Abstract
Mycobacterium avium subsp. paratuberculosis is the cause of Johne's disease in cattle and other ruminants. M. avium subsp. paratuberculosis infection of the bovine host is not well understood; however, it is assumed that crossing the bovine intestinal mucosa is important in order for M. avium subsp. paratuberculosis to establish infection. To examine the ability of M. avium subsp. paratuberculosis to infect bovine epithelial cells in vitro, Madin-Darby bovine kidney (MDBK) epithelial cells were exposed to M. avium subsp. paratuberculosis. It was observed that bacteria can establish infection and replicate within MDBK cells. M. avium subsp. paratuberculosis also has been reported to infect mammary tissue and milk, and we showed that M. avium subsp. paratuberculosis infects bovine mammary epithelial cells (MAC-T cell line). Using polarized MAC-T cell monolayers, it was also determined that M. avium subsp. paratuberculosis crosses apical and basolateral surfaces with approximately the same degree of efficiency. Because M. avium subsp. paratuberculosis can be delivered to the naïve host by milk, it was investigated whether incubation of M. avium subsp. paratuberculosis with milk has an effect on invasion of MDBK cells. M. avium subsp. paratuberculosis exposed to milk entered epithelial cells with greater efficiency than M. avium subsp. paratuberculosis exposed to broth medium or water (P < 0.01). Growth of M. avium subsp. paratuberculosis within MAC-T cells also resulted in augmented ability to subsequently infect bovine MDBK cells (P < 0.001). Microarray analysis of intracellular M. avium subsp. paratuberculosis RNA indicates the increased transcription of genes which might be associated with an invasive phenotype.
Mycobacterium avium subsp. paratuberculosis is the etiologic agent of Johne's disease in cattle and other ruminants. It is assumed that M. avium subsp. paratuberculosis infects the young calf by crossing the intestinal barrier. Previous work (3, 26) has indicated that the interaction of M. avium subsp. paratuberculosis with bovine epithelial cells is a complex process which might involve participation of several bacterial and host factors. For example, it has been reported that both in calves and in mice, challenge by the gastrointestinal route results in M. avium subsp. paratuberculosis infecting M cells in the Peyer's patches (23, 26). Recently, Secott and colleagues have suggested that the invasion of the intestinal mucosa by M. avium subsp. paratuberculosis is secondary to the binding to fibronectin (26). In addition, Bannantine and colleagues demonstrated a role for a 35-kDa M. avium subsp. paratuberculosis protein in the invasion of cultured bovine epithelial cells (5). The 35-kDa protein is exposed in the outer layer of M. avium subsp. paratuberculosis and has also been associated with Mycobacterium avium invasion of human intestinal cells (22).
After M. avium subsp. paratuberculosis crosses the intestinal mucosa, the infection spreads to other organs, leading to the advanced stages of disease. Several studies have reported the presence of M. avium subsp. paratuberculosis in the mammary glands and in milk of symptomatic or asymptomatic animals (6, 28-30). This observation raises the possibility that the mammary gland could be a reservoir for M. avium subsp. paratuberculosis. The environment inside the mammary gland is believed to be hyperosmolar (milk) and hypoxic (28). In addition, M. avium subsp. paratuberculosis organisms infecting mammary epithelial cells might suffer the influence of the intracellular environment. In fact, M. avium subsp. paratuberculosis may remain in contact with either the intracellular or milk environment for periods of up to 24 h before being excreted. Previous studies using Mycobacterium avium subsp. avium have shown that the environment to which the bacterium is exposed in the host can influence the expression of genes associated with its ability to enter epithelial cells. Incubation of M. avium subsp. avium in low oxygen tension or increased osmolarity conditions significantly enhances its ability to enter intestinal epithelial cells (8). A previous study on interactions between M. avium subsp. avium and environmental amoebae has shown that infection of amoebae results in enhanced virulence (14). Similar findings were reported regarding the M. avium subsp. paratuberculosis 35-kDa protein (5), which was shown to be expressed under anaerobic and hyperosmolar conditions (5).
It is reasonable to hypothesize that bacterial exposure to the complex conditions in the mammary gland or mammary gland epithelial cells may have an effect on virulence gene expression and, therefore, on the efficacy of M. avium subsp. paratuberculosis invasion of the intestinal mucosa.
The objectives of this work were to determine whether M. avium subsp. paratuberculosis has the ability to enter cultured bovine epithelial cells in vitro and whether this characteristic can be influenced by exposure to milk or the intracellular environment of mammary epithelial cells.
MATERIALS AND METHODS
Cell lines.
A bovine epithelial cell line (Madin-Darby bovine kidney [MDBK]) was purchased from American Type Culture Collection (ATCC, Manassas, VA) and maintained on Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated fetal bovine serum. A bovine mammary epithelial cell line (MAC-T) was kindly provided by Lewis Sheffield (Department of Dairy Science, University of Wisconsin). MAC-T cells were maintained on Dulbecco's modified Eagle's medium with 10% heat-inactivated fetal bovine serum, 5 μg/ml insulin, and 1 μg/ml hydrocortisone.
Bacteria.
M. avium subsp. paratuberculosis ATCC 19698, a bovine clinical isolate from an animal with Johne's disease, was purchased from ATCC. It was grown at 37οC on modified Middlebrook 7H11 agar supplemented with Mycobactin J (2 mg/liter) and oleic acid-albumin-dextrose-catalase (OADC) (10%, vol/vol) or in 7H9 broth with Mycobactin J (2 mg/liter) and OADC (10%, vol/vol). For the invasion assay, individual colonies were selected, and a bacterial suspension was prepared using Hanks' balanced salt solution (HBSS) to give turbidity equivalent to a 0.5 McFarland standard. The M. avium subsp. paratuberculosis suspension (5 ml) was then passed through a 30-gauge needle 10 times, and clumps in the final suspension were allowed to settle. The top 1 ml out of the 5-ml suspension containing dispersed bacteria was used for the invasion assay.
