Abstract
The relative contributions of antibody-induced complement-mediated bacterial lysis and antibody/complement-mediated phagocytosis to host immunity against meningococcal infections are currently unclear. Further, the in vivo effector functions of antibodies may vary depending on their specificity and Fc heavy-chain isotype. In this study, a mouse immunoglobulin G2a (mIgG2a) monoclonal antibody (MN12H2) to meningococcal outer membrane protein PorA (P1.16), its human IgG subclass derivatives (hIgG1 to hIgG4), and an mIgG2a monoclonal antibody (Nmb735) to serogroup B capsular polysaccharide (B-PS) were evaluated for passive protection against meningococcal serogroup B strain 44/76-SL (B:15:P1.7,16) in an infant rat infection model. Complement component C6-deficient (PVG/c−) rats were used to assess the importance of complement-mediated bacterial lysis for protection. The PorA-specific parental mIgG2a and the hIgG1 to hIgG3 derivatives all induced efficient bactericidal activity in vitro in the presence of human or infant rat complement and augmented bacterial clearance in complement-sufficient HsdBrlHan:WIST rats, while the hIgG4 was unable to do so. In C6-deficient PVG/c− rats, lacking complement-mediated bacterial lysis, the augmentation of bacterial clearance by PorA-specific mIgG2a and hIgG1 antibodies was impaired compared to that in the syngeneic complement-sufficient PVG/c+ rat strain. This was in contrast to the case for B-PS-specific mIgG2a, which conferred similar protective activity in both rat strains. These data suggest that while anti-B-PS antibody can provide protection in the infant rats without membrane attack complex formation, the protection afforded by anti-PorA antibody is more dependent on the activation of the whole complement pathway and subsequent bacterial lysis.
Meningitis and septicemia caused by Neisseria meningitidis (meningococcus) continue to cause morbidity and mortality worldwide. The important role of specific antibody and an intact complement system for protection against this pathogen is highlighted by the peak incidence of meningococcal infections in young children devoid of specific antibody (7), the inverse relation between the age-related decrease in the incidence of disease with the acquisition of serum bactericidal activity (BA) (7), and the increased frequency of systemic neisserial infections among persons with deficiencies in C3, alternative pathway, and especially late complement pathway components (C5 to C9) (4). Thus, BA, i.e., the ability of specific antibody to lyse bacterial cells in vitro in the presence of intact complement, has been considered crucial for evaluation of protection against meningococcal disease. Immunoglobulin G (IgG) antibodies to the outer membrane protein PorA, a major component of serogroup B outer membrane vesicle vaccines (5, 16) and an important antigen for meningococcal typing (1, 20), are frequently bactericidal and confer protection in an animal model of meningococcal infection (15, 18, 22). Good correlation between BA and protective activity in an infant rat model has been reported for mouse anti-PorA monoclonal antibodies (MAbs) (26). The protective activity of anti-PorA MAbs of human origin has not been measured in animal models of meningococcal infection. On the other hand, it has been recently shown that natural human antibodies to serogroup C capsular polysaccharide and serogroup B capsular polysaccharide (B-PS) can confer protection in vivo even in the absence of BA in vitro (27, 30).
Besides BA, several reports suggest that opsonophagocytic activity (OA) also is an important defense mechanism against meningococcal infections, especially those caused by serogroup B organisms (17, 19, 23). While BA is dependent on antibody-mediated deposition of the membrane attack complex on bacterial membranes through the activation of the whole complement cascade (C1 to C9), IgG-mediated phagocytosis is not. IgG-mediated phagocytosis is, however, amplified by complement activation but requires only deposition of opsonically active C3 split products (C3b and iC3b) on the bacterial surface. IgG and deposited C3 fragments can therefore function in concert as opsonins, targeting the invading pathogen for ingestion and killing by professional phagocytes through binding to Fcγ receptor (FcγR) and complement receptor. Increased OA has been shown in human sera taken at convalescence and after vaccination with serogroup B outer membrane vesicle vaccine (8, 9, 14, 24). These opsonic antibodies are directed to a variety of meningococcal surface antigens (13, 14), including the PorA protein. The relative importance of OA and BA for protection in vivo, however, has been difficult to define.
To study the in vitro effector functions of anti-PorA antibodies in more detail, a panel of mouse-human chimeric MAbs of all the four human IgG subclasses (hIgG1 to hIgG4) with identical variable (V) genes against the P1.16 epitope on PorA protein were generated from mouse IgG2a (mIgG2a) MAb MN12H2 (10) and characterized for their effector functions in vitro (29). While isotypes hIgG1 to hIgG3 mediated efficient bacterial lysis (relative activity, hIgG1 = hIgG3 > hIgG2) and phagocytosis (relative activity, hIgG3 > hIgG1 ≫ hIgG2), hIgG4 had undetectable activity in these assays. How these differences in functional activities in vitro are reflected in protection in vivo is not known.
