with the histological studies.
Editor: A. Casadevall
REFERENCES
- 1.Bromuro, C., A. Torosantucci, P. Chiani, S. Conti, L. Polonelli, and A. Cassone. 2002. Interplay between protective and inhibitory antibodies dictates the outcome of experimentally disseminated candidiasis in recipients of a Candida albicans vaccine. Infect. Immun. 70:5462-5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burnie, J., and R. Matthews. 2004. Genetically recombinant antibodies: new therapeutics against candidiasis. Expert. Opin. Biol. Ther. 4:233-241. [DOI] [PubMed] [Google Scholar]
- 3.Caesar, T. C., and J. E. Cutler. 1997. A monoclonal antibody to Candida albicans enhances mouse neutrophil candidacidal activity. Infect. Immun. 65:5354-5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casadevall, A. 1995. Antibody immunity and invasive fungal infections. Infect. Immun. 63:4211-4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Bernardis, F., M. Boccanera, D. Adriani, E. Spreghini, G. Santoni, and A. Cassone. 1997. Protective role of antimannan and anti-aspartyl proteinase antibodies in an experimental model of Candida albicans vaginitis in rats. Infect. Immun. 65:3399-3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.García Ruíz, J. C., E. Amutio, and J. Pontón. 2004. Infección fúngica invasora en pacientes inmunocomprometidos. Rev. Iberoam. Micol. 21:55-62. [PubMed] [Google Scholar]
- 7.Han, Y., and J. Cutler. 1995. Antibody response that protects against disseminated candidiasis. Infect. Immun. 63:2714-2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han, Y., T. R. Kozel, M. X. Zhang, R. S. MacGill, R. J. Carroll, and J. E. Cutler. 2001. Complement is essential for protection by an IgM and an IgG3 monoclonal antibody against experimental, hematogenously disseminated candidiasis. J. Immunol. 167:1550-1557. [DOI] [PubMed] [Google Scholar]
- 9.Han, Y., R. P. Morrison, and J. E. Cutler. 1998. A vaccine and monoclonal antibodies that enhance mouse resistance to Candida albicans vaginal infection. Infect. Immun. 66:5771-5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han, Y., M. A. Ulrich, and J. E. Cutler. 1999. Candida albicans mannan extract-protein conjugates induce a protective immune response against experimental candidiasis. J. Infect. Dis. 179:1477-1484. [DOI] [PubMed] [Google Scholar]
- 11.Kozel, T. R., R. S. MacGill, A. Percival, and Q. Zhou. 2004. Biological activities of naturally occurring antibodies reactive with Candida albicans mannan. Infect. Immun. 72:209-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matthews, R. C., J. P. Burnie, D. Howat, T. Rowland, and F. Walton. 1991. Autoantibody to heat-shock protein 90 can mediate protection against systemic candidosis. Immunology 74:20-24. [PMC free article] [PubMed] [Google Scholar]
- 13.Moragues, M. D., M. J. Omaetxebarria, N. Elguezabal, M. J. Sevilla, S. Conti, L. Polonelli, and J. Pontón. 2003. A monoclonal antibody directed against a Candida albicans cell wall mannoprotein exerts three anti-C. albicans activities. Infect. Immun. 71:5273-5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mossmann, T. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65:55-63. [DOI] [PubMed] [Google Scholar]
- 15.Taborda, C. P., and A. Casadevall. 2001. Immunolglobulin M efficacy against Cryptococcus neoformans: mechanism, dose dependence, and prozone-like effects in passive protection experiments. J. Immunol. 166:2100-2107. [DOI] [PubMed] [Google Scholar]
- 16.Taborda, C. P., J. Rivera, O. Zaragoza, and A. Casadevall. 2003. More is not necessarily better: prozone-like effects in passive immunization with IgG. J. Immunol. 170:3621-3630. [DOI] [PubMed] [Google Scholar]
- 17.Zaragoza, O., C. de Virgilio, J. Pontón, and C. Gancedo. 2002. Disruption in Candida albicans of the TPS2 gene encoding trehalose-6-phosphate phosphatase causes aggregation and decreases infectivity. Microbiology 148:1281-1290. [DOI] [PubMed] [Google Scholar]