Abstract
Haemophilus ducreyi, the etiologic agent of the sexually transmitted genital ulcer disease chancroid, has been shown to associate with dermal collagen fibers within infected skin lesions. Here we describe NcaA, a previously uncharacterized outer membrane protein that is important for H. ducreyi collagen binding and host colonization. An H. ducreyi strain lacking the ncaA gene was impaired in adherence to type I collagen but not fibronectin (plasma or cellular form) or heparin. The mutation had no effect on serum resistance or binding to HaCaT keratinocytes or human foreskin fibroblasts in vitro. Escherichia coli expressing H. ducreyi NcaA bound to type I collagen, demonstrating that NcaA is sufficient to confer collagen attachment. The importance of NcaA in H. ducreyi pathogenesis was assessed using both swine and human experimental models of chancroid. In the swine model, 20% of lesions from sites inoculated with the ncaA mutant were culture positive for H. ducreyi 7 days after inoculation, compared to 73% of wild-type-inoculated sites. The average number of CFU recovered from mutant-inoculated lesions was also significantly reduced compared to that recovered from wild-type-inoculated sites at both 2 and 7 days after inoculation. In the human challenge model, 8 of 30 sites inoculated with wild-type H. ducreyi progressed to the pustular stage, compared to 0 of 30 sites inoculated with the ncaA mutant. Together these results demonstrate that the collagen binding protein NcaA is required for H. ducreyi infection.
Haemophilus ducreyi is the etiologic agent of the sexually transmitted genital ulcer disease (GUD) chancroid. Chancroid is rare in the United States and Western Europe. It is prevalent in tropical resource-poor regions and is endemic in certain areas of Africa and Asia (11, 38, 39, 47), where there is also a significant confluence of chancroid infection and human immunodeficiency virus (HIV) type 1 seropositivity (17, 30, 40). In these regions, case-control studies have shown that the relative risk of acquiring HIV infection for GUD patients ranged from an odds ratio of 3 to 18.2 (20, 49, 60). Per individual sexual act, GUD produces a 4- to 23-fold enhancement of HIV acquisition (22, 33), and GUD in an HIV-positive person doubles the relative risk per coital act of viral transmission to an HIV-negative partner (22, 61). Furthermore, HIV-positive chancroid patients often present ulcers that are more severe, longer lasting, and more difficult to cure (31, 45). These observations outline the strong synergistic relationship between GUD and HIV (23, 24). Aggressive control of chancroid, a prevalent and highly transmissible GUD, may have the potential to curtail the global spread of HIV (34, 37, 42).
Chancroid presents as a painful genital ulcer with a ragged erythematous border. This lesion emits a purulent exudate replete with macrophages, neutrophils, T and B lymphocytes, and bacteria (both H. ducreyi and other opportunistic species) (32). H. ducreyi does not cause disseminated infection in healthy or immunocompromised individuals and appears to be well adapted to survive in the skin of its obligate human host (59). However, the mechanism by which H. ducreyi colonizes skin remains poorly understood. H. ducreyi attaches to the HaCaT keratinocyte cell line in vitro (62), and this association is dependent upon expression of the outer membrane protein DsrA (15). DsrA also confers resistance to complement-mediated killing by normal human serum (19). H. ducreyi lacking DsrA is attenuated in both human and swine experimental chancroid models (12), but it is not clear how the keratinocyte attachment or serum resistance properties of DsrA specifically contribute to this phenotype. H. ducreyi also adheres to human foreskin dermal fibroblasts and forms microcolonies in vitro; this aggregative-adhesive phenotype depends on expression of the fimbria-like protein encoded by the flp gene cluster (41). Human volunteers inoculated with an flp mutant display lesions that fail to progress from the papular to the pustular stage, and live H. ducreyi was recovered from only 1 of 31 of these sites (53), suggesting that the above-described phenotype contributes to H. ducreyi survival in skin.