In this study, the parental P1.16 PorA-specific mIgG2a MAb MN12H2 (10), the hIgG1 to hIgG4 isotypes derived from it (29), and the B-PS-specific mIgG2a MAb Nmb735 (6) were assessed for protective activity in an infant rat infection model (21, 26). We had two major objectives: first, to assess the influence of antibody isotype on protection by comparing the protective activities of the P1.16 PorA-specific hIgG1 to hIgG4 isotypes in complement sufficient animals, and second, to assess the importance of complement-mediated bacterial lysis for protection in vivo. This was done by comparing the protective activities of the B-PS specific mIgG2a antibody and the PorA-specific mIgG2a and hIgG1 antibodies in complement-sufficient and C6-deficient animals (12) with otherwise syngeneic backgrounds.
(Part of these data were presented at the 13th International Pathogenic Neisseria Conference in Oslo, Norway [M. Toropainen, L. Saarinen, G. Vidarsson, J. G. J. Van de Winkel, M. R. Daha, and H. Käyhty, Abstr. 13th Int. Pathogenic Neisseria Conf., p. 200, 2002.)
MATERIALS AND METHODS
Monoclonal antibodies.
The production and in vitro characteristics of the mouse-human chimeric P1.16 PorA-specific hIgG1 to hIgG4 antibodies have been described elsewhere (29). On a weight basis, all isotypes showed equal binding to Alexa488-labeled H44/76 cells as detected by fluorescence-activated cell sorting after incubation with phycoerythrin-labeled rabbit anti-human κ-chain antibodies (Southern Biotechnology Associates, Birmingham, AL). The parental P1.16-specific mIgG2a antibody MN12H2 (10) was a gift from B. Kuipers (NVI, Bilthoven, The Netherlands), and the B-PS-specific mIgG2a antibody Nmb735 (formerly MAb 735) (6) was from H.-P. Harthus (Dade Behring, Marburg, Germany).
Bacterial strain and growth conditions.
The 44/76-SL (B:15:P1.7,16:L3,7) meningococcal strain has been described previously (5). For passive-protection studies, the strain was rat passaged three times and stored in skim milk at −70°C. The resulting strain expressed the L3,7 immunotype as determined by colony blotting with L3,7,9 (4A8-B2) (28)- and L8 (MN43F8.10)-specific monoclonal antibodies (both received from B. Kuipers, NVI). The inoculum for rat protection and BA assays was prepared from brain heart infusion broth (Difco)-grown, early-log-phase rat-passaged 44/76-SL bacteria as previously described (25). The number of viable bacteria in the challenge dose was determined by counting the CFU after serial 10-fold dilution of the suspension in phosphate-buffered saline and plating onto supplemented proteose-peptone agar plates.
Bactericidal activity assay.
The BA of antibodies was determined in sterile 96-well flat-bottomed microtiter plates (Nunclon, Roskilde, Denmark). Normal human serum or pooled serum from 5- to 6-day-old HsdBrlHan:WIST rat pups (all MAbs) or PVG/OlaHsd rat pups (PorA-specific mIgG2a and hIgG1 MAbs) was used as the exogenous complement source. The complement sources did not show significant killing alone (<5% reduction in CFU after 60 min of incubation) at the final concentration of 20% used. The reaction mixture had a final volume of 50 μl. Hanks' balanced salt solution containing 0.9 mM Mg2+, 1.4 mM Ca2+, and 0.1% bovine serum albumin (Sigma), pH 7.2, was the dilution buffer. A mixture containing antibodies in appropriate concentrations (25 μl), bacteria (15 μl, containing approximately 100 CFU), and complement serum (10 μl) was incubated in duplicate wells at 37°C on a rotatory shaker at 220 rpm for 60 min. Control wells contained (i) bacteria with buffer, (ii) bacteria with buffer and complement, and (iii) bacteria with buffer and MAb. After incubation, the reaction was stopped by placing the plates on ice, and 25-μl samples taken from each well were allowed to run down in ∼8-cm lanes on supplemented GC (Difco) agar plates. After incubation overnight at 37°C in 5% CO2, the colonies were counted, and the results were expressed as the lowest antibody concentration giving 90% killing of the inoculum at 60 min compared to the number of CFU at time zero.
Experimental animals.
Outbred HsdBrlHan:WIST and inbred PVG/OlaHsd (designated PVG/c+) rats with normal complement activity were obtained from Harlan Nederland (Horst, The Netherlands). Complement component C6-deficient PVG rats (designated PVG/c−) (12) were received from M. R. Daha (Department of Nephrology, Leiden University Medical Center, Leiden, The Netherlands). The lack of lytic activity in PVG/c− rats was confirmed with a total hemolytic complement activity assay in agarose plates (11).
Infant rat protection assay and definition of protection.