Invasion assay.
The invasion assays were carried out using the methods described by Bermudez and Young and by Sangari et al. (10, 24). Briefly, 24-well tissue culture plates (Corning Costar, New York) were seeded with 104 MDBK or MAC-T cells, and monolayers were grown in an atmosphere of 5% CO2 at 37οC until confluence. Before infection, the medium was replaced with fresh culture medium. Monolayers were then infected with approximately 2 × 106 bacteria (approximate ratio, 10 bacteria:1 cell) and incubated at 37οC in 5% CO2 for up to 4 h, depending upon the experimental design. After incubation, monolayers were washed three times with HBSS and then treated with 200 μg/ml of amikacin for 2 h at 37οC to kill extracellular bacteria, as shown in the M. avium system (8, 10) and confirmed using M. paratuberculosis (data not shown). Following incubation, the supernatant was removed and cell monolayers were washed three times with HBSS. To lyse the cells, wells were treated with 0.5 ml Triton X-100 (0.1%) for 10 min. Subsequently, 0.5 ml 7H9 broth containing Mycobactin J (2 mg/liter) and OADC (10%, vol/vol) was added to each well, and cells were disrupted by vigorous pipetting. Lysates were collected, serially diluted, and plated onto 7H11 agar for colony count. The percentage of invasion was calculated as the fraction of the inoculated bacteria that was recovered from the cell lysate.
M. avium subsp. paratuberculosis survival in MDBK and MAC-T cells.
Confluent monolayers of bovine epithelial cells were incubated with approximately 106 M. avium subsp. paratuberculosis organisms for 2 h. After the incubation period, extracellular bacteria were killed by amikacin treatment (as described above), the supernatant was removed, and fresh culture medium was added to the monolayers. Infected cells were incubated for up to 4 days. At the end of the incubation period, monolayers were lysed, the lysate was diluted, and the number of intracellular bacteria was determined by plating cell lysates onto 7H11 agar.
Translocation assay.
The translocation assay was performed using the Transwell 2-chamber culture system (Corning Costar, New York). Monolayers were established either on the top or bottom sides of the membrane by seeding it with 1 × 105 MAC-T cells. Some monolayers had their apical surface exposed to the medium in the bottom chamber, whereas others had their basolateral side exposed to the medium in the top chamber. The culture medium was changed daily, and the integrity of the monolayers was determined by the following methods: (i) microscopic observation, (ii) measuring the transepithelial resistance, and (iii) the trypan blue (0.25%) permeability assay (optical density at 580 nm), as previously described (9, 24). Trypan blue (0.25%) was added to the monolayer, and 3 h later the supernatant of the lower chamber was obtained for spectrometer reading. Controls included medium only (baseline). The top chamber was infected with 3 × 107 bacteria as described above. After 1, 2, 3, and 4 days infection, 600 μl of filtrate was collected from the bottom chamber, and fresh culture medium (600 μl) was replenished. The ability to translocate was calculated as the cumulative percentage of the initial inoculum recovered in the bottom chamber at each time point.
Passage of M. avium subsp. paratuberculosis in MAC-T cells.
Confluent monolayers of MAC-T cells in cell culture flasks (Corning Costar, New York) were inoculated with approximately 107 M. avium subsp. paratuberculosis bacteria. After 2 h of contact time, extracellular bacteria were removed, followed by amikacin treatment as described previously, and new medium was added to the monolayer. Internalized bacteria were allowed to grow at 37°C for a total of 1 or 4 days. At the end of the incubation period, MAC-T cells were lysed by adding 0.5 ml Triton X-100 (0.1%) for 10 min. Lysates were then submitted to differential centrifugation as reported previously (8). The M. avium subsp. paratuberculosis cells were collected into 7H9 broth containing Mycobactin J (2 mg/liter) and OADC (10%, vol/vol). The bacteria were recovered by centrifugation at 3,500 × g for 30 min at 4°C to maintain their phenotype and then used in the invasion assays.
Preexposure of M. avium subsp. paratuberculosis to milk and milk components.
Approximately 107 M. avium subsp. paratuberculosis organisms were inoculated into 5 ml of raw milk (from a pool of several cows) containing polymyxin B (5.5 mg/liter), amphotericin (11 mg/liter), carbenicillin (25 mg/liter), and trimethoprim (2.5 mg/liter). Incubation was carried out at 37°C. After 24 h, M. avium subsp. paratuberculosis was recovered by centrifugation at 3,500 × g for 30 min at 5°C. Subsequently, bacteria were resuspended in HBSS, quantified by both McFarland turbidity standard and colony count, and used for infection of MDBK cells. Sterile water and 7H9 broth treated in the same manner as milk were used as controls. To determine the role of milk components, casein and serum protein fractions were separated by centrifuging milk at 90,000 × g for 2 h at 5°C (16). The casein fraction formed the pellet, while serum protein and the lactose fraction remained in the supernatant. The volumes of the casein fraction and serum-protein-plus-lactose fractions were brought to the level of the original volume of milk by adding water. The casein fraction with 0.9% NaCl was prepared by addition of NaCl to the casein fraction. The 4.8% lactose sample was prepared by dissolving lactose in water.
RNA isolation and DNA microarray.
An M. avium subsp. paratuberculosis whole genome microarray, containing 70-mer oligonucleotides representing 98% of M. avium subsp. paratuberculosis coding sequences identified in the M. avium subsp. paratuberculosis K-10 genome, was provided by the National Animal Disease Center, Ames, Iowa. For RNA isolation, M. avium subsp. paratuberculosis bacteria were kept in contact with confluent monolayers of MAC-T cells grown in T75 flasks for 2 h. Extracellular bacteria were removed by washing, followed by amikacin treatment for 2 h. Intracellular bacteria were recovered after 24 h by lysing MAC-T cells with sterile water. Cell debris and nuclear fractions were removed by low-speed centrifugation at 500 rpm for 5 min at 4°C (20). The bacterial pellet was recovered from the supernatant after centrifugation at 3,000 × g for 10 min at 4°C. Pelleted bacteria were stored at −70°C. The RNA extraction procedure used a combination of guanidine-thiocyanate-based buffer (TRIzol; Invitrogen, Carlsbad, CA) and rapid mechanical cell lysis, as described previously (18). Briefly, 1 ml TRIzol reagent was added to the bacterial pellet and gently mixed. The mixture was added to a (2-ml) screw-cap tube containing 0.4 ml glass beads (0.1 mm). Cell disruption was carried out in a bead beater using three beatings for 30 s each. The TRIzol solution was transferred to a heavy phase lock gel (Eppendorf, Hamburg, Germany) after centrifugation at 3,000 rpm for 90 s at 4°C. Subsequently, 300 μl of chloroform:isoamyl alcohol (24:1) was added to the heavy phase lock gel tube. This was followed by rapid mixing for 15 s. Mixing was continued periodically for 2 additional min, followed by centrifugation at 6,000 rpm for 10 min at 4°C. The aqueous layers were precipitated with cold isopropanol. Isopropanol was then carefully removed without disturbing the RNA pellet, and the final washing was done in 80% ethanol. The RNA was stored at −70°C until further use. RNA quality was determined spectrophotometrically (optical density at 260/280 nm). A ratio of ≥2.0 was considered significant. RNA was treated with DNase as per the manufacturer's instructions (QIAGEN, Valencia, CA). The RNA-DNA hybridization and microarray analysis were carried out at the Central Service Laboratory, Oregon State University, Corvallis, Oregon. RNA prepared from M. avium subsp. paratuberculosis bacteria incubated in 7H9 broth served as a control, whereas M. avium subsp. paratuberculosis bacteria incubated in MAC-T cells served as the treatment. For the DNA microarray, the Array 900 MDX (Gemisphere, Hatfield, PA) labeling and detection system was used as per instructions. Microarray analysis was carried out as follows. Briefly, RNA samples were reverse transcribed using deoxynucleoside triphosphates and a random primer. The cDNA was labeled at the 3′ end with a terminal deoxynucleotidyltransferase tailing reaction followed by ligation to the 3DNA capture sequence. The tagged cDNA was purified using a PCR purification kit (QIAGEN, Valencia, CA), and then successive hybridization of cDNA and 3DNA was carried out using the M. avium subsp. paratuberculosis genome microarray.
RT-PCR.
cDNA amplification for genes that have been shown to be upregulated by DNA array was carried out as previously described (22). We used specific primers for five selected genes: MAP0482, MAP0706, MAP2450c, MAP2751, and MAP3305c. PCR amplification was carried out at 95°C for 3 min (1 cycle); 95°C for 3 s, 62°C for 30 s, and 72°C for 2 min in a linear range (35 cycles); and then 72°C for 10 min (1 cycle). Equivalent amounts of cDNA were used for the reverse transcriptase (RT)-PCR. The products obtained by PCR amplification were quantified using the Kodak EDEAS 290 system and the Kodak ID image analysis software (Eastman Kodak Company, Rochester, NY). The 16S cDNA was used as a control.
Transmission electron microscopy.
Approximately 5 × 104 MAC-T cells were grown in T25 tissue culture flasks (Corning Costar, New York) until confluence was established and then inoculated with approximately 107 M. avium subsp. paratuberculosis bacteria. Incubation was carried out for 24 h under 5% CO2 at 37°C. After the incubation period, MAC-T cells were treated with trypsin (0.5%) for 10 min and subsequently fixed in 0.1 M cacodylate buffer (pH 7.3) containing 3% glutaraldehyde and 2% paraformaldehyde. After fixing, cells were pelleted and surrounded by 1% agarose, cut into quarters, and processed as per the following schedule: 0.1 M cacodylate buffer (2×), 30 min at room temperature (RT); 1% osmium tetroxide in 0.1 M cacodylate buffer, 1 h at RT; 0.1 M cacodylate buffer (2×), 30 min at RT; acetone (10%, 30%, 50%, 70%, 80%, 95%, and 100%), all 10 min at RT; 3:1 acetone:resin, 30 min at RT; 1:1 acetone:resin, 30 min at RT; 1:3 acetone:resin, 30 min at RT; 100% resin, 1 h at 30°C; and 100% resin, 20 h at RT. Fresh resin was placed in polymerization capsules for 24 h at 60°C. The blocks were sectioned on an MT-5000 ultramicrotome (Sorvall, Newton, CO) using a diamond knife, and sections were placed on 300-mesh copper grids. Grids were stained with saturated uranyl acetate and lead citrate and viewed using a Zeiss 10A transmission electron microscope (Carl Zeiss, Thornwood, NY).
Statistical analysis.
Each experiment was repeated at least three times, and significant differences were determined using Student's t test (unpaired) and analysis of variance. A P value of <0.05 was considered significant.
RESULTS
M. avium subsp. paratuberculosis invasion and survival in MDBK cells.
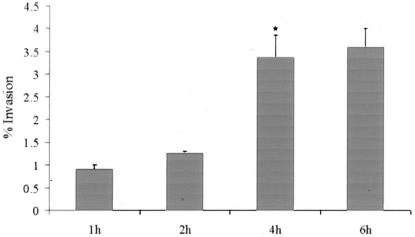
Invasion assays were carried out to examine the ability of M. avium subsp. paratuberculosis to enter bovine epithelial cells using MDBK cells as a model of intestinal mucosa. The results shown in Fig. 1 indicate that M. avium subsp. paratuberculosis can invade MDBK cells. The efficiencies of invasion at 1-h and 2-h time points were comparable; however, a significant increase (P < 0.05) in invasion was observed after 6 h of contact time.
FIG. 1.
Invasion of bovine epithelial cells (MDBK) by M. avium subsp. paratuberculosis. The percentage of invasion was defined as the fraction of inoculated bacteria that became internalized after the incubation period. Values represent the means of three experiments ± standard errors of the means (SEM). *, P < 0.05 compared with the percent invasion at 2 h.
The ability of M. avium subsp. paratuberculosis to survive inside MDBK cells was evaluated by incubating intracellular bacteria for 24 h or 96 h after invasion. The number of M. avium subsp. paratuberculosis bacteria increased at 96 h of infection compared with the inoculum taken at 2 h (Table 1), suggesting that M. avium subsp. paratuberculosis is able to survive inside MDBK cells.
TABLE 1.
Intracellular survival of M. avium subsp. paratuberculosis in bovine epithelial cells (MDBK)
| Time (h)b | Mean no. of intracellular bacteria ± SEM (CFU/ml)a |
|---|---|
| 2 | 2.36 × 103 ± 0.44 × 103 |
| 24 | 2.81 × 103 ± 0.39 × 103 |
| 96 | 3.26 × 103 ± 0.43 × 103 |
Values represent means of three experiments ± SEM. A P value of >0.05 resulted for the comparison between the values for 24 h and 96 h and the value for 2 h.
MDBK cells were exposed to M. avium subsp. paratuberculosis for 2 h. Extracellular bacteria were removed in all experiments after 2 h. Internalized bacteria were allowed to grow for 24 h or 96 h.
Mammary gland as a reservoir for M. avium subsp. paratuberculosis.
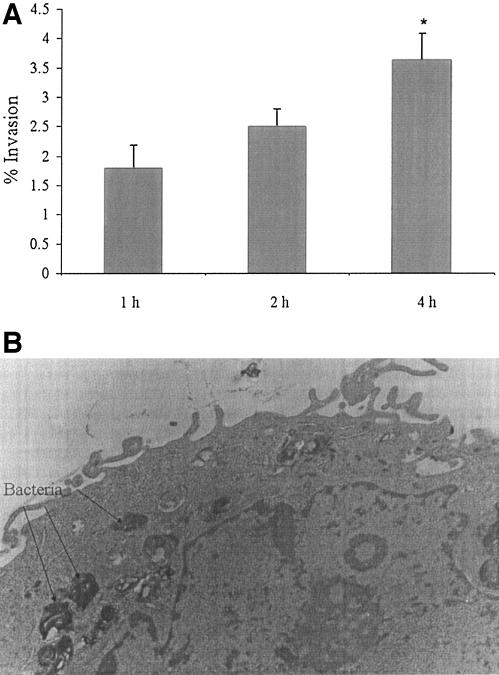
The results of M. avium subsp. paratuberculosis invasion of the MAC-T cell line are shown in Fig. 2A. M. avium subsp. paratuberculosis entered MAC-T cells, although the efficiency of invasion did not change during the first 2 h of incubation. However, invasion was greater at 4 h (P < 0.05) than at 2 h. M. avium subsp. paratuberculosis could be observed within vacuoles by using transmission electron microscopy (Fig. 2B). The survival experiments whose results are shown in Table 2 indicate that M. avium subsp. paratuberculosis is able to persist over time within MAC-T cells.
FIG. 2.
(A) Invasion of bovine mammary epithelial cells (MAC-T) by M. avium subsp. paratuberculosis. The percent invasion was defined as the fraction of inoculated bacteria that became internalized after the incubation period. Values represent the means of three experiments ± SEM. *, P ≤ 0.05 compared with the percent invasion at 2 h. (B) Representative transmission electron micrograph of mammary epithelial cells (MAC-T) after 24 h of infection by M. avium subsp. paratuberculosis bacteria (arrows). Bacterial cells can be seen within vacuoles. Magnification, ×10,000.
TABLE 2.
Intracellular survival of M. avium subsp. paratuberculosis in bovine mammary epithelial cells (MAC-T)
| Time of infection (h)b | Mean no. of intracellular bacteria ± SEM (CFU/ml)a |
|---|---|
| 2 | 0.91 × 104 ± 0.25 × 104 |
| 24 | 0.96 × 104 ± 0.28 × 104 |
| 96 | 1.29 × 104 ± 0.39 × 104 |
Values represent means of three experiments ± SEM.
MAC-T cells were exposed to M. avium subsp. paratuberculosis for 2 h. Extracellular bacteria were removed in all experiments after 2 h. Internalized bacteria were allowed to grow for 24 h or 96 h.
In the mammary gland, the apical surface of epithelial cells faces the alveolar lumen, whereas the basolateral side faces the bloodstream. To investigate whether there was a preferential route for entry, invasion assays were performed using polarized cell monolayers. M. avium subsp. paratuberculosis crossed the MAC-T cells' polarized monolayers equally well from the apical and basolateral surfaces (Table 3). These results in vitro indicate that infection of mammary epithelial cells could potentially occur across both membrane surfaces.
TABLE 3.
Translocation of M. avium subsp. paratuberculosis across polarized monolayersb of bovine mammary epithelial cellsc (MAC-T)
| Invasion surface |
M. avium subsp. paratuberculosis inoculum recovered after designated incubation period (%)a
|
|||
|---|---|---|---|---|
| 1 day | 2 days | 3 days | 4 days | |
| Apical | 0.11 ± 0.02 | 0.25d ± 0.03 | 0.35d ± 0.04 | 0.49d ± 0.05 |
| Basolateral | 0.15 ± 0.02 | 0.26e ± 0.01 | 0.34d ± 0.02 | 0.47d ± 0.03 |
Translocation percentage was defined as the percentage of M. avium subsp. paratuberculosis inoculum that was recovered from the bottom chamber of the Transwell apparatus. The results represent the means of three experiments within rows ± SEM.
Monolayer integrity was verified by a trypan blue dye exclusion assay and by measuring transepithelial resistance.
MAC-T cells were exposed to M. avium subsp. paratuberculosis for 1, 2, 3, or 4 days from either the apical or the basolateral surface.
P < 0.05, cumulative percent translocation after 2, 3, and 4 days compared with that after 1 day within individual rows.
P < 0.01, cumulative percent translocation after 2 days compared with that after 1 day within individual row.
Incubation with milk and efficiency of invasion.
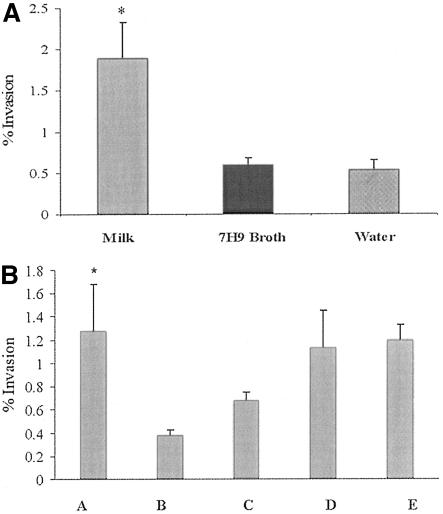
Since M. avium subsp. paratuberculosis in the mammary gland may be exposed to milk, we attempted to evaluate the effect of incubation of M. avium subsp. paratuberculosis in milk on the ability to enter bovine epithelial cells. M. avium subsp. paratuberculosis bacteria were exposed to milk (increased osmolarity conditions), 7H9 broth (iso-osmolar medium), or water (hypo-osmolar medium) at 37°C for 24 h in the presence of antibiotics (polymyxin B [5.5 mg/liter], amphotericin [11 mg/liter], carbenicillin [25 mg/liter], and trimethoprim [2.5 mg/liter]). The antibiotics were used to prevent the growth of other microorganisms present in milk. After incubation, the ability to enter MDBK cells was evaluated. The efficiency of invasion was significantly greater when M. avium subsp. paratuberculosis was preincubated in milk than when M. avium subsp. paratuberculosis was exposed to other environments (Fig. 3A).
FIG. 3.
(A) Ability of M. avium subsp. paratuberculosis to invade MDBK epithelial cells following exposure to three different environments: milk, 7H9 broth, or water for 24 h at 37°C. Values are the means of three experiments ± SEM. *, P < 0.01 compared with 7H9 broth or water. (B) Invasion of bovine epithelial cells (MDBK) by M. avium subsp. paratuberculosis. Prior to invasion, M. avium subsp. paratuberculosis bacteria were incubated in the presence of milk (column A), casein (column B), casein plus 0.9% NaCl (column C), serum protein plus lactose (column D), or lactose (4.8% solution) (column E) for 24 h at 37°C. Values represent the means of three experiments ± SEM. *, P < 0.05 for difference between the invasion percent for milk (column A) and casein (column B) casein plus NaCl (column C).
To determine whether a specific milk component was associated with increased invasion, M. avium subsp. paratuberculosis was exposed to different milk components prior to invasion of MDBK cells. Figure 3B shows that the efficiency of invasion of M. avium subsp. paratuberculosis after incubation in casein alone (low-osmolar medium) was lower than with milk (P < 0.05). However, differences were not significant when milk was compared to casein with 0.9% NaCl, lactose, or lactose plus serum protein (hyperosmolar conditions) (Fig. 3B).
Intracellular phenotype.
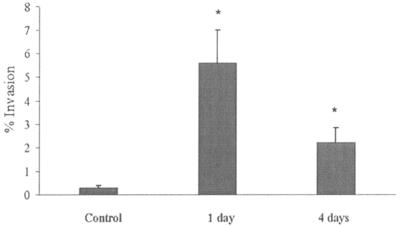
Because M. avium subsp. paratuberculosis is encountered intracellularly during infection and is potentially eliminated from the mammary gland within detached epithelial cells, it is of paramount importance to determine whether M. avium subsp. paratuberculosis infection of mammary epithelial cells impacts its ability to enter bovine MDBK epithelial cells. MAC-T cells infected with M. avium subsp. paratuberculosis were maintained for 1 or 4 days in culture before they were lysed. Intracellular bacteria recovered after lysis were then used to infect MDBK cells. Results shown in Fig. 4 indicate that the efficiency of invasion by M. avium subsp. paratuberculosis collected from infected MAC-T cells was approximately 10-fold greater than that of M. avium subsp. paratuberculosis incubated in medium.
FIG. 4.
Invasion of bovine epithelial cells (MDBK) by M. avium subsp. paratuberculosis after passage in MAC-T cells. M. avium subsp. paratuberculosis bacteria were incubated in MAC-T cells for 1 or 4 days and then used to infect MDBK epithelial cells. M. avium subsp. paratuberculosis incubated in medium was used as a control. Values represent the means of three experiments ± SEM. *, P < 0.001 compared with the invasion percent for the control.
DNA microarray.
To determine whether M. avium subsp. paratuberculosis genes are upregulated during the intracellular stage in mammary epithelial cells, DNA microarray analysis was carried out. MAC-T cells were infected for 24 h and M. avium subsp. paratuberculosis RNA was obtained. The 24-h time point was chosen based on the greater invasion of MDBK cells. The results in Table 4 indicate that upregulation of a number of M. avium subsp. paratuberculosis genes occurs during infection of MAC-T cells.
TABLE 4.
DNA microarray profile showing differential expression of M. avium subsp. paratuberculosis genes
| Gene | Fold increase in DNA arraya | Function(s) or characteristic(s) | Fold increase in RT-PCR |
|---|---|---|---|
| MAP2450c | 6.35 | atpC; probable ATP synthase, M. leprae | 7.1 |
| MAP3305c | 6.35 | Conserved hypothetical protein, M. leprae | 6.8 |
| MAP0482 | 5.76 | Putative transcription regulator, Nocardia farcinica | 6.3 |
| MAP2751 | 5.47 | Unique to M. avium subsp. paratuberculosis | 5.5 |
| MAP0706 | 5.47 | Probable cytoplasmic peptidase, Listeria monocytogenes | 5.8 |
| MAP0741c | 4.91 | Possible oxidoreductase | NDb |
| MAP3404 | 4.78 | Biotin carboxyl bifunctional carrier protein; essential gene in M. tuberculosis | ND |
| MAP1259 | 4.49 | Probable transcription regulatory protein in M. tuberculosis | ND |
| MAP2708c | 4.33 | Probable glutamine amidotransferase | ND |
| MAP3224 | 4.33 | Possibly involved in secretion of β-lactamase in M. tuberculosis | ND |
| MAP1695c | 4.25 | Transcription factor mediated by hypoxic conditions in M. tuberculosis; essential gene in M. tuberculosis | ND |
| MAP2652c | 4.02 | Probable phosphate acetyl transferase | ND |
| MAP2524c | 3.89 | Oxidoreductase | ND |
| MAP0462 | 3.79 | ureC; urease alpha subunit; essential gene in M. tuberculosis | ND |
| MAP0369 | 3.72 | Probable nitrate reductase | ND |
| MAP1758c | 3.56 | nrtC; possible acyl coenzyme A dehydrogenase | ND |
| MAP3374 | 3.43 | Probable F 420 biosynthesis protein | ND |
| MAP0392c | 3.23 | Probable bifunctional membrane associated penicillin binding protein | ND |
| MAP4310c | 3.08 | Possible acyl coenzyme A dehydrogenase | ND |
M. avium subsp. paratuberculosis bacteria were incubated intracellularly in MAC-T cells for 1 day prior to RNA microarray analysis. The expression was compared to that for bacteria grown in 7H9 broth.
ND, not done.
M. avium subsp. paratuberculosis whole genome microarray analysis identified 20 genes that showed gene expression threefold or higher than control. The in silico analysis of these genes suggests a diverse array of regulatory, metabolic, and candidate virulence-associated factors. For example, genes MAP0482, MAP1695c, MAP3404, MAP1259, and MAP2652c encode transcription-regulatory proteins. Among other differentially expressed genes, MAP0392 belongs to an operon encoding an upstream transcription-regulatory protein. MAP0462, which encodes a urease alpha subunit, and MAP3404, encoding a biotin carboxyl bifunctional carrier protein, are essential genes for survival based on their homology with Mycobacterium tuberculosis (25). Among other differentially expressed genes are MAP2450c (encoding a probable ATP synthase), MAP0462 (encoding a tRNA synthetase), and MAP3224 (involved in secretion of β-lactamase). Finally, MAP2751, which has previously been shown to be present uniquely in M. avium subsp. paratuberculosis (4) and has no known function, also showed increased transcription within MAC-T cells. The expression of five of those genes was evaluated using RT-PCR and shown to correspond to the DNA array analysis (Table 4).
DISCUSSION
Mycobacterial invasion of intestinal epithelial cells is a complex event, requiring participation of several bacterial and host factors. However, M. avium subsp. paratuberculosis infection is difficult to study in large animal model systems. Tissue culture cell models have been shown to be useful to obtain insights into the host-pathogen interactions. For example, Madin-Darby canine kidney cells have been used by several laboratories to study bacterial pathogenesis (17, 20). In addition, a number of studies employed cultured epithelial cells to examine the interaction between the bacteria and host cells (8, 9, 14). Earlier work has established MDBK epithelial cells as a model of bovine intestinal mucosa (5). When confluent monolayers of MDBK cells were exposed to M. avium subsp. paratuberculosis, it was observed that M. avium subsp. paratuberculosis was capable of invading epithelial cells. Since epithelial cells are not phagocytic in nature, M. avium subsp. paratuberculosis invasion of MDBK cells indicates that the bacteria might trigger their own uptake, probably by inducing cytoskeleton reorganization. We did not observe significant replication of M. avium subsp. paratuberculosis inside cells after 4 days, which should be due to the long replication time (15), which might be even longer when inside epithelial cells.
Previous work has demonstrated the recovery of M. avium subsp. paratuberculosis from various sites in the body during advanced stages of infection. For instance, M. avium subsp. paratuberculosis has been isolated from milk, colostrum, and mammary lymph nodes from both asymptomatic and symptomatic cows (28-30), making it plausible that mammary epithelial cells could be a site of M. avium subsp. paratuberculosis infection. Our results demonstrated that M. avium subsp. paratuberculosis infects MAC-T cells in vitro and that the infection is possible from both the apical and basolateral surfaces with comparable efficiency. Therefore, the implication of the observation is that infection in mammary gland tissue may potentially occur by either the systemic or the ascending route. Similar findings have been reported for other pathogens such as Streptococcus dysgalactiae and Staphylococcus aureus (1, 2). The results of transmission electron microscopy (Fig. 2B) confirmed that M. avium subsp. paratuberculosis, once inside MAC-T cells, is encountered within cytoplasmic vacuoles, similar to what has been described for M. avium subsp. paratuberculosis in macrophages (15) and other mycobacteria in epithelial cells (8). Furthermore, it was observed that M. avium subsp. paratuberculosis survived within mammary epithelial cells for several days in vitro. Collectively, these findings support the idea that the infection of the mammary gland can occur through the systemic route and that mammary gland epithelial cells may serve as a reservoir for M. avium subsp. paratuberculosis and a potential source of infection for young calves.
It is assumed that the intracellular environment in the mammary gland has high osmolarity, while the mammary gland milk is also a hyperosmolar fluid in nature. M. avium subsp. paratuberculosis incubated in milk prior to infecting MDBK epithelial cells became significantly more invasive than M. avium subsp. paratuberculosis that had been previously incubated in broth or water. The augmented ability to invade cells was then attributed to the hyperosmolar conditions of milk, a hypothesis that was strengthened by the observation that M. avium subsp. paratuberculosis incubated in four different hyperosmolar milk fractions acquired a similar phenotype. It appears that the environment with high osmolarity may serve as a trigger for expression of invasion-related determinants. In fact, osmolarity has been shown to be associated with the expression of virulence determinants in a number of bacteria, e.g., the toxR gene in Vibrio cholerae and ompR genes in Salmonella and Shigella (11, 13, 21). Previously, it was also shown that when M. avium was preincubated under high osmolarity conditions, a change in phenotype was induced, resulting in enhanced efficiency in entering human intestinal epithelial cells (8). These studies also showed that the invasive phenotype was likely to be related to the upregulation of genes involved in invasion, since incubation under high-osmolarity conditions in the presence of subinhibitory concentrations of amikacin, which inhibits protein synthesis, failed to result in expression of the invasive phenotype (8).
Prior incubation of M. avium subsp. paratuberculosis in MAC-T cells enhanced the efficiency of invasion of MDBK cells. DNA microarray analysis of M. avium subsp. paratuberculosis genes regulated during MAC-T cell infection showed that several genes had their expression altered. The upregulated M. avium subsp. paratuberculosis genes, for example, MAP0482, MAP1695c, MAP3404, MAP1259, MAP2652c, and MAP0392, encode proteins with transcription-regulatory functions. MAP0482 encodes a putative transcriptional regulator in Nocadia farcinica. The upstream gene MAP0483 encodes a transcription-regulatory protein in M. tuberculosis. Another transcription protein, encoded by MAP1695c, acts as a cochaperone in M. tuberculosis (19, 27). The upstream gene is for Hsp18, a stress protein induced by anoxia. Homology with M. tuberculosis suggests that the MAP1695c operon encodes a response regulator having an important role. MAP3404 belongs to an operon having an upstream sigma factor. MAP0392 encodes a probable bifunctional membrane-associated penicillin binding protein (PonA2, murine polymerase) under the control of the transcription-regulatory protein encoded by MAP0393. Studies with M. smegmatis and M. tuberculosis have suggested that transposon disruption of ponA resulted in a penicillin-binding-deficient mutant that was sensitive to beta-lactam antibiotics and grew slowly in culture (7, 12). The genes identified may be associated with other functions, such as intracellular survival. Efficiency of invasion among intracellular bacteria peaks at 24 h, probably reflecting the fact that the mammary gland is emptied at least once a day. The function of the majority of the identified genes is unknown, and further studies are necessary to understand the role of the identified genes in intracellular survival.
In summary, we have examined the different conditions of M. avium subsp. paratuberculosis invasion and survival. A working model can be identified from the present results. Infection of the mammary gland and milk are observed in the majority of the infected cows. Therefore, it is plausible to hypothesize that M. avium subsp. paratuberculosis (within or outside of cells) fed to calves in milk is an organism with the ability to cross the intestinal barrier with efficiency, compared with organisms present in the water. Fecal material may be another important source of M. avium subsp. paratuberculosis expressing an invasive phenotype. Future work will address the role of genes upregulated within milk and mammary epithelial cells and will attempt to put together the 35-kDa protein, identified previously (5), and the present model of infection.
Acknowledgments
We thank Denny Weber for preparing the manuscript.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Almeida, R. A., K. R. Matthews, E. Cifrian, A. J. Guidry, and S. P. Oliver. 1996. Staphylococcus aureus invasion of bovine mammary epithelial cells. J. Dairy Sci. 79:1021-1026. [DOI] [PubMed] [Google Scholar]
- 2.Almeida, R. A., and S. P. Oliver. 1995. Invasion of bovine mammary epithelial cells by Streptococcus dysgalactiae. J. Dairy Sci. 78:1310-1317. [DOI] [PubMed] [Google Scholar]
- 3.Bannantine, J. P., R. G. Barletta, J. R. Stabel, M. L. Paustian, and V. Kapur. 2004. Application of the genome sequence to address concerns that Mycobacterium avium subspecies paratuberculosis might be a foodborne pathogen. Foodborne Pathog. Dis. 1:3-15. [DOI] [PubMed] [Google Scholar]
- 4.Bannantine, J. P., J. K. Hansen, M. L. Paustian, A. Amonsin, L.-L. Li, J. R. Stabel, and V. Kapur. 2004. Expression and immunogenicity of proteins encoded by sequences specific to Mycobacterium avium subsp. paratuberculosis. J. Clin. Microbiol. 42:106-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bannantine, J. P., J. F. J. Huntley, E. Miltner, J. R. Stabel, and L. E. Bermudez. 2003. The Mycobacterium avium subsp. paratuberculosis 35 kDa protein plays a role in invasion of bovine epithelial cells. Microbiology 149:2061-2069. [DOI] [PubMed] [Google Scholar]
- 6.Barrington, G. M., J. M. Gay, I. S. Eriks, W. C. Davis, J. F. Evermann, C. Emerson, J. L. O'Rourke, M. J. Hamilton, and D. S. Bradway. 2003. Temporal patterns of diagnostic results in serial samples from cattle with advanced paratuberculosis infections. J. Vet. Diagn. Investig. 15:195-200. [DOI] [PubMed] [Google Scholar]
- 7.Basu, J., S. Mahapatra, M. Kundu, S. Mukhopadhyay, M. Nguyen-Distèche, P. Dubois, B. Joris, J. Van Beeumen, S. T. Cole, P. Chakrabarti, and J.-M. Ghuysen. 1996. Identification and overexpression in Escherichia coli of a Mycobacterium leprae gene, pon1, encoding a high-molecular-mass class A penicillin-binding protein, PBP1. J. Bacteriol. 178:1707-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bermudez, L. E., M. Petrofsky, and J. Goodman. 1997. Exposure to low oxygen tension and increased osmolarity enhance the ability of Mycobacterium avium to enter intestinal epithelial (HT-29) cells. Infect. Immun. 65:3768-3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bermudez, L. E., F. J. Sangari, P. Kolonoski, M. Petrofsky, and J. Goodman. 2002. The efficiency of the translocation of Mycobacterium tuberculosis across a bilayer of epithelial and endothelial cells as a model of the alveolar wall is a consequence of transport within mononuclear phagocytes and invasion of alveolar epithelial cells. Infect. Immun. 70:140-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bermudez, L. E., and L. S. Young. 1994. Factors affecting invasion of HT-29 and HEp-2 epithelial cells by organisms of the Mycobacterium avium complex. Infect. Immun. 62:2021-2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernardini, M. L., A. Fontaine, and P. J. Sansonetti. 1990. The two-component regulatory system OmpR-EnvZ controls the virulence of Shigella flexneri. J. Bacteriol. 172:6274-6281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Billman-Jacobe, H., R. E. Haites, and R. L. Coppel. 1999. Characterization of a Mycobacterium smegmatis mutant lacking penicillin binding protein 1. Antimicrob. Agents Chemother. 43:3011-3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chatfield, S. N., C. J. Dorman, C. Hayward, and G. Dougan. 1991. Role of ompR-dependent genes in Salmonella typhimurium virulence: mutants deficient in both OmpC and OmpF are attenuated in vivo. Infect. Immun. 59:449-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cirillo, J. D., S. Falkow, L. S. Tompkins, and L. E. Bermudez. 1997. Interaction of Mycobacterium avium with environmental amoebae enhances virulence. Infect. Immun. 65:3759-3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris, N. B., and R. G. Barletta. 2001. Mycobacterium avium subsp. paratuberculosis in veterinary medicine. Clin. Microbiol. Rev. 14:489-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holt, C. 1998. Casein micelle substructure and calcium phosphate interactions studied by Sephacryl column chromatography. J. Dairy Sci. 81:2994-3003. [Google Scholar]
- 17.Leung, K. Y., and B. B. Finlay. 1991. Intracellular replication is essential for the virulence of Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 88:11470-11474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahenthiralingam, E. 1998. Extraction of RNA from mycobacteria. Methods Mol. Biol. 101:65-75. [DOI] [PubMed] [Google Scholar]
- 19.Mao, Q., D. Ke, X. Feng, and Z. Chang. 2001. Preheat treatment for Mycobacterium tuberculosis Hsp16.3: correlation between a structural phase change at 60 degrees C and a dramatic increase in chaperone-like activity. Biochem. Biophys. Res. Commun. 284:942-947. [DOI] [PubMed] [Google Scholar]
- 20.McGarvey, J. A., D. Wagner, and L. E. Bermudez. 2004. Differential gene expression in mononuclear phagocytes infected with pathogenic and non-pathogenic mycobacteria. Clin. Exp. Immunol. 136:490-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miltner, E., K. Daroogheh, P. K. Mehta, S. L. Cirillo, J. D. Cirillo, and L. E. Bermudez. 2005. Identification of Mycobacterium avium genes that affect invasion of the intestinal epithelium. Infect. Immun. 73:4214-4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Momotani, E., D. L. Whipple, A. B. Thiermann, and N. F. Cheville. 1988. Role of M cells and macrophages in the entrance of Mycobacterium paratuberculosis into domes of ileal Peyer's patches in calves. Vet. Pathol. 25:131-137. [DOI] [PubMed] [Google Scholar]
- 24.Sangari, F. J., J. Goodman, and L. E. Bermudez. 2000. Mycobacterium avium enters intestinal epithelial cells through the apical membrane, but not by the basolateral surface, activates small GTPase Rho and, once within epithelial cells, expresses an invasive phenotype. Cell. Microbiol. 2:561-568. [DOI] [PubMed] [Google Scholar]
- 25.Sassetti, C. M., and E. J. Rubin. 2003. Genetic requirements for mycobacterial survival during infection. Proc. Natl. Acad. Sci. USA 100:12989-12994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Secott, T. E., T. L. Lin, and C. C. Wu. 2004. Mycobacterium avium subsp. paratuberculosis fibronectin attachment protein facilitates M-cell targeting and invasion through a fibronectin bridge with host integrins. Infect. Immun. 72:3724-3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stewart, G. R., L. Wernisch, R. Stabler, J. A. Mangan, J. Hinds, K. G. Laing, D. B. Young, and P. D. Butcher. 2002. Dissection of the heat-shock response in Mycobacterium tuberculosis using mutants and microarrays. Microbiology 148:3129-3138. [DOI] [PubMed] [Google Scholar]
- 28.Streeter, R. N., G. F. Hoffsis, S. Bech-Nielsen, W. P. Shulaw, and D. M. Rings. 1995. Isolation of Mycobacterium paratuberculosis from colostrum and milk of subclinically infected cows. Am. J. Vet. Res. 56:1322-1324. [PubMed] [Google Scholar]
- 29.Sweeney, R. W., R. H. Whitlock, and A. E. Rosenberger. 1992. Mycobacterium paratuberculosis cultured from milk and supramammary lymph nodes of infected asymptomatic cows. J. Clin. Microbiol. 30:166-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor, T. K., C. R. Wilks, and D. S. McQueen. 1981. Isolation of Mycobacterium paratuberculosis from the milk of a cow with Johne's disease. Vet. Rec. 109:532-533. [PubMed] [Google Scholar]