Despite the observation that H. ducreyi adheres to skin epithelial cells and fibroblasts in vitro, direct association between H. ducreyi and these types of cells within the chancroid lesion has not been proven. H. ducreyi was, however, shown to bind the extracellular matrix (ECM) proteins fibronectin, laminin, and collagen types I and III in vitro (9). In early-stage lesions derived from the human challenge model, H. ducreyi colocalized with macrophages and neutrophils within the pustule and also associated with fibrin and collagen in the dermis directly beneath the pustule (8). These findings suggest that adherence to ECM proteins, which comprise a major structural component of skin, may be an important contributing factor in H. ducreyi skin colonization.
We recently found that protection against H. ducreyi infection in the swine model of chancroid coincides with the emergence of H. ducreyi-specific bactericidal serum antibodies and that transfer of this immune serum protects naïve pigs against H. ducreyi challenge (16). Antibodies present in this immune serum recognized a distinct subset of H. ducreyi outer membrane proteins. Here we describe one of these proteins, termed NcaA, and its role in mediating binding of H. ducreyi to collagen binding and in H. ducreyi pathogenesis.
MATERIALS AND METHODS
Bacterial growth conditions.
H. ducreyi strains were grown on chocolate agar containing 1.5% Bacto agar, 2.5% brain heart infusion (BHI), 1% bovine hemoglobin, 1% IsoVitaleX (Becton Dickinson, Cockeysville, MD), 5% newborn calf serum, and 5% fetal bovine serum (Life Technologies, Inc., Rockville, MD) at 35°C in a humidified incubator with 5% CO2. Escherichia coli strains were propagated in LB broth or agar at 37°C. Ampicillin, kanamycin, vancomycin, or chloramphenicol was added to media when indicated to a final concentration of 100, 40, 3, or 1 μg/ml, respectively.
Radioimmunoprecipitation of H. ducreyi outer membrane proteins.
H. ducreyi 35000HP was grown overnight on chocolate agar as a confluent lawn, harvested, and resuspended in phosphate-buffered saline (PBS). This suspension was added to a precoated IODO-GEN tube (Pierce Biotechnology, Rockford, IL) with 1.0 mCi of 125NaI. Labeling was quenched after 3 min with an excess of GC medium base broth. Surface-iodinated bacteria were washed and mixed with preimmune or H. ducreyi-immune pig serum, incubated for 15 min at room temperature with occasional mixing, again washed with PBS, and pelleted. The resulting cell pellet was then resuspended in radioimmunoprecipitation buffer (2% Zwittergent 3-14, 50 mM Tris [pH 8.0], 5 mM EDTA, 0.15 M NaCl) and incubated with rocking for 30 min at 37°C. Insoluble matter was removed by centrifugation (5 min at 14,000 × g), and the supernatant containing solubilized antibody-labeled outer membranes was mixed with protein G-agarose (Sigma Chemical, St. Louis, MO). After overnight rocking at 4°C, the slurry was washed three times with radioimmunoprecipitation buffer and once with distilled H2O. The resulting pellet was resuspended in Laemmli sample buffer containing β-mercaptoethanol, boiled, and resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Resolved bands of iodinated (outer membrane) proteins bound by preimmune or H. ducreyi-immune pig serum were visualized by autoradiography.
Identification, cloning, and mutagenesis of ncaA.