The passive-protection experiments were done as described previously (25). In brief, 5- to 7-day-old infant rats (n = 5 or 6/group) were injected intraperitoneally with antibodies (0.1 ml/pup), diluted in saline, 1 to 2 h before the intraperitoneal bacterial challenge with approximately 106 CFU/pup in a final volume of 0.1 ml. Saline was used as a negative control for protection. Development of bacteremia was assessed by culturing blood samples taken 6 h after challenge. The limit of detection for blood cultures was 1 × 103 CFU/ml. Animals with sterile cultures were assigned a value of 0.3× the detection limit, i.e., 3 × 102 CFU/ml blood. Protection was defined as a statistically significant (P < 0.05) reduction in geometric mean (GM) blood bacterial density compared to control animals treated with saline. All experimental protocols were reviewed by the Institutional Laboratory Animal Committee and approved by the Provincial Board of Southern Finland.
Statistical methods.
The log-transformed blood culture data were subjected to one-way analysis of variance (ANOVA) (SPSS Inc., Chicago, Illinois), followed by appropriate post hoc tests (least significant difference [LSD]) if indicated. Statistical significance between BAs obtained with rat and human complement was analyzed with a paired-sample t test, using untransformed data. For all comparisons, a P value of <0.05 was considered significant.
RESULTS
Bactericidal activity.
The species of the complement source used in BA assays is known to affect the results greatly (34). Thus, the BAs of the MAbs were first reassessed using infant rat serum collected from 5- to 6-day-old HsdBrlHan:WIST rats or human serum as the exogenous complement source (Table 1). The results were similar to those obtained previously using human complement (29). Thus, B-PS-specific mouse MAb Nmb735 (not determined previously) and the parental PorA-specific mouse MAb MN12H2 (both IgG2a isotypes) were the most potent antibodies in this assay, followed by human PorA-specific IgG isotypes in the order of activity of hIgG1 = hIgG3 > hIgG2. The hIgG4 isotype failed to show any BA at the concentrations of up to 20 μg/ml tested (Table 1). Approximately threefold-higher antibody concentrations were needed for similar BAs (≥90% killing of the inoculum) when human complement was used, compared to rat, but this difference did not reach statistical significance (P = 0.06, paired one-sided t test).
TABLE 1.
Bactericidal activities of monoclonal antibodies
| Monoclonal antibody
|
Bactericidal activity (μg/ml)a
|
|||
|---|---|---|---|---|
| Nameb | Specificity | Origin | Infant rat complement | Human complement |
| Nmb735 (IgG2a) | Group B PS | Mouse | 0.027 | 0.08 |
| MN12H2 (IgG2a) | P1.16 PorA | Mouse | 0.027 | 0.08 |
| hIgG1 | P1.16 PorA | Mouse-human chimera | 0.25 | 0.74 |
| hIgG2 | P1.16 PorA | Mouse-human chimera | 0.74 | 2.2 |
| hIgG3 | P1.16 PorA | Mouse-human chimera | 0.25 | 0.74 |
| hIgG4 | P1.16 PorA | Mouse-human chimera | >20 | >20 |
Lowest antibody concentration needed for ≥90% killing of strain 44/76-SL (B:15:P1.7,16) bacteria in the presence of infant rat complement (HsdBrlHan:WIST) or human complement. The complement source (20% infant rat serum or human serum) did not show significant killing alone.
hIgG1 to hIgG4, P1.16 PorA-specific human IgG1 to IgG4 isotypes derived from MN12H2.
Effect of C6 deficiency on disease outcome in the infant rats.
Next, the protective activities of MAbs were assessed in an infant rat model of meningococcal infection. In these studies, one outbred rat strain (HsdBrlHan:WIST) and two syngeneic inbred rat strains, the complement-sufficient (PVG/c+) and the C6-deficient (PVG/c−) PVG strains, were used.
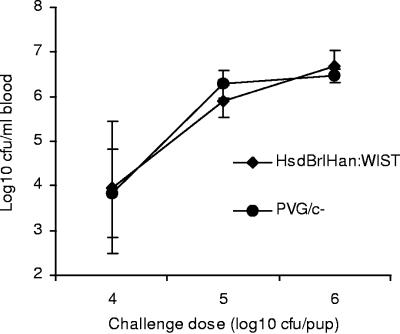
As the effect of C6 deficiency on the outcome of infection in nonimmune infant rats was not known, in preliminary studies several challenge doses (104 to 106 CFU/pup) of strain 44/76-SL were tested and the development of bacteremia (GM CFU per milliliter of blood) in PVG/c− rats compared to that in complement-sufficient HsdBrlHan:WIST rats. No significant differences between rat strains were found (Fig. 1). On the basis of these results, a dose of approximately 106 CFU/pup, resulting in a GM blood bacterial density of approximately 106 CFU/ml at 6 h postinfection, was chosen for protection studies with all rat strains.
FIG. 1.
Development of bacteremia in complement-sufficient (HsdBrlHan:WIST) and C6-deficient (PVG/c−) infant rats as a function of bacterial challenge dose. The results are given as GM bacteremia level (CFU per milliliter of blood) in each group of six animals. Error bars indicate standard deviations.
Effect of antibody isotype on protection.
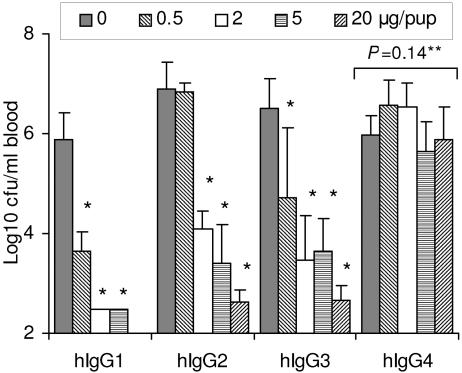
To asses the influence of antibody isotype on protection, the protective activities of PorA-specific hIgG1 to hIgG4 isotypes in complement-sufficient HsdBrlHan:WIST rats were assessed (Fig. 2). The results resembled those obtained in the BA assay. Thus, hIgG1 and hIgG3 exhibited similar protective activities, while a fourfold-higher antibody dose (2 versus 0.5 μg/pup) of hIgG2 was needed for equal protective activity (Fig. 2). The nonbactericidal hIgG4 failed to show significant protective activity at the antibody doses of up to 20 μg/pup tested (Fig. 2).
FIG. 2.
Protective activity of P1.16 PorA-specific hIgG1 to hIgG4 isotypes in complement-sufficient HsdBrlHan:WIST infant rats. The results are given as GM bacteremia level (CFU per milliliter of blood) in each group of five or six animals. Error bars indicate upper 95% confidence levels. The different isotypes were tested in separate experiments. *, P < 0.05 compared to control animals not given antibody, LSD. **, P value obtained with one-way ANOVA.
Importance of complement-mediated bacterial lysis for protection.
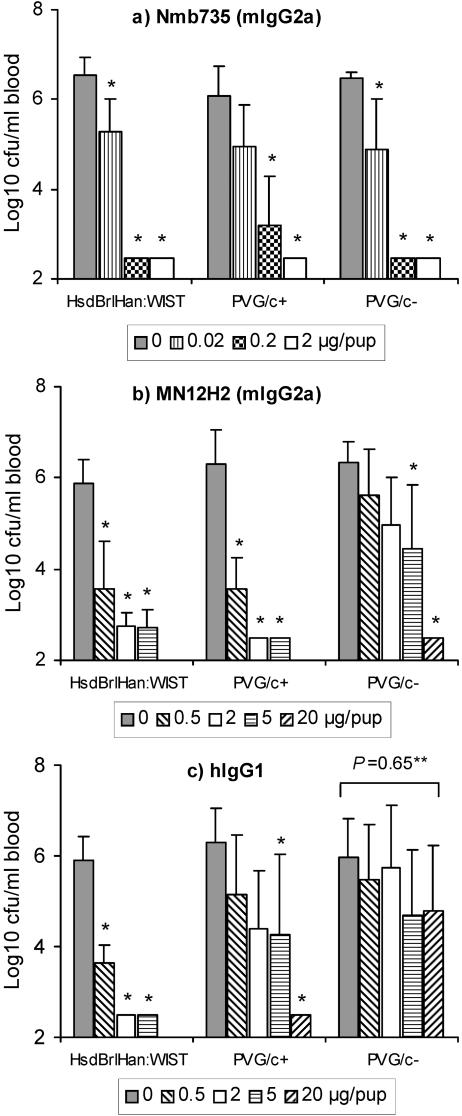
To assess the importance of antibody-induced complement-mediated bacterial lysis for protection, the B-PS-specific mIgG2a antibody Nmb735, the parental PorA-specific mIgG2a MN12H2, and the hIgG1 isotype derived from it were tested for protective activity in complement-sufficient (PVG/c+) and C6-deficient (PVG/c−) PVG rats of otherwise syngeneic background. For comparison, data obtained with the HsdBrlHan:WIST rat strain are included.
The B-PS-specific MAb Nmb735 was equally protective in all rat strains, with the lowest tested dose of 0.02 μg/pup conferring protection compared to control animals receiving saline (Fig. 3a). This was in contrast to PorA-specific MAb MN12H2, of which a 10-fold-higher antibody dose (5 versus 0.5 μg/pup) was needed for protection in the C6-deficient PVG/c− rats compared to complement-sufficient PVG/c+ or HsdBrlHan:WIST rats (Fig. 3b). The PorA-specific hIgG1 isotype failed to show any protective activity in PVG/c− rats at the doses up to 20 μg/pup tested (Fig. 3c). Similar results were obtained with the hIgG2 to hIgG4 isotypes (data not shown).
FIG. 3.
Protective activities of monoclonal antibodies in complement-sufficient (HsdBrlHan:WIST and PVG/c+) and C6-deficient (PVG/c−) infant rats. a) Nmb735, B-PS-specific mouse IgG2a antibody; b) MN12H2, P1.16 PorA-specific mouse IgG2a antibody; c) hIgG1, P1.16 PorA-specific human IgG1 isotype derived from MN12H2. The results are given as GM bacteremia level (CFU per milliliter of blood) in each group of five or six animals. Error bars indicate upper 95% confidence levels. *, P < 0.05 compared to control animals not given antibody, LSD. **, P value obtained with one-way ANOVA.
With Nmb735 and MN12H2, no significant differences in protective activity between the two complement sufficient rat strains were detected (Fig. 3a and b). This was in contrast to the hIgG1 isotype, of which a 10-fold-higher antibody dose (5 versus 0.5 μg/pup) was needed for equal protection in PVG/c+ compared to HsdBrlHan:WIST rats (Fig. 3c).
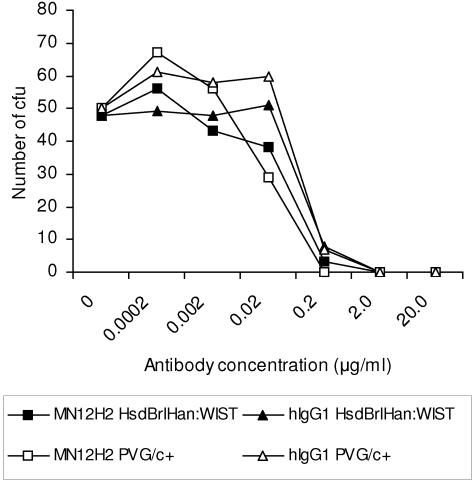
To study the possibility that rat strain-specific differences in complement activity might explain the lower protective activity of the hIgG1 isotype in PVG/c+ compared to HsdBrlHan:WIST rats, the in vitro BAs of the parental PorA-specific MAb MN12H2 and the hIgG1 isotype were reassessed using pooled sera from the rat strains as the exogenous complement source. No significant differences were detected between sera from the different strains (Fig. 4). As expected, the C6-deficient serum from PVG/c− rats failed to show any bactericidal activity up to 80% concentration (data not shown).
FIG. 4.
Bactericidal activities of PorA-specific monoclonal antibodies in the presence HsdBrlHan:WIST or PVG/c+ infant rat complement. MN12H2, mouse IgG2a isotype; hIgG1, human IgG1 isotype derived from MN12H2.
DISCUSSION
While there is evidence supporting the importance of both antibody-induced BA (4, 7) and OA (9, 19, 24) for protection against serogroup B meningococcal disease, their relative contributions to protective immunity have been difficult to delineate. The effector functions of antibodies may also vary depending on their specificity and Fc heavy-chain isotype. In this study, we addressed these questions by assessing the protective activity of PorA-specific and B-PS-specific mIgG2a antibodies in complement-sufficient and C6-deficient infant rats. Human IgG1 to IgG4 isotypes with identical V genes against the P1.16 epitope on the PorA outer membrane protein of N. meningitidis (29) were used to asses the influence of antibody isotype on protection. Our results showed that while the parental PorA-specific mIgG2a MAb MN12H2 and the human IgG1 to IgG3 isotypes derived from it efficiently enhanced bactericidal activity in vitro and augmented bacterial clearance in vivo in complement-sufficient rats, the augmentation of bacterial clearance by mIgG2a and hIgG1 antibodies was impaired in C6-deficient animals. This was in contrast to a bactericidal, B-PS-specific mIgG2a MAb, Nmb735, which conferred similar protective activity in complement-sufficient and C6-deficient animals.
Similar to the case in our previous studies (25, 26) with an outbred HsdCpb:WU rat strain, the B-PS-specific mouse MAb Nmb735 and the parental PorA-specific mouse MAb MN12H2 efficiently induced BA in vitro and augmented bacterial clearance in vivo in both complement-sufficient HsdBrlHan:WIST and PVG/c+ rat strains. This was in contrast to the PorA-specific hIgG1 isotype, of which a 10-fold-higher antibody dose (5.0 versus 0.5 μg/pup) was needed for protection in PVG/c+ compared to HsdBrlHan:WIST animals. As no rat strain-specific differences in the BAs in vitro were detected, the reason for the lower protective activity of this MAb in PVG/c+ animals remains to be fully evaluated. At least two possibilities exist to explain this difference between the BA in vitro and protection in vivo. First, the sensitivity of the BA assay (using 20% infant rat serum an as exogenous complement source) may not have been satisfactory to detect rat strain-specific differences in complement activity. Second, there may be strain-specific differences in the ability of rat phagocyte FcγR to bind antibody of human origin.
We found the relative protective activities of the PorA-specific MAbs to be mIgG2a = hIgG1 = hIgG3 > hIgG2 (hIgG4 had undetectable activity) in complement-sufficient HsdBrlHan:WIST rats and mIgG2a > hIgG1 in PVG/c+ rats. These results were consistent with the in vitro BA data in the presence of human complement (reference 29 and this study) or infant rat complement (this study), but the complete lack of protection by the hIgG1 isotype in C6-deficient PVG/c− rats was somewhat at variance with the previous phagocytic activity data (29) obtained using human polymorphonuclear leukocytes as the effector cells and 44/76-SL bacteria as the target. A 10-fold-higher antibody dose of the parental mouse mIgG2a MAb MN12H2 was needed for protection in the C6-deficient PVG/c− rat strain than in the isogenic complement-sufficient PVG/c+ rat strain. Possibly, an antibody dose higher than the 20 μg/pup tested would also have been needed for the hIgG1 isotype to confer protection in the PVG/c− rat strain.
In contrast to the results with PorA-specific mIgG2a and hIgG1 MAbs, but similar to our previous studies with polyclonal, nonbactericidal B-PS-specific IgM antibodies of human origin (27), the B-PS-specific mIgG2a was equally protective in complement-sufficient and C6-deficient animals. These findings emphasize the importance of capsular polysaccharide-specific antibodies over subcapsular ones for protection in late complement component-deficient individuals (2). Why antibodies to capsular compared to subcapsular antigen were more efficient in conferring protection in C6-deficient animals (this study) and also opsonophagocytosis in vitro (2) is not clear but has been suggested to arise from to the more exposed nature of the polysaccharide capsule and anticapsular antibodies and/or C3 fragments deposited by it on the bacterial surface (2, 3), where they are readily recognized by phagocytic cell FcRs and/or complement receptors. Despite the apparent dominance of anti-PorA antibody-mediated protection through complement-mediated bacterial lysis, this does not seem to be a common feature of all subcapsular meningococcal antigens. It has recently been demonstrated that despite the absence of BA in vitro, antibodies to other subcapsular antigens, such as transferrin binding protein A (33), genome-derived neisserial antigen 2132 (31), and genome-derived neisserial antigen 1870 (32), are able to confer protection in animal models of meningococcal infection. For the last two antigens, antibody-induced deposition of C3 fragments (C3b and iC3b) on the bacterial surface predicted well their ability to confer protection in vivo (31, 32), suggesting that OA was responsible for bacterial clearance.
To conclude, we have shown that while B-PS-specific antibody can confer protection in the infant rat without membrane attack complex formation, the protection afforded by PorA antibody is more dependent on the activation of the whole complement pathway and subsequent bacterial lysis. The relevance of these findings to protection in humans, and thus the validity of the infant rat model to assess the protective activity of antibodies of human origin in general, remain open until the rat homologs of human FcRs and their ligand specificities have been fully characterized. Further, taking into consideration the importance of complement system for innate and specific immunity against meningococcal disease, a careful comparison of the rat and human complement systems would be needed to assess the validity of this infection model in general.
Acknowledgments
We thank M. R. Daha for kindly providing the C6-deficient rat strain, H.-P. Harthus for providing Nmb735, B. Kuipers for providing MN12H2, H. Jarva for performing complement assays, L. Pyhälä for providing facilities for experimental work with animals, and the staff at the animal facilities of the National Public Health Institute in Helsinki and Kuopio, Finland, for comprehensive care of the animals.
This work was funded partially by the World Health Organization, Global Program for Vaccines and Immunization, Vaccine Research and Development (contracts V23/181/74, V23/181/118), and the National Meningitis Trust, United Kingdom.
Editor: J. N. Weiser
REFERENCES
- 1.Abdillahi, H., and J. T. Poolman. 1988. Neisseria meningitidis group B serosubtyping using monoclonal antibodies in whole-cell ELISA. Microb. Pathog. 4:27-32. [DOI] [PubMed] [Google Scholar]
- 2.Andreoni, J., H. Käyhty, and P. Densen. 1993. Vaccination and the role of capsular polysaccharide antibody in prevention of recurrent meningococcal disease in late complement component-deficient individuals. J. Infect. Dis. 168:227-231. [DOI] [PubMed] [Google Scholar]
- 3.Brown, E. J., K. A. Joiner, R. M. Cole, and M. Berger. 1983. Localization of complement component 3 on Streptococcus pneumoniae: anticapsular antibody causes complement deposition on the pneumococcal capsule. Infect. Immun. 39:403-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Figueroa, J., J. Andreoni, and P. Densen. 1993. Complement deficiency states and meningococcal disease. Immunol. Res. 12:295-311. [DOI] [PubMed] [Google Scholar]
- 5.Fredriksen, J. H., E. Rosenqvist, E. Wedege, K. Bryn, G. Bjune, L. O. Frøholm, A. K. Lindbak, B. Mogster, E. Namork, U. Rye, G. Stabbetorp, R. Winsnes, B. Aase, and O. Closs. 1991. Production, characterization and control of MenB-vaccine “Folkehelsa”: an outer membrane vesicle vaccine against group B meningococcal disease. NIPH Ann. 14:67-79. [PubMed] [Google Scholar]
- 6.Frosch, M., I. Gorgen, G. J. Boulnois, K. N. Timmis, and D. Bitter-Suermann. 1985. NZB mouse system for production of monoclonal antibodies to weak bacterial antigens: isolation of an IgG antibody to the polysaccharide capsules of Escherichia coli K1 and group B meningococci. Proc. Natl. Acad. Sci. USA 82:1194-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldschneider, I., E. C. Gotschlich, and M. S. Artenstein. 1969. Human immunity to the meningococcus. I. The role of humoral antibodies. J. Exp. Med. 129:1307-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halstensen, A., B. Haneberg, L. O. Frøholm, V. Lehmann, C. E. Frasch, and C. O. Solberg. 1984. Human opsonins to meningococci after vaccination. Infect. Immun. 46:673-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halstensen, A., H. Sjursen, S. E. Vollset, L. O. Frøholm, A. Naess, R. Matre, and C. O. Solberg. 1989. Serum opsonins to serogroup B meningococci in meningococcal disease. Scand. J. Infect. Dis. 21:267-276. [DOI] [PubMed] [Google Scholar]
- 10.Jiskoot, W., P. Hoogerhout, E. C. Beuvery, J. N. Herron, and D. J. Crommelin. 1991. Preparation and application of a fluorescein-labeled peptide for determining the affinity constant of a monoclonal antibody-hapten complex by fluorescence polarization. Anal. Biochem. 196:421-426. [DOI] [PubMed] [Google Scholar]
- 11.Lachmann, P. J., and M. J. Hobart. 1978. Complement technology, p. 5A12. In D. M. Weir (ed.), Handbook of experimental immunology, 3rd ed., vol. 1. Immunochemistry. Blackwell Scientific Publications, Oxford, United Kingdom. [Google Scholar]
- 12.Leenaerts, P. L., R. K. Stad, B. M. Hall, B. J. Van Damme, Y. Vanrenterghem, and M. R. Daha. 1994. Hereditary C6 deficiency in a strain of PVG/c rats. Clin. Exp. Immunol. 97:478-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lehmann, A. K., A. R. Gorringe, K. M. Reddin, K. West, I. Smith, and A. Halstensen. 1999. Human opsonins induced during meningococcal disease recognize transferrin binding protein complexes. Infect. Immun. 67:6526-6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lehmann, A. K., A. Halstensen, I. S. Aaberge, J. Holst, T. E. Michaelsen, S. Sornes, L. M. Wetzler, and H. Guttormsen. 1999. Human opsonins induced during meningococcal disease recognize outer membrane proteins PorA and PorB. Infect. Immun. 67:2552-2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Milagres, L. G., M. C. A. Gorla, C. T. Sacchi, and M. M. Rodrigues. 1998. Specificity of bactericidal antibody response to serogroup B meningococcal strains in Brazilian children after immunization with an outer membrane vaccine. Infect. Immun. 66:4755-4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peeters, C. C., H. C. Rumke, L. C. Sundermann, E. M. Rouppe van der Voort, J. Meulenbelt, M. Schuller, A. J. Kuipers, P. van der Ley, and J. T. Poolman. 1996. Phase I clinical trial with a hexavalent PorA containing meningococcal outer membrane vesicle vaccine. Vaccine 14:1009-1015. [DOI] [PubMed] [Google Scholar]
- 17.Platonov, A. E., G. A. Shipulin, I. V. Vershinina, J. Dankert, J. G. van de Winkel, and E. J. Kuijper. 1998. Association of human Fc gamma RIIa (CD32) polymorphism with susceptibility to and severity of meningococcal disease. Clin. Infect. Dis. 27:746-750. [DOI] [PubMed] [Google Scholar]
- 18.Rosenqvist, E., E. A. Høiby, E. Wedege, K. Bryn, J. Kolberg, A. Klem, E. Ronnild, G. Bjune, and H. Nokleby. 1995. Human antibody responses to meningococcal outer membrane antigens after three doses of the Norwegian group B meningococcal vaccine. Infect. Immun. 63:4642-4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ross, S. C., P. J. Rosenthal, H. M. Berberich, and P. Densen. 1987. Killing of Neisseria meningitidis by human neutrophils: implications for normal and complement-deficient individuals. J. Infect. Dis. 155:1266-1275. [DOI] [PubMed] [Google Scholar]
- 20.Russell, J. E., K. A. Jolley, I. M. Feavers, M. C. Maiden, and J. Suker. 2004. PorA variable regions of Neisseria meningitidis. Emerg. Infect. Dis. 10:674-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saukkonen, K. 1988. Experimental meningococcal meningitis in the infant rat. Microb. Pathog. 4:203-211. [DOI] [PubMed] [Google Scholar]
- 22.Saukkonen, K., H. Abdillahi, J. T. Poolman, and M. Leinonen. 1987. Protective efficacy of monoclonal antibodies to class 1 and class 3 outer membrane proteins of Neisseria meningitidis B:15:P1.16 in infant rat infection model: new prospects for vaccine development. Microb. Pathog. 3:261-267. [DOI] [PubMed] [Google Scholar]
- 23.Schlesinger, M., R. Greenberg, J. Levy, H. Käyhty, and R. Levy. 1994. Killing of meningococci by neutrophils: effect of vaccination on patients with complement deficiency. J. Infect. Dis. 170:449-453. [DOI] [PubMed] [Google Scholar]
- 24.Sjursen, H., R. Bjerknes, A. Halstensen, A. Naess, L. O. Frøholm, E. Rosenqvist, and C. O. Solberg. 1987. Serum opsonins to group B meningococci. Acta Pathol. Microbiol. Immunol. Scand. C 95:283-289. [DOI] [PubMed] [Google Scholar]
- 25.Toropainen, M., H. Käyhty, L. Saarinen, E. Rosenqvist, E. A. Høiby, E. Wedege, T. Michaelsen, and P. H. Mäkelä. 1999. The infant rat model adapted to evaluate human sera for protective immunity to group B meningococci. Vaccine 17:2677-2689. [DOI] [PubMed] [Google Scholar]
- 26.Toropainen, M., L. Saarinen, P. van der Ley, B. Kuipers, and H. Käyhty. 2001. Murine monoclonal antibodies to PorA of Neisseria meningitidis show reduced protective activity in vivo against B:15:P1.7,16 subtype variants in an infant rat infection model. Microb. Pathog. 30:139-148. [DOI] [PubMed] [Google Scholar]
- 27.Toropainen, M., L. Saarinen, E. Wedege, K. Bolstad, T. E. Michaelsen, A. Aase, and H. Käyhty. 2005. Protection by natural human immunoglobulin M antibody to meningococcal serogroup B capsular polysaccharide in the infant rat protection assay is independent of complement-mediated bacterial lysis. Infect. Immun. 73:4694-4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verheul, A. F., A. J. Kuipers, A. K. Braat, H. A. Dekker, C. C. Peeters, H. Snippe, and J. T. Poolman. 1994. Development, characterization, and biological properties of meningococcal immunotype L3,7,(8),9-specific monoclonal antibodies. Clin. Diagn. Lab. Immunol. 1:729-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vidarsson, G., W. L. van Der Pol, J. M. van Den Elsen, H. Vile, M. Jansen, J. Duijs, H. C. Morton, E. Boel, M. R. Daha, B. Corthesy, and J. G. van De Winkel. 2001. Activity of human IgG and IgA subclasses in immune defense against Neisseria meningitidis serogroup B. J. Immunol. 166:6250-6256. [DOI] [PubMed] [Google Scholar]
- 30.Welsch, J. A., and D. Granoff. 2004. Naturally acquired passive protective activity against Neisseria meningitidis group C in the absence of serum bactericidal activity. Infect. Immun. 72:5903-5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Welsch, J. A., G. R. Moe, R. Rossi, J. Adu-Bobie, R. Rappuoli, and D. M. Granoff. 2003. Antibody to genome-derived neisserial antigen 2132, a Neisseria meningitidis candidate vaccine, confers protection against bacteremia in the absence of complement-mediated bactericidal activity. J. Infect. Dis. 188:1730-1740. [DOI] [PubMed] [Google Scholar]
- 32.Welsch, J. A., R. Rossi, M. Comanducci, and D. M. Granoff. 2004. Protective activity of monoclonal antibodies to genome-derived neisserial antigen 1870, a Neisseria meningitidis candidate vaccine. J. Immunol. 172:5606-5615. [DOI] [PubMed] [Google Scholar]
- 33.West, D., K. Reddin, M. Matheson, R. Heath, S. Funnell, M. Hudson, A. Robinson, and A. Gorringe. 2001. Recombinant Neisseria meningitidis transferrin binding protein A protects against experimental meningococcal infection. Infect. Immun. 69:1561-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zollinger, W. D., and R. E. Mandrell. 1983. Importance of complement source in bactericidal activity of human antibody and murine monoclonal antibody to meningococcal group B polysaccharide. Infect. Immun. 40:257-264. [DOI] [PMC free article] [PubMed] [Google Scholar]