Abstract
Cytokine mRNA expression in biopsies of Mycobacterium ulcerans-infected human tissue was investigated using real-time PCR, and the findings were correlated with the clinical stages of disease and histopathologies. A broad range of cytokine mRNAs were detected in 16 early nodules and 28 late-stage ulcers, including those for the Th1 cytokines tumor necrosis factor alpha (TNF-α) and gamma interferon (IFN-γ) and the Th2 cytokine interleukin 10 (IL-10). IFN-γ was strongly expressed in both nodules and ulcers, suggesting that a Th1 response begins early in the disease. There was a significantly higher expression of IL-8 and other proinflammatory cytokines in results from 32 biopsies with neutrophilia than in those from 12 biopsies without acute inflammation. Ten tissue samples containing granulomas showed high mRNA expression for IFN-γ, IL-1β, IL-12p35, IL-12p40, IL-15, and TNF-α relative to 34 tissue samples without granulomas. These results suggest that the human immune response to M. ulcerans is similar to that seen with some other mycobacteria despite the presence of the toxin mycolactone in the tissues.
Buruli ulcer is caused by infection with Mycobacterium ulcerans, which secretes mycolactone (a polyketide toxin) in subcutaneous fatty tissue, causing extensive necrosis. On mouse fibroblast L929 cells, mycolactone causes a cytopathic effect that is characterized by cytoskeletal rearrangement with subsequent rounding up and detachment from tissue culture plates within 48 h (11, 22). It also causes cell cycle arrest in the G0/G1 phase of the cell cycle within 48 h, and ultimately, cells die by apoptosis (11, 12). When injected into guinea pigs intradermally, mycolactone causes lesions similar to those produced by the injection of live organisms, but an isogenic M. ulcerans mutant that does not produce toxin is nonvirulent in the guinea pig model (12). The histopathology of Buruli ulcer has been studied extensively (4, 16, 18, 25). In the early stages, the histological examination of nodules shows extensive subcutaneous necrosis, within which there are abundant clumps of extracellular acid-fast bacilli (AFB). There is a mixture of acute and chronic inflammation in long-standing lesions as in early lesions, but granulomas are also seen frequently and the number of AFB is greatly reduced (5, 8, 18, 37). These pathological findings suggest that cell-mediated immunity plays a role in healing, and this possibility is supported by studies of the delayed hypersensitivity response in patients with Buruli ulcer to a crude preparation of M. ulcerans sonicate (burulin) given intradermally (35) (7). Patients with early M. ulcerans disease did not react, whereas a positive response was elicited from 50% of patients with healing lesions, indicating the development of T-cell sensitization (35). When semiquantitative reverse transcription-PCR (RT-PCR) was used to quantify gamma interferon (IFN-γ) and interleukin 10 (IL-10) mRNA levels in diseased tissue, high IFN-γ but low IL-10 mRNA levels were found in nodular lesions compared with low IFN-γ and high IL-10 mRNA levels in ulcerated lesions, suggesting that there was suppression of IFN-γ and increased IL-10 in longer-established lesions (32). Although the natural history of the human disease is not well described, it was shown in a study of clofazamine treatment for nodular lesions that 30% of nodules in the placebo group healed spontaneously (34). This raises the possibility that early development of an adequate IFN-γ response can lead to healing. We have shown that an IFN-γ response to stimulation of whole blood with M. ulcerans sonicate was demonstrable in patients with early lesions, increased in patients with ulcers, and persisted in subjects with healed lesions (30). Mycolactone has profound effects on cytokine production in vitro (27), and therefore the development of the local immune response in infected human tissue is of particular interest.
We have investigated the cytokine response in tissue from M. ulcerans disease patients from three districts of endemicity in Ghana using quantitative real-time PCR and correlated our findings with the clinical stages of disease and the histopathologies.
MATERIALS AND METHODS
Recruitment of patients took place between September 2003 and September 2004 after informed consent. The study protocol was approved by the ethics review committees at the School of Medical Sciences, Kwame Nkrumah University of Science and Technology (SMS-KNUST), Kumasi, Ghana, and St. George's Hospital in London (United Kingdom).
Skin punch biopsies.
This study took place in parallel with an investigation of the feasibility of punch biopsies for diagnosing M. ulcerans disease (29). Multiple punch biopsies were taken using sterile disposable 4- or 6-mm punch biopsy needles (Stiefel Laboratories, United Kingdom) with the patient anesthetized immediately before surgical excision of the lesion, which is the usual form of treatment for the disease. Biopsies were taken within 1 to 2 cm of the center of a nodule or from the edge of the viable tissue when there was ulceration. Each individual biopsy taken for diagnostic confirmation by microscopy and culture, histology, and PCR (29) was placed in a sterile, 2-ml skirted tube with an “O” ring (Sarstedt, United Kingdom). A further biopsy was taken for RNA extraction, placed in a 2-ml cryovial (Nalgene), and frozen within 2 min. All tissues for RNA extraction were stored at −70°C.
Diagnosis.
Punch biopsy specimens for each study subject were processed for microscopy, culture, histology, and PCR for diagnostic confirmation as described elsewhere (29). Briefly, homogenized tissue was stained by the Ziehl-Neelsen technique and 1 ml was decontaminated by the modified Petroff method (31) for 10 min and inoculated on Lowenstein-Jensen slopes. Cultures were incubated at 31°C and examined weekly for 6 months before they were discarded. An independent semiquantitative assessment of acute inflammation (neutrophils), chronic inflammation (lymphocytes, plasma cells, and eosinophils), and granulomas was made histologically on each skin section after hematoxylin and eosin staining. Inflammatory cells and granulomas were graded as present or absent. DNA extraction for PCR was performed by the guanidinium thiocyanate diatoms technique (3), and PCR was performed targeting the IS2404 insertion sequence as described elsewhere (29).
Whole-blood assay and ELISA.
Twelve milliliters of venous blood was obtained by venesection from M. ulcerans disease patients and controls in sodium heparin Vacutainer tubes (Becton Dickinson, United Kingdom). The whole-blood assay was performed in a laboratory at Komfo Anokye Teaching Hospital, Kumasi, as described elsewhere (30). Briefly, whole blood was aseptically distributed, 1 ml per well, in duplicate in 24-well tissue culture plates (Falcon; Becton Dickinson, United Kingdom). Cell cultures were incubated with M. ulcerans sonicate prepared from an African isolate, M. ulcerans 1 (KIT, Royal Tropical Institute, Amsterdam, The Netherlands), at a final concentration of 10 μg per ml. Sonification was carried out with a Branson 250 Sonifier at 50% duty cycle using a small probe in a cup horn container and four cycles of 15 min interspersed with 5-min breaks with continuous cooling. One set of tissue culture wells was unstimulated to serve as background controls. Gentamicin was added to each well at 10 μg/ml (Sigma, United Kingdom). The plates were gently swirled on a flat surface and incubated at 37°C with 5% CO2 for 24 h. Plasma supernatants (200 to 300 μl per well) were collected and stored at −70°C. Enzyme-linked immunosorbent assays (ELISAs) for IFN-γ were carried out on samples that were clear and nonhemolyzed. OptEIA sets for human IFN-γ-containing components that are necessary to develop enzyme-linked immunosorbent assays and recommended buffers and solutions were obtained from BD Biosciences, Pharmingen (San Diego, Calif.). The manufacturer's recommended assay procedure was used.
RNA extraction and removal of DNA.
Each frozen tissue sample was placed in a 2-ml skirted, screw-cap, microcentrifuge tube with an “O” ring seal containing 0.5 ml of 0.1-mm silica/ceramic beads, 500 μl acid-phenol, 100 μl of chloroform-isoamylalcohol (24:1), and 500 μl detergent solution (10% Teepol HB7 in 500 mM sodium acetate, pH 4.0) (tissue joke). This was processed in a Ribolyser reciprocal shaker (Hybaid, United Kingdom) at the 6.5 power setting for 45 seconds to homogenize tissue and centrifuged in a microcentrifuge (Eppendorf) at 15,000 × g for 10 min. The upper aqueous layer (about 500 μl) containing RNA was put in a fresh microcentrifuge tube and reextracted twice with 500 μl of chloroform-isoamylalcohol. RNA was precipitated by the addition (to the aqueous phase) of 1/10 of the sample volume of 3 M sodium acetate at pH 4.8 to 5.2 and 2.5 times the combined volume of 100% ethanol and transported to the United Kingdom on ice packs for storage at −70°C. Extracts were spun for 15 min at 15,000 × g to pellet the precipitated RNA and washed twice with 500 μl 70% ethanol, and RNA was redissolved in 100 μl RNase-free water. To remove contaminating DNA, RNA cleanup and DNase treatment were carried out on columns (QIAGEN, United Kingdom) at room temperature using the RNeasy mini protocol according to the manufacturer's instructions.
Extracted RNA yield and quality.
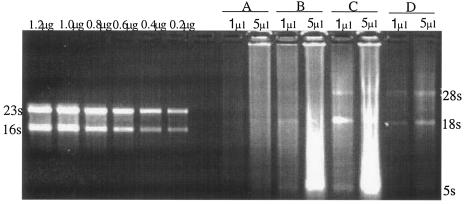
RNA from biopsies was intact after extraction with tissue joke. Figure 1 shows typical RNA obtained from four biopsies quantified by separation alongside 0.2 to 1.2 μg of Escherichia coli 16S and 23S rRNA standards. A total of 1 to 30 μg of total RNA was obtained from each skin sample. To demonstrate that RNA was free of DNA contamination, 1 μl of cDNA prepared with or without reverse transcriptase was amplified for the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene. Samples that were reverse transcribed resulted in a 203-bp product of the GAPDH gene. Samples that were not reverse transcribed gave a negative result, indicating that the RNA was free of DNA contamination. Only samples free of DNA contamination were analyzed.
FIG. 1.
Quantification of extracted RNA. One- and 5-μl portions of a total of 100 μl were separated by electrophoresis on a 2% agarose gel stained with ethidium bromide alongside 0.2 μg, 0.4 μg, 0.6 μg, 0.8 μg, 1.0 μg, and 1.2 μg of E. coli 23S/16S rRNA standards. Sections A to D represent RNA samples of four different subjects; the 28S, 18S, and 5S ribosomal bands are indicated.
Reverse transcription-PCR.
To synthesize cDNA, total RNA (140 ng) obtained from skin tissue was incubated with random primers (250 ng), 10 mM deoxynucleoside triphosphate mix (10 mM each dATP, dGTP, dCTP, and dTTP), made up to 12 μl with sterile RNase-free water, and incubated at 65°C for 5 min, after which it was quickly cooled on ice. A total of 4 μl 5× first-strand buffer (250 mM Tris-HCl [pH 8.3], 375 mM KCl, 15 mM MgCl2), 2 μl of 0.1 M dithiothreitol, and 1 μl RNaseOUT RNase inhibitor (40 U/μl; Invitrogen, United Kingdom) were added. The mix was incubated at 42°C for 2 min after which it was further incubated at 25°C for 10 min, 42°C for 50 min, and 70°C for 15 min with 1 μl of Superscript II reverse transcriptase (Invitrogen) and then at 37°C for 20 min with 1 μl of E. coli RNase H (2 units). A minus RT reaction was included for each sample to ascertain the absence of contaminating DNA.
A 1-μl sample of cDNA generated as above was amplified. Reaction mixtures contained 10 mM Tris, 50 mM KCl, 0.01% gelatin, 1.5 mM MgCl2, 0.1% Triton X-100 each; 0.125 mM (each) dATP, dCTP, dGTP, and dTTP; 1 μM each of GAPDH forward primer (CGAACCACTTTGTCAAGCTCA) and reverse primer (AGGGGAGATTCAGTGTGGTG); and 1 unit of SuperTaq polymerase (HT Biotechnology Ltd, United Kingdom) per 50 μl reaction volume. Mixtures with cDNA were placed in a thermocycler preheated to 94°C for 1 min. Cycling parameters were 30 cycles of denaturation at 94°C for 1 min, annealing at 55°C for 1 min, extension at 72°C for 1 min, and a final extension for 7 min at 72°C. Amplified products were separated by 2% agarose gel electrophoresis and visualized by a transilluminator/camera combination. Minus RT reactions that produced a signal were considered DNA contaminated, and RNA cleanup was repeated. Reverse transcription reactions that produced a 203-bp GAPDH product and were negative in the absence of reverse transcriptase were considered suitable for real-time PCR.
Real-time PCR.
PCR mixtures and cDNA for patients and the “calibrator” were loaded into the cytokine gene expression plate 1 arrayed with primers and probes for 12 human cytokines (IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-8, IL-10, IL-12p35, IL-12p40, IL-15, IFN-γ, and tumor necrosis factor alpha [TNF-α]) according to the manufacturer's instructions (Applied Biosystems). Primers and probes for an endogenous control (18S rRNA) were included in each well and simultaneously amplified along with the target amplicon to normalize for the amount of RNA added to a reaction. The 18S rRNA endogenous control was amplified in the VIC dye layer, and the target was amplified in the 6-carboxyfluorescein (FAM) dye layer. The endogenous control was primer limited to prevent it from competing for common reagents with the amplification of the target cytokine sequence. Amplicons included in this multiplexed PCR had equivalent efficiencies approaching 100%, and this property was used in the calculation of relative quantification values for the cytokine (24).
The PCR mixture for each well contained 25 μl TaqMan Universal PCR mixture (AmpliTaqGold, DNA polymerase, AmpErase UNG, dNTP with dUTP, and optimized buffer components), 20 μl RNase-free water, and 5 μl of cDNA. cDNA mixtures were loaded in duplicate, with each plate taking an array of three patient samples and one calibrator. Cycling conditions for the ABI PRISM 7700 sequence detection system were 50°C for 2 min for AmpErase UNG incubation, 95°C for 10 min for AmpliTaqGold DNA polymerase activation, 50 cycles of 95°C for 15 s for denaturation, and 60°C for 1 min for annealing and extension.
Quantification of cytokine mRNA.
The cycle threshold (CT), at which a significant amount of amplified target was generated in the ABI PRISM 7700 sequence detection system, was established. To normalize for the input RNA, the CT value generated for the 18S rRNA endogenous control in the VIC dye layer for each well was deducted from the CT value for target in the FAM dye layer, resulting in ΔCT. The average ΔCT value for the calibrator was deducted from ΔCT for each sample, generating a ΔΔCT value. The relative quantity of target normalized to an endogenous reference and relative to a calibrator is given by 2−AverageΔΔCT (24).
Samples were defined as negative when the signal did not attain threshold levels up to 36 cycles for the FAM dye layer and up to 23 cycles for the VIC dye layer as suggested by the manufacturer. Cytokines whose calibrator failed to amplify were excluded since relative quantification values could not be generated from such wells.
RESULTS
Punch biopsies taken from 44 subjects, 16 with nodules and 28 with ulcers, were confirmed to show M. ulcerans disease by a combination of histology analysis, AFB detection, culture, and PCR. The patients' ages ranged from 5 to 54 years (median, 9 years) for nodular and 5 to 38 years (median, 10 years) for ulcerated lesions (Table 1).
TABLE 1.
Characteristics of confirmed Buruli ulcer patients
| Characteristica | Nodules | Ulcers |
|---|---|---|
| No. of patients | 16 | 28 |
| Median age (yr) (range) | 9 (5-54) | 10 (5-38) |
| M:F ratio | 6:10 | 17:11 |
| Mean duration of skin lesion (mo) ± SE | 1.9 ± 0.3 | 3.8 ± 0.7 |
| Range of duration (mo) | 0.3-6.0 | 0.5-12.0 |
| Site of lesion | 13 | 12 |
| Upper limb | ||
| Lower limb | 1 | 14 |
| Trunk | 2 | 1 |
| Head/neck | 0 | 1 |
F, female; M, male; SE, standard error of the mean.
Expression of cytokine mRNA in tissues of Buruli nodules and ulcers.
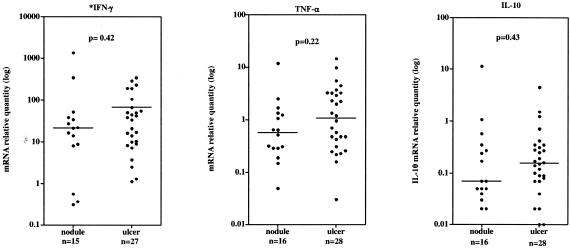
Table 2 shows the quantities of mRNA relative to the calibrator expressed in nodules and ulcers. Although the median relative quantities of most cytokines were higher for ulcers than for nodules, only IL-8 expression was significantly higher in ulcers. Figure 2 shows that there was a wide range of expression of IFN-γ, TNF-α, and IL-10 in nodules and ulcers relative to a calibrator RNA. Most lesions showed substantial IFN-γ expression, with the widest range of expression occurring in nodules. mRNA expression for IL-4 and IL-5 in most nodules and ulcers was below the detection level of this assay. Significant IL-4 mRNA was detected in only two nodules and two ulcers, in which relative quantities were 1.91 and 0.55 for nodules and 1.16 and 0.44 for ulcers.
TABLE 2.
Cytokine mRNA expression in nodular and ulcerative Buruli lesions determined by real-time PCR
| Target cytokine or chemokine | Median relative quantity of cytokine mRNA (range) |
P valuea | |
|---|---|---|---|
| Nodules (n = 16) | Ulcers (n = 28) | ||
| IFN-γb | 21.63 (0.31-1,380) | 33.82 (1.13-341.3) | 0.42 |
| TNF-α | 0.58 (0.05-11.7) | 0.96 (0.03-14.4) | 0.22 |
| IL-12p35 | 3.19 (0-35.3) | 3.49 (0-334.3) | 0.47 |
| IL-12p40 | 1.01 (0-109.9) | 3.24 (0-149.6) | 0.09 |
| IL-1β | 266.9 (9.3-37,120) | 430.5 (3.7-348,300) | 0.17 |
| IL-15 | 18.51(4.5-225.2) | 19.23 (3.3-318.5) | 0.81 |
| IL-8 | 78.55 (4.9-18,950) | 456.7 (17.5-77,400) | 0.004 |
| IL-10 | 0.07 (0.02-11.24) | 0.16 (0.01-4.45) | 0.43 |
P values refer to comparisons between 16 nodules and 28 ulcers for each cytokine using the Mann-Whitney test. A P value of <0.05 was considered a significant difference.
IFN-γ results for 1 nodule and 1 ulcer were not available.
FIG. 2.
Median relative quantity of mRNA for cytokines IFN-γ, TNF-α, and IL-10 in nodular and ulcerated Buruli lesions determined by real-time PCR. Threshold cycles of amplification for each cytokine target were normalized to 18S rRNA and quantified relative to a control human RNA. The median relative quantity is indicated by a horizontal line. P values refer to comparisons between nodules and ulcers for each cytokine using the Mann- Whitney test. A P value of <0.05 was considered a significant difference. *, an IFN-γ result not available for one nodule and one ulcer.
The relationship between tissue cytokine expression and an acute inflammatory or a granulomatous response.
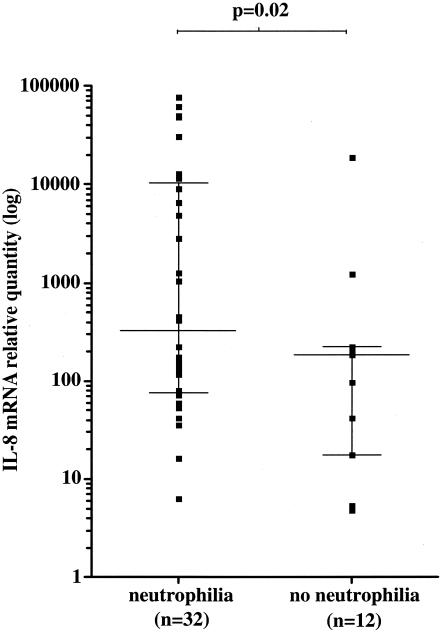
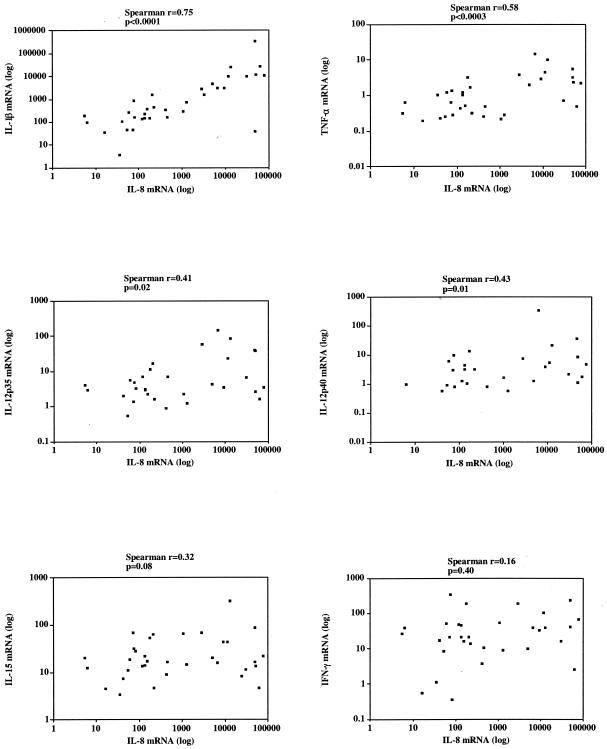
Six out of 12 Buruli nodules and 22 out of 28 Buruli ulcers had histological neutrophilia. There was no clinical evidence of secondary bacterial infection in any of the lesions. Cytokines and chemokines thought to be associated with an acute inflammatory response were correlated with the presence of histological neutrophilia as a marker of an acute inflammatory response. There was a significant difference between IL-8 mRNA expression in 32 tissues with neutrophilia (10,680 ± 2,966, mean ± standard error of the mean) and 12 without neutrophilia (1,773 ± 1,565, mean ± standard error of the mean) (P = 0.008) (Fig. 3). Interleukin 8 was coexpressed with IL-1β, TNF-α, IL-12p35, and IL-12p40 in tissues with neutrophilia. There was a strong positive correlation of IL-8 and IL-1β (Spearman r = 0.75) (P < 0.0001) and TNF-α (Spearman r = 0.58) (P < 0.0003) and significant correlation with IL-12p35 (Spearman r = 0.41) (P = 0.02) and IL12p40 (Spearman r = 0.43) (P = 0.01). In contrast, there was no correlation between the expression of IL-8 and IL-15 (Spearman r = 0.32) (P = 0.08) or IFN-γ (Spearman r = 0;16, P = 0.40) (Fig. 4).
FIG. 3.
Median relative quantity (horizontal lines) of IL-8 mRNA determined by real-time PCR for M. ulcerans disease tissues with histological neutrophilia and without neutrophilia. Values represent threshold cycles of amplification normalized to 18S rRNA and relative to a calibrator reference human RNA. The median plus or minus the interquartile range is indicated for each group (vertical lines). Statistical differences were determined using the Mann-Whitney nonparametric test. A P value of <0.05 was considered a significant difference.
FIG. 4.
The correlation of IL-8 mRNA expression with the expression of other proinflammatory cytokines in tissue biopsies taken from M. ulcerans disease patients with histological neutrophilia. There was a significant positive Spearman's correlation between IL-8 and IL-1β, TNF-α, IL-12p35, and IL-12p40 but no correlation with IL-15 and IFN-γ. A P value of <0.05 was considered a significant difference. Each point represents the value for a single individual.
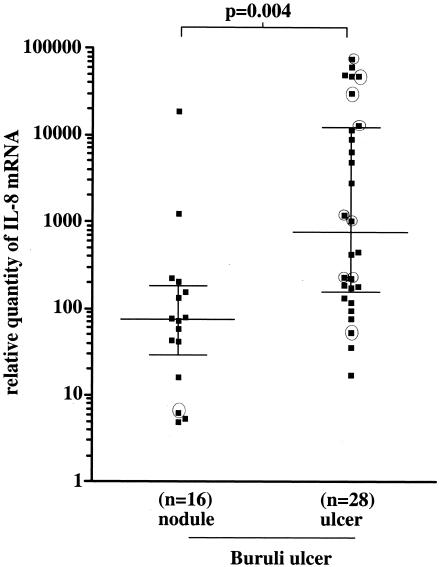
IL-8 expression was significantly higher in the 28 ulcers than in the 16 nodules (Fig. 5). Nine patients had received antimycobacterial drugs (rifampin, 10 mg/kg of body weight, and streptomycin, 15 mg/kg) for 2 to 8 weeks prior to biopsy. Of these, six patients with ulcers had relative tissue IL-8 mRNA levels above the median for the group and levels of other neutrophil-associated cytokines, IL-1β and TNF-α, were also higher (Table 3). One patient with a nodule who had received antibiotics for 6 weeks had low mRNA expression of neutrophil-associated cytokines; this patient had severe disease with a previous Buruli ulcer scar and a concurrent active Buruli ulcer.
FIG. 5.
Median relative quantity (horizontal lines) of IL-8 mRNA expression in nodular and ulcerated forms of Buruli ulcer. Each point represents the value for a single individual. Statistical differences were determined using the Mann-Whitney nonparametric test. A P value of <0.05 was considered a significant difference. ○, patients who had received antibiotic treatment before skin biopsy.
TABLE 3.
Expression of neutrophil-associated cytokine mRNAs in untreated Buruli lesions compared with ulcers treated with the combination streptomycin (15 mg/kg) and rifampin (10 mg/kg) daily for 2 to 8 weeksa
| Cytokine | Median relative quantity of cytokine mRNA (range)b |
||
|---|---|---|---|
| Buruli noduleb |
Buruli ulcer after antibiotics (n = 9) | ||
| No antibiotic treatment (n = 15) | No antibiotic treatment (n = 19) | ||
| IL-8 | 80.7 (4.9-18,950) | 231.5 (17.5-62,000) | 5,961.0 (41.5-77,400) |
| IL-1β | 312 (9.3-37,120) | 430.5 (3.7-348,300) | 4,928 (108.8-11,710) |
| TNF-α | 0.64 (0.05-11.7) | 0.96 (0.03-14.4) | 1.37 (0.23-4.5) |
| IL-12p35 | 3.03 (0-35.3) | 4.23 (0-334.3) | 3.06 (0.9-23.7) |
| IL-12p40 | 1.06 (0-109.9) | 3.27 (0-149.6) | 0.97 (0-5.3) |
Ulcers were treated with streptomycin (15 mg/kg) and rifampin (10 mg/kg) daily for 2 to 8 weeks. There were no significant differences between ulcers treated with antibiotic and those that were untreated (Mann-Whitney P value, >0.05).
One patient with a nodule was excluded because he had received antibiotics for 6 weeks. He had severe disease with a previous Buruli ulcer scar and a concurrent active Buruli ulcer and he had low mRNA expression of neutrophil associated cytokines.
Median relative quantities of mRNA for cytokines and chemokines thought to be associated with granuloma formation were quantified and correlated with the presence of histological granulomas in tissues containing granulomas relative to tissues without granulomas. Table 4 shows that mRNA expression for IFN-γ, IL-1β, IL-8, IL-12p35, IL12p40, IL-15, TNF-α, and IL-10 was significantly higher in 10 tissue biopsy samples containing granulomas than in 34 samples without granulomas.
TABLE 4.
Relative expression of cytokine mRNA in tissue from Buruli ulcers with or without granulomas
| Target cytokinea | Relative quantity of cytokine mRNA (range) |
P valueb | |
|---|---|---|---|
| Granulomas present (n = 10) | Granulomas absent (n = 34) | ||
| IFN-γ | 79.4 (14.0-1,380) | 16.9 (0.3-341.3) | 0.006 |
| TNF-α | 3.3 (0.31-11.7) | 0.5 (0.03-3.2) | 0.005 |
| IL-12p35 | 21.2 (1.7-110.0) | 2.6 (0-11.1) | 0.0001 |
| IL-12p40 | 6.6 (1.2-35.3) | 1.3 (0-13.6.0) | 0.001 |
| IL-1β | 6,158 (13.2-348,300) | 278.4 (3.7-26,710) | 0.02 |
| IL-15 | 42.0 (4.8-318.5) | 16.9 (3.4-65.8) | 0.02 |
| IL-8 | 7,156 (117.8-48,980) | 134.8 (4.9-77,400) | 0.01 |
| IL-10 | 0.32 (0.07-1.50) | 0.09 (0.01-11.0) | 0.02 |
An IFN-γ result was not available for two subjects.
P values were obtained by the Mann-Whitney test. A P value of <0.05 was considered significant.
Correlation between tissue IFN-γ mRNA expression and secretion of IFN-γ in whole blood stimulated with M. ulcerans 1 sonicate antigens.
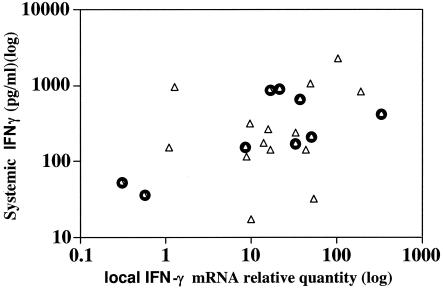
Peripheral blood and a punch biopsy of a Buruli lesion were taken concurrently from 23 patients with M. ulcerans disease. Whole peripheral blood was stimulated with M. ulcerans sonicate antigens, and IFN-γ secretion was measured by ELISA as described previously (30). Figure 6 shows that there was a weak correlation between tissue IFN-γ mRNA expression and the systemic IFN-γ response (Spearman r = 0.40) but it was not statistically significant (P = 0.06). When only the 9 patients with nodules were included, there was a significant correlation (Spearman r = 0.53) (P < 0.05), whereas 14 patients with ulcers did not show a significant correlation (Spearman r = 0.25) (P = 0.40).
FIG. 6.
IFN-γ mRNA expression levels measured at the site of disease by real-time PCR were correlated with IFN-γ secretion measured by ELISA after overnight stimulation of whole blood by M. ulcerans 1 sonicate in 23 Buruli patients, 14 patients with ulcers (▵), and 9 with nodules ( ). Each square represents the results for one patient. There was no significant correlation between tissue IFN-γ mRNA expression and the systemic IFN-γ response for the group as a whole (Spearman r = 0.40) (P = 0.06) or for patients with ulcers (Spearman r = 0.25) (P = 0.40), but the correlation was significant for patients with nodules (Spearman r = 0.53) (P < 0.05). A P value of <0.05 was considered significant.
DISCUSSION
This study showed that a broad range of cytokines were expressed in M. ulcerans-infected tissues, including Th1 cytokines TNF-α and IFN-γ. The data suggest that patients were able to mount a Th1 response to M. ulcerans in infected tissue despite the presence of mycolactone, which is known to influence cytokine production in vitro (27). These results are similar to our recent findings that, after stimulation with M. ulcerans antigens, blood from patients with active Buruli lesions produced both IFN-γ and IL-10. IFN-γ production was higher in patients with ulcers than in those with early lesions, and it reached a peak in healed Buruli ulcers, whereas IL-10 production did not persist in patients with healed lesions (30). IL-10 is produced by Th2 cells but also by others, such as activated B cells and some Th1 cells (19), so it is not certain whether IL-10 was present as a regulator of a Th1 response or as a component of a Th2 response. In Buruli lesions from the present group of patients, IFN-γ was strongly expressed in both nodules and ulcers, suggesting that a Th1 response begins early in the disease. In other mycobacterial diseases, such as tuberculosis and leprosy, a Th1 response has been associated with protection (1, 21). Self healing of early M. ulcerans lesions was observed in placebo recipients during a trial of clofazamine therapy (34), so one can speculate that patients able to mount a strong Th1 response at an early stage control the infection and do not develop ulcerative disease. It is interesting to note that two of the highest IFN-γ results were in nodules, but there is no way of knowing if these lesions would have healed without any intervention since they were excised.
Only one other group has studied cytokine mRNA in M. ulcerans-infected human tissue. Semiquantitative assessment using RT-PCR showed high IFN-γ and low IL-10 mRNA expression in five nodules from patients in French Guyana relative to low IFN-γ and high IL-10 expression in nine ulcers, suggesting that, during the course of M. ulcerans infection, there was increased production of IL-10, perhaps leading to the suppression of IFN-γ production (32). We did not find a low IFN-γ expression in most ulcers, but there were wide variations between individual tissue samples, implying that large patient numbers need to be studied as was the case here. Differences in the study populations are unlikely to account for the different results in these two studies since the disease course is similar in West Africa and French Guyana. There may be a relationship between tissue IFN-γ expression and natural healing of ulcers, but since treatment is available, it is not possible, for ethical reasons, to study this.
Measurement of mRNA using real-time PCR is more accurate than that using other methods available at present, but it does not give an absolute measure of individual cytokines since the results relate to the amount of mRNA for each cytokine present in a reference sample (24). However, there was more than a log difference between mRNA values for IFN-γ and both TNF-α and IL-10, suggesting that IFN-γ was more strongly expressed. IL-8 was also strongly expressed, particularly in tissues containing neutrophils. Immunocytochemistry would be needed to show which cells were producing this chemokine, but whether the cells were neutrophils or macrophages, the correlation between the expression of IL-8 and that of IL-1β, TNF-α, IL-12p35, and IL-12p40 suggests that it was related to the initiation of a Th1 response. It is well recognized that both ulcers and nodules contain neutrophils (16), but in our study, ulcers showed significantly higher expression of IL-8 compared with that of nodules; the strongest IL-8 responses were observed in patients who had received a combination of rifampin and streptomycin immediately prior to biopsy, suggesting that, once mycolactone production has ceased, neutrophils and/or monocytes acquire the ability to phagocytose organisms and to recruit more inflammatory cells by chemotaxis. Buruli ulcers become painful and inflamed when there is secondary bacterial infection, and while this was not observed in any lesions included in the study, the possibility that tissue neutrophilia was related to the presence of other bacteria cannot be entirely ruled out.
It was only possible to take one biopsy for histology and one from an adjacent area for cytokine studies, but the large number of patients studied probably compensates for variations in histology which occur from one part of a lesion to another and for variations in cytokine values between individuals which may be due either to differences in the immune responses of subjects or to sampling.
In the early stages of M. ulcerans disease, histological examination shows extensive areas of subcutaneous fatty necrosis containing abundant clumps of extracellular acid-fast bacilli with little inflammation. Inflammation is separate from areas of necrosis, and particularly in long-standing and healing lesions, there is a mixture of acute and chronic inflammation, sometimes including granulomas, and the number of AFB is greatly reduced (5, 8, 18, 37). The presence of granulomas in M. ulcerans-infected lesions has been frequently associated in the literature with the healing phase of the disease (17, 28, 36). M. ulcerans lesions with granulomas expressed significantly higher mRNA levels of IFN-γ, IL-12p35, IL-12p40, IL-15, IL-8, IL-1β, and TNF-α compared with those of lesions with no granulomas, suggesting the importance of these cytokines for containment of the disease. Expression of most of these cytokines has been associated with granuloma formation in tuberculosis and leprosy (2, 9, 10, 23, 39). Interleukin-1β was important in the early recruitment stages of granuloma formation, while TNF-α may take part in later maintenance or effector functions of granulomas (26, 33). Interleukin 15 has not been investigated previously in this context, but it was expressed more strongly in tuberculoid leprosy than in lepromatous leprosy, suggesting it also has a role in containment of infection (20).
There was some correlation between the results for the expression of IFN-γ and IL-10 mRNA in tissue and those for the secretion of IFN-γ and IL-10 in whole blood stimulated with M. ulcerans sonicate in 23 patients from whom both samples were taken, so the local immune response was broadly reflected in the systemic response. This may be helpful for future studies in which it is not possible to assess the tissue cytokine profile. Our results after whole blood was stimulated with M. ulcerans sonicate (30) differed from those obtained when peripheral blood mononuclear cells (PBMC) from 10 Australian subjects with healed M. ulcerans disease and 4 with active disease were stimulated for 6 days with live M. ulcerans or live Mycobacterium bovis. They produced less IFN-γ than PBMC from healthy tuberculin-positive individuals, suggesting there was T-cell anergy to mycobacterial antigens (13). Gooding et al. further described a patient with acquired T-helper lymphocyte anergy to mycobacteria following infection with M. ulcerans (15). Using reverse transcription and PCR, they showed that after stimulation with live M. ulcerans or M. bovis, PBMC of Buruli patients expressed mainly the Th2 cytokines IL-4, IL-5, IL-6, and IL-10, whereas unaffected contacts responded with Th1 cytokines IFN-γ and IL-12 (14). In similar experiments using PBMC from patients in French Guyana stimulated with whole-killed M. ulcerans or M. bovis, five patients with early nodular disease showed predominantly Th1 cytokine profiles, while nine patients with ulcers had Th2 cytokine profiles (32). These experiments, which are the only other ones using M. ulcerans antigens, differed from ours in several ways, including the antigen preparations, the cells stimulated, and the duration of stimulation, but perhaps the most important difference was the number of subjects studied since it is clear that there were large variations between subjects. Westenbrink et al. showed higher IFN-γ production after stimulation with tuberculin in patients with late Buruli ulcer lesions than in controls, but no specific M. ulcerans antigens were used in that study (38). To date, mycolactone has not been quantified in infected tissues, but by extrapolation from the guinea pig model, it is likely to be present in the areas of subcutaneous fatty necrosis where clumps of AFB are frequently observed. Overall, the present results suggest that patients with M. ulcerans disease are able to mount the type of immune response that has been described for other mycobacteria, such as Mycobacterium tuberculosis, despite the presence of mycolactone in the tissues. It is thought that most Buruli ulcers heal eventually without treatment, and the rate at which this occurs may be determined by the development of the above-described pattern of immune response.
It is not known how the immune response is initiated since mycolactone inhibits the phagocytosis of M. ulcerans. There is recent evidence that mouse macrophages can sometimes phagocytose M. ulcerans, but they soon died and may not have had enough time to initiate an immune response (6). It is perhaps more likely that dead organisms or their antigens are processed by macrophages in sites adjacent to the infected areas permeated by mycolactone. Effector cells generated as a result would not be able to function in the presence of mycolactone, and infection could be eliminated only if mycolactone production ceased. More studies of the production of mycolactone in infected tissues and how it can be inhibited are needed.
Acknowledgments
This study was supported by a grant from the Wellcome Trust. R. Phillips had a Wellcome Trust Training Research Fellowship Award for Research into Infectious Diseases for Scientists from Tropical and Developing Countries.
We thank Ohene Adjei and Edwin Ampadu, Ghana National Buruli Ulcer Program, for their assistance with this study.
Editor: J. L. Flynn
REFERENCES
- 1.Alcais, A., M. Mira, J. L. Casanova, E. Schurr, and L. Abel. 2005. Genetic dissection of immunity in leprosy. Curr. Opin. Immunol. 17:44-48. [DOI] [PubMed] [Google Scholar]
- 2.Aung, H., Z. Toossi, S. M. McKenna, P. Gogate, J. Sierra, E. Sada, and E. A. Rich. 2000. Expression of transforming growth factor-β but not tumor necrosis factor-α, interferon-γ, and interleukin-4 in granulomatous lung lesions in tuberculosis. Tuber. Lung Dis. 80:61-67. [DOI] [PubMed] [Google Scholar]
- 3.Boom, R., C. J. Sol, M. M. Salimans, C. L. Jansen, P. M. Wertheim-van Dillen, and J. van der Noordaa. 1990. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 28:495-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burchard, G. D., and M. Bierther. 1986. Buruli ulcer: clinical pathological study of 23 patients in Lambarene, Gabon. Trop. Med. Parasitol. 37:1-8. [PubMed] [Google Scholar]
- 5.Connor, D. H., and H. F. Lunn. 1965. Mycobacterium ulcerans infection (with comments on pathogenesis). Int. J. Lepr. 33(Suppl.):698-709. [PubMed] [Google Scholar]
- 6.Coutanceau, E., L. Marsollier, R. Brosch, E. Perret, P. Goossens, M. Tanguy, S. T. Cole, P. L. Small, and C. Demangel. 2005. Modulation of the host immune response by a transient intracellular stage of Mycobacterium ulcerans: the contribution of endogenous mycolactone toxin. Cell. Microbiol. 7:1187-1196. [DOI] [PubMed] [Google Scholar]
- 7.Dobos, K. M., E. A. Spotts, B. J. Marston, C. R. Horsburgh, Jr., and C. H. King. 2000. Serologic response to culture filtrate antigens of Mycobacterium ulcerans during Buruli ulcer disease. Emerg. Infect. Dis. 6:158-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dodge, O. G. 1964. Mycobacterial skin ulcers in Uganda: histopathological and experimental aspects. J. Pathol. Bacteriol. 88:169-174. [DOI] [PubMed] [Google Scholar]
- 9.Fenhalls, G., L. Stevens, J. Bezuidenhout, G. E. Amphlett, K. Duncan, P. Bardin, and P. T. Lukey. 2002. Distribution of IFN-γ, IL-4 and TNF-α protein and CD8 T cells producing IL-12p40 mRNA in human lung tuberculous granulomas. Immunology 105:325-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fenhalls, G., L. Stevens, L. Moses, J. Bezuidenhout, J. C. Betts, P. van Helden, P. T. Lukey, and K. Duncan. 2002. In situ detection of Mycobacterium tuberculosis transcripts in human lung granulomas reveals differential gene expression in necrotic lesions. Infect. Immun. 70:6330-6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.George, K. M., L. P. Barker, D. M. Welty, and P. L. Small. 1998. Partial purification and characterization of biological effects of a lipid toxin produced by Mycobacterium ulcerans. Infect. Immun. 66:587-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.George, K. M., D. Chatterjee, G. Gunawardana, D. Welty, J. Hayman, R. Lee, and P. L. Small. 1999. Mycolactone: a polyketide toxin from Mycobacterium ulcerans required for virulence. Science 283:854-857. [DOI] [PubMed] [Google Scholar]
- 13.Gooding, T. M., P. D. Johnson, D. E. Campbell, J. A. Hayman, E. L. Hartland, A. S. Kemp, and R. M. Robins-Browne. 2001. Immune response to infection with Mycobacterium ulcerans. Infect. Immun. 69:1704-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gooding, T. M., P. D. Johnson, M. Smith, A. S. Kemp, and R. M. Robins-Browne. 2002. Cytokine profiles of patients infected with Mycobacterium ulcerans and unaffected household contacts. Infect. Immun. 70:5562-5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gooding, T. M., A. S. Kemp, R. M. Robins-Browne, M. Smith, and P. D. Johnson. 2003. Acquired T-helper 1 lymphocyte anergy following infection with Mycobacterium ulcerans. Clin. Infect. Dis. 36:1076-1077. [DOI] [PubMed] [Google Scholar]
- 16.Guarner, J., J. Bartlett, E. A. Whitney, P. L. Raghunathan, Y. Stienstra, K. Asamoa, S. Etuaful, E. Klutse, E. Quarshie, T. S. van der Werf, W. T. van der Graaf, C. H. King, and D. A. Ashford. 2003. Histopathologic features of Mycobacterium ulcerans infection. Emerg. Infect. Dis. 9:651-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayman, J. 1993. Out of Africa: observations on the histopathology of Mycobacterium ulcerans infection. J. Clin. Pathol. 46:5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayman, J., and A. McQueen. 1985. The pathology of Mycobacterium ulcerans infection. Pathology 17:594-600. [DOI] [PubMed] [Google Scholar]
- 19.Janeway, C. A., P. Travers, M. Walport, and M. Shlomchik. 2005. Immunobiology, 6th ed. Garland Science Publishing, New York, N.Y.
- 20.Jullien, D., P. A. Sieling, K. Uyemura, N. D. Mar, T. H. Rea, and R. L. Modlin. 1997. IL-15, an immunomodulator of T cell responses in intracellular infection. J. Immunol. 158:800-806. [PubMed] [Google Scholar]
- 21.Kaufmann, S. H. 2002. Protection against tuberculosis: cytokines, T cells, and macrophages. Ann. Rheum Dis. 61(Suppl. 2):ii54-ii58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krieg, R. E., W. T. Hockmeyer, and D. H. Connor. 1974. Toxin of Mycobacterium ulcerans. Production and effects in guinea pig skin. Arch. Dermatol. 110:783-788. [DOI] [PubMed] [Google Scholar]
- 23.Little, D., S. Khanolkar-Young, A. Coulthart, S. Suneetha, and D. N. Lockwood. 2001. Immunohistochemical analysis of cellular infiltrate and gamma interferon, interleukin-12, and inducible nitric oxide synthase expression in leprosy type 1 (reversal) reactions before and during prednisolone treatment. Infect. Immun. 69:3413-3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 25.MacCallum, P., J. C. Tolhurst, G. Buckle, and H. A. Sissons. 1948. A new mycobacterial infection in man. J. Pathol. Bacteriol. 60:93-122. [PubMed] [Google Scholar]
- 26.Myatt, N., G. Coghill, K. Morrison, D. Jones, and I. A. Cree. 1994. Detection of tumour necrosis factor alpha in sarcoidosis and tuberculosis granulomas using in situ hybridisation. J. Clin. Pathol. 47:423-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pahlevan, A. A., D. J. Wright, C. Andrews, K. M. George, P. L. Small, and B. M. Foxwell. 1999. The inhibitory action of Mycobacterium ulcerans soluble factor on monocyte/T cell cytokine production and NF-κB function. J. Immunol. 163:3928-3935. [PubMed] [Google Scholar]
- 28.Palenque, E. 2000. Skin disease and nontuberculous atypical mycobacteria. Int. J. Dermatol. 39:659-666. [DOI] [PubMed] [Google Scholar]
- 29.Phillips, R., C. Horsfield, S. Kuijper, A. Lartey, I. Tetteh, S. Etuaful, B. Nyamekye, P. Awuah, K. M. Nyarko, F. Osei-Sarpong, S. Lucas, A. H. Kolk, and M. Wansbrough-Jones. 2005. Sensitivity of PCR targeting the IS2404 insertion sequence of Mycobacterium ulcerans in an assay using punch biopsy specimens for diagnosis of Buruli ulcer. J. Clin. Microbiol. 43:3650-3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phillips, R., C. Horsfield, S. Kuijper, S. Sarfo, J. Obeng-Baah, S. Etuaful, B. Nyamekye, P. Awuah, K. Nyarko, F. Osei-Sarpong, S. Lucas, A. H. J. Kolk, and M. Wansbrough-Jones. 2006. Cytokine response to antigen stimulation of whole blood from patients with Mycobacterium ulcerans disease compared to that from patients with tuberculosis. Clin. Vaccine Immunol. 13:253-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Portaels, F., P. Johnson, and W. M. Meyers. 2001. Buruli ulcer: Diagnosis of Mycobacterium ulcerans disease. [Online.] http://www.who.int/burul:/information/diagnosis/en/index15.html. World Heath Organization, Geneva, Switzerland.
- 32.Prevot, G., E. Bourreau, H. Pascalis, R. Pradinaud, A. Tanghe, K. Huygen, and P. Launois. 2004. Differential production of systemic and intralesional gamma interferon and interleukin-10 in nodular and ulcerative forms of Buruli disease. Infect. Immun. 72:958-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Remick, D. G., W. E. Scales, M. A. May, M. Spengler, D. Nguyen, and S. L. Kunkel. 1988. In situ hybridization analysis of macrophage-derived tumor necrosis factor and interleukin-1 mRNA. Lab. Investig. 59:809-816. [PubMed] [Google Scholar]
- 34.Revill, W. D., R. H. Morrow, M. C. Pike, and J. Ateng. 1973. A controlled trial of the treatment of Mycobacterium ulcerans infection with clofazimine. Lancet ii:873-877. [DOI] [PubMed] [Google Scholar]
- 35.Stanford, J. L., W. D. Revill, W. J. Gunthorpe, and J. M. Grange. 1975. The production and preliminary investigation of Burulin, a new skin test reagent for Mycobacterium ulcerans infection. J. Hyg. 74:7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stienstra, Y., W. T. van der Graaf, G. J. te Meerman, T. H. The, L. F. de Leij, and T. S. van der Werf. 2001. Susceptibility to development of Mycobacterium ulcerans disease: review of possible risk factors. Trop. Med. Int. Health 6:554-562. [DOI] [PubMed] [Google Scholar]
- 37.Uganda Buruli Group. 1970. Clinical features and treatment of pre-ulcerative Buruli lesions (Mycobacterium ulcerans infection). Report II of the Uganda Buruli Group. Br. Med. J. 2:390-393. [PMC free article] [PubMed] [Google Scholar]
- 38.Westenbrink, B. D., Y. Stienstra, M. G. Huitema, W. A. Thompson, E. O. Klutse, E. O. Ampadu, H. M. Boezen, P. C. Limburg, and T. S. van der Werf. 2005. Cytokine responses to stimulation of whole blood from patients with Buruli ulcer disease in Ghana. Clin. Diagn. Lab. Immunol. 12:125-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamamura, M., K. Uyemura, R. J. Deans, K. Weinberg, T. H. Rea, B. R. Bloom, and R. L. Modlin. 1991. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science 254:277-279. [DOI] [PubMed] [Google Scholar]