H. ducreyi outer membrane proteins were resolved by SDS-PAGE and transferred to a polyvinylidene difluoride membrane. Protein bands were excised and subjected to Edman degradation at the UNC Protein Sequencing & Peptide Synthesis Facility (University of North Carolina, Chapel Hill). Amino acid sequences were compared using EMBOSS pairwise alignment algorithms hosted by the European Bioinformatics Institute. (http://www.ebi.ac.uk/emboss/align/index.html). The ncaA gene was amplified by PCR from H. ducreyi 35000HP genomic DNA by using forward primer 5′CTAGGCTAATGAGAGGTATATCG3′ and reverse primer 5′TTGTACGCATCGCTTGTTCC3′. The resulting product was cloned into pCR2.1 (Invitrogen, Carlsbad, CA) and was verified by automated DNA sequencing (UNC Genome Analysis Facility, Chapel Hill, NC). To produce an inactivated allele of ncaA, a 134-bp segment of ncaA between two NdeI sites was excised and replaced with a chloramphenicol acetyltransferase gene (cat) cassette derived from pUNCH40 (57). The cat-interrupted ncaA gene (ncaA::cat) was subcloned into plasmid pRSM2072 (a gift of Robert S. Munson, Jr.), and the resulting construct was used as a suicide vector to perform allelic exchange in H. ducreyi as described by Bozue et al. (14). H. ducreyi 35000HP was transformed with pRSM2072-ncaA::cat and plated on chloramphenicol to select cointegrates. Cointegrates were then plated on medium containing chloramphenicol and X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) (40 μg/ml) to counterselect for excision of the pRSM2072 vector backbone and the wild-type ncaA allele. Southern blot and PCR analyses of the mutant confirmed that a single copy of the cat cassette had been inserted into ncaA (data not shown). Transcriptions of genes adjacent to the ncaA locus (Fig. 1) in 35000HP and 35000HPncaA were found equivalent by reverse transcriptase PCR, showing that ncaA inactivation did not exert a negative polar effect on local transcription. To examine whether 35000HPncaA had phenotypes other than that caused by the mutation, 35000HPncaA was compared to the master 35000HP stock used to infect human volunteers. The phenotypes included comparisons of growth rates in broth and outer membrane protein (OMP) and lipooligosaccharide (LOS) profiles, as previously described (21). 35000HP and 35000HPncaA had similar generation times in broth (data not shown). OMPs and LOSs from 35000HP and 35000HPncaA were analyzed by SDS-PAGE, followed by Coomassie blue and silver staining. The OMP and LOS profiles demonstrated no difference between the parent and the mutant (data not shown). Because NcaA is a low-abundance protein as determined by Coomassie blue staining, ablation of NcaA protein expression in the mutant strain was confirmed by Western blot analysis with H. ducreyi-immune pig serum. Whole-cell lysates of 35000HP and 35000HPncaA were heated in sample buffer containing SDS and β-mercaptoethanol and then resolved by SDS-PAGE and blotted to nitrocellulose. The blot was probed with H. ducreyi-immune pig serum (1:1,000), followed by peroxidase-conjugated rabbit anti-pig immunoglobulin (1:32,000; Sigma Chemical, St. Louis, MO) and chemiluminescence detection (SuperSignal Pico; Pierce Biotechnology, Rockford, IL).
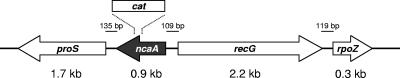
FIG. 1.
The H. ducreyi 35000HP ncaA locus and neighboring genes. RNA transcripts of prolyl-tRNA ligase (proS, HD1919), ATP-dependent DNA helicase (recG, HD1921), and RNA polymerase ω subunit (rpoZ, HD1923) were detected by reverse transcription-PCR and found to be produced in both 35000HP and 35000HPncaA (in which the ncaA gene is insertionally inactivated by cat as shown).
Serum susceptibility assay.
Serum susceptibility assays were performed as previously described (19). Briefly, bacteria grown on solid medium for 16 to 18 h were collected and suspended in 2 ml of BHI, vortexed for 5 seconds, and allowed to settle for 5 min. After settling, the upper 1 ml was removed and the cell density was adjusted such that the final concentration of bacteria was approximately 1 × 103 to 5 × 103 CFU/ml. Bacteria were mixed with fresh or heat-inactivated normal swine serum to a final concentration of 25% or 50%. Following incubation for 45 min (35°C, 5% CO2), the bacterial suspensions were plated to count viable CFU. Percent survival was calculated by dividing the number of CFU from fresh serum by the number from heat-inactivated serum and multiplying by 100. Assays were performed in triplicate and on three separate days.
Fluorescein labeling of bacterial strains.
Overnight cultures of H. ducreyi grown on chocolate agar or of E. coli grown in LB broth were collected and washed three times in PBS, followed by passage through a 30-gauge needle to disperse large aggregates. Carboxyfluorescein diacetate succinimidyl ester (Molecular Probes, Eugene, OR) in dimethyl sulfoxide was added to a final concentration of 12 μM, and the suspension was incubated with gentle mixing at 35°C for 25 min. H. ducreyi labeling reactions were quenched with an equal volume of BHI medium supplemented with 1% IsoVitaleX (Becton Dickinson, Cockeysville, MD), 1% hemoglobin, (Becton Dickinson, Cockeysville, MD), and 10% fetal bovine serum (Life Technologies, Rockville, MD). E. coli labeling reactions were quenched by addition of bovine serum albumin to a final concentration of 1%. The resulting fluorescein-labeled bacteria were collected by centrifugation at 1,040 × g for 10 min and resuspended in LB (E. coli) or in BHI supplemented with 1% IsoVitaleX and 1% hemoglobin (H. ducreyi). Bacteria were then incubated for 40 to 60 min at 35°C with shaking. The doubling times of fluorescein-labeled and unlabeled H. ducreyi were similar (data not shown).
Extracellular matrix protein attachment assay.
White 96-well plates (Fluoronunc; Nalge Nunc, Rochester, NY) were coated with bovine or human type I collagen (Chondrex, Redmond, WA), human cellular fibronectin, or human plasma fibronectin (Sigma-Aldrich, St. Louis, MO) diluted in collagen dilution buffer (Chondrex, Redmond, WA) or 50 mM sodium carbonate, pH 9.6 (fibronectins). Similar results were obtained in binding assays using bovine- and human-derived type I collagens. Plates were incubated overnight at 35°C (fibronectin) or 4°C (collagen), washed three times with PBS containing 0.05% Tween 20, and blocked with 300 μl of 0.5% bovine serum albumin (Sigma-Aldrich, St. Louis, MO) in PBS for 2 hours at 35°C. Wells were washed three times in PBS. Fluorescein-labeled or nonlabeled bacteria in BHI (200 μl) were added to appropriate wells. Separate aliquots of the bacterial suspensions were serially diluted and plated to determine CFU input. After 4 h at 35°C, the wells were washed three times with PBS containing 0.05% Tween 20, and 200 μl of PBS was added to all wells prior to reading fluorescence. Fluorescence emission at 520 nm was measured following excitation at 490 nm, using a microplate spectrometer (model LS50B; Perkin-Elmer Ltd., Beconsfield, Buckinghamshire, England). Standard curves were established for each strain in each assay to allow calculation of attached CFU from fluorescence intensity.
Animal care.
Pigs were housed in an American Association for Accreditation of Laboratory Animal Care-accredited P2 containment facility at North Carolina State University College of Veterinary Medicine. Animals were sedated for all procedures with 0.3 ml per 22.7 kg of body weight of an anesthetic formulation consisting of: Telazol (tiletamine HCl [50 mg/ml] and zolazepam HCl [50 mg/ml]; Fort Dodge Laboratories, Fort Dodge, IA), 50 mg/ml ketamine HCl (Fort Dodge Laboratories, Fort Dodge, IA), and 50 mg/ml xylazine (Miles Laboratories, Shawnee Mission, KS). Atropine sulfate (Phoenix Scientific Inc., Joseph, MO) was given at 0.5 to 1 ml to slow salivary and bronchial secretion. Pigs were maintained in the same room but in separate enclosures during the course of the study.
Virulence testing in pigs.
Inoculum preparation, inoculation, and biopsy procedures were previously described (27, 52). Briefly, H. ducreyi grown on chocolate agar was suspended in PBS and passed through a 30-gauge needle to disperse aggregates. Multi-Test skin test applicators (Lincoln Diagnostics, Decatur, Ill.) were loaded with 10 μl of inoculum and pressed into the dorsal side of the ear. Each round of inoculations entailed four to six application sites per ear per strain tested. Biopsies of experimental lesions collected 2 and 7 days after inoculation using 6-mm skin biopsy punches (Acuderm, Ft. Lauderdale, FL) were minced and plated on chocolate agar containing vancomycin to detect viable H. ducreyi within lesions.
Virulence testing in human volunteers.
Healthy adult male and female volunteers over 18 years of age were recruited for the study. Subjects gave informed consent for participation and for HIV serology, in accordance with the human experimentation guidelines of the U.S. Department of Health and Human Services and the Institutional Review Board of Indiana University-Purdue University of Indianapolis. The experimental challenge protocol, preparation and inoculation of the bacteria, calculation of the estimated delivered dose (EDD), surface cultures, and clinical observations were done exactly as described previously (4, 5, 54, 55, 58). Each subject was inoculated on one arm with three identical doses of the parent strain and on the other arm with three doses of twofold serial dilutions of the mutant. Subjects were observed until they reached a clinical end point, defined as either 14 days after inoculation, development of a pustule that was either painful or greater than 4 mm in diameter, or resolution of infection at all sites. Once a clinical end point was achieved, the code was broken and sites with clinical disease, if present, were biopsied. The subjects were then treated with two doses of oral ciprofloxacin (5, 55).
To confirm that the inocula were correct and that no phenotypic changes occurred during infection, individual colonies from the inocula, surface cultures, and biopsy specimens were picked, suspended in freezing medium, and frozen in 96-well plates. The colonies were scored for susceptibility to chloramphenicol. If available, sufficient colonies (n ≥ 30) from an individual specimen were scored so that there was a 95% probability that ≤11% of the colonies would have the incorrect phenotype (1).
Statistical analysis.
Data from the pig model were analyzed using SigmaStat version 2.0 (Jandel Scientific, San Rafael, CA) and SAS version 9 (SAS Institute Inc., Cary, NC). Numbers of viable wild-type and mutant H. ducreyi CFU recovered from lesion biopsies were analyzed using Poisson regression. Percentages of culture-positive biopsies were analyzed via logistic regression modeling of probabilities of recovery. Both regression models were adjusted for variability among pigs by using generalized estimating equations (GEE). Comparisons of papule and pustule formation rates between the two strains in the human challenge model were performed using a logistic regression model with GEE to account for the correlation among sites within the same subject, as described previously (53). The GEE sandwich estimate for the standard errors was used to calculate 95% confidence intervals for these rates. When a rate was zero, the exact binomial confidence intervals were calculated based on the number of subjects rather than the number of sites. The z-significance test of two means was used to compare the adherence of different strains of bacteria to type I collagen, and the t test was used to determine the significance of NcaA-expressing E. coli bound to collagen.
RESULTS
Identification of a putative oligomeric coiled adhesin in H. ducreyi.
We recently demonstrated that pigs repeatedly inoculated with H. ducreyi develop a modest antibody-mediated protective response against reinfection (16). Targets of this antibody response were identified by immunoprecipitation of 125I-labeled H. ducreyi OMPs with immune sera from multiply inoculated pigs. Seven distinct H. ducreyi outer membrane proteins were precipitated exclusively by immune sera (data not shown). One of these proteins, with an apparent molecular mass of >100 kDa, was isolated and identified via N-terminal amino acid sequencing and comparison to the published H. ducreyi genome sequence. The N-terminal sequence, ITTESIPT, matched predicted amino acids 21 to 28 of a previously uncharacterized H. ducreyi OMP (hypothetical protein HD1920; BLAST accession no. NP_874254) with a predicted molecular mass of ∼33 kDa. We named this protein NcaA.
The NcaA amino acid sequence is similar to those of the H. ducreyi OMP DsrA (25.1% identity and 42.6% similarity), Yersinia enterocolitica YadA (23.0% identity and 39.9% similarity), Moraxella catarrhalis UspA proteins (21.0 to 22.0% identity and 35.6 to 39.3% similarity), and Escherichia coli Eib (20.7 to 27.2% identity and 38.0 to 43.2% similarity) proteins. These proteins have been described as oligomeric coiled adhesins (Oca) (48), a subfamily of the type Vc surface-attached oligomeric autotransporter family (25). They are typified by a signature C-terminal 9-amino-acid stretch of alternating hydrophobic amino acids terminating with phenylalanine or tryptophan which comprises an outer membrane localization motif. The C-terminal region of these proteins also appears to mediate the formation of multimers that can remain stable even when heated with detergent and reducing agents (48). The disparity between the hypothetical molecular weight of monomeric NcaA and its dramatically slower migration in SDS-PAGE (Fig. 2) is consistent with similar proteins of this nature. The sequence of the 79 C-terminal amino acids of NcaA is 98% similar to the consensus Oca sequence and also possesses the 9-amino-acid terminus motif. Oca proteins share other properties in addition to the C-terminal signature sequence, including involvement in adherence, binding of ECM proteins, and resistance to complement-mediated killing. These common functions led us to investigate whether NcaA mediates adherence to eukaryotic cells and ECM proteins.
FIG. 2.
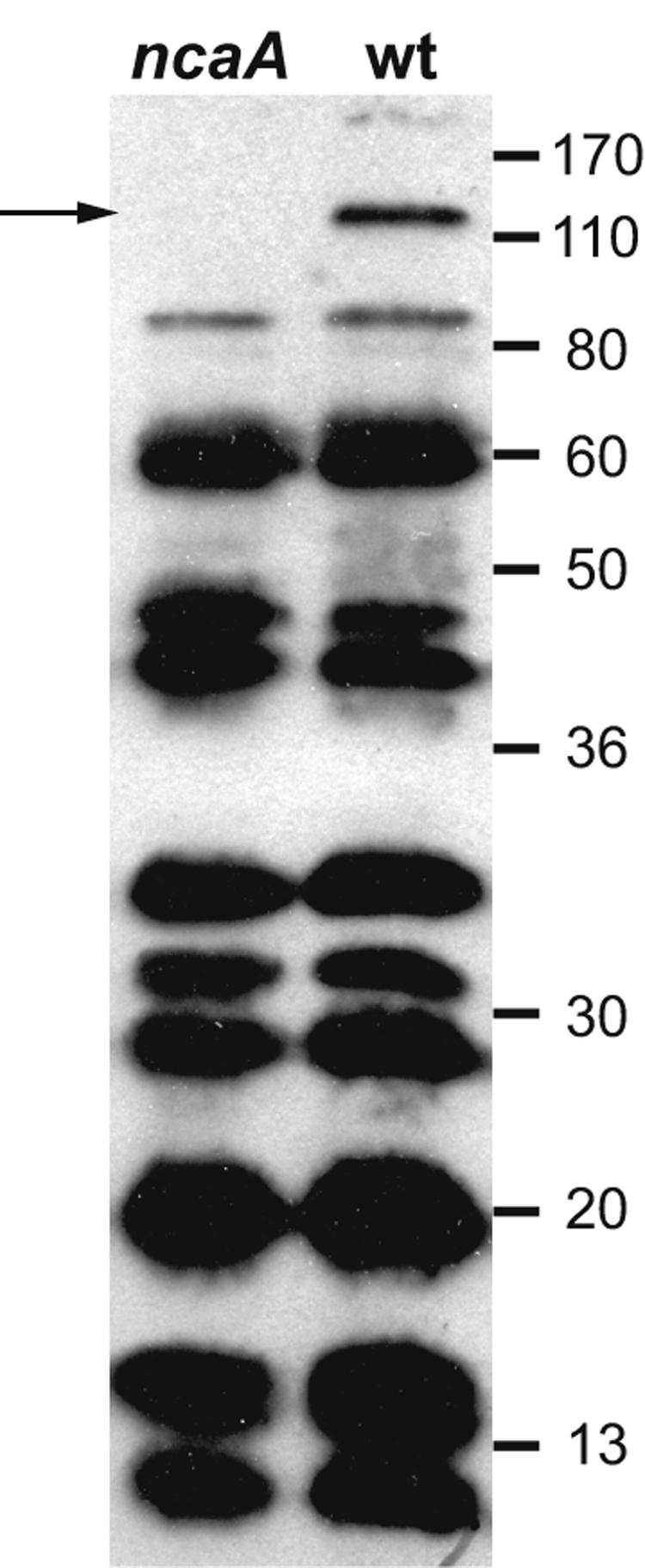
Immunoblot of H. ducreyi 35000HP (wild type [wt]) and 35000HPncaA (ncaA) whole-cell lysates with immune pig serum. The arrow indicates the position of an apparently multimeric form of NcaA, which migrated as a >100-kDa protein despite heating in the presence of SDS and β-mercaptoethanol. Other bands represent the constellation of H. ducreyi proteins recognized by polyclonal immune pig serum. Numbers indicate molecular mass standards in kilodaltons. The immunoblot film was scanned with an HP ScanJet 3970. The contrast of the scanned image was adjusted using the auto contrast command within Adobe Photoshop CS, and annotations were added with Adobe Illustrator CS.
Extracellular matrix protein binding properties of NcaA.
To determine whether NcaA binds ECM proteins, we compared the ECM binding properties of wild-type H. ducreyi 35000HP with those of a mutant unable to express NcaA due to insertion-deletion inactivation of the ncaA gene. Fluorescein-labeled 35000HP and the ncaA mutant (35000HPncaA) were incubated in ECM-coated microplate wells. Assays were performed by incubating a bacterial suspension of constant density in wells coated with a range of ECM concentrations from 0 to 80 μg/ml. A minimum H. ducreyi density of 107 CFU/ml was sufficient to ensure that binding saturation was achieved at the highest ECM concentrations. Bacterial binding was determined by measuring the fluorescence intensity of each well following incubation and washing and then relating that intensity to an assay-specific standard curve correlating fluorescence to CFU.
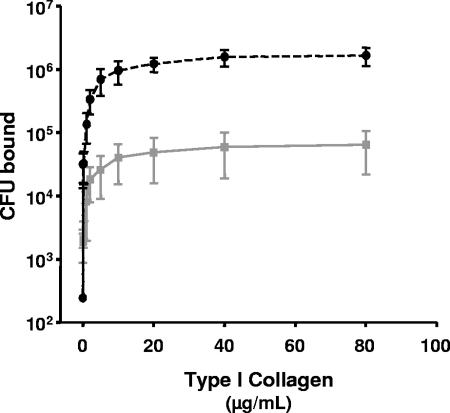
Binding of the ncaA mutant strain to type I human collagen was significantly less than that of its isogenic parent strain H. ducreyi 35000HP (Fig. 3), with statistically significant differences in attachment at all ECM concentrations tested except 0.002 μg/ml. In contrast, there was no difference in the level of binding of 35000HP or 35000HPncaA to heparin (an analog of the cell surface receptor heparan sulfate proteoglycan), plasma fibronectin, or cellular fibronectin (data not shown). These data indicate that NcaA promotes specific adherence to type I collagen and not to related ECM proteins or cell surface adhesion molecules.
FIG. 3.
Adherence of fluorescently labeled ncaA mutant (gray plot) or wild-type (black dashed plot) H. ducreyi to immobilized type I collagen. Adherence at each concentration of collagen was tested eight times in each assay, and adherence assays were performed three times for each strain. The data presented were averaged from the three assays, and error bars depict standard errors of the means. All values except the lowest (0.002 μg/ml collagen) were significantly different (all P < 0.05).
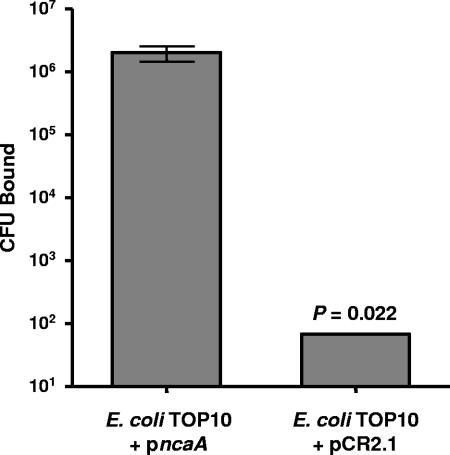
To determine if NcaA was sufficient to confer adherence to type I collagen, we tested the binding phenotype of E. coli expressing H. ducreyi NcaA. E. coli TOP10 containing the ncaA gene in pCR2.1 (TOP10 pncaA) or with pCR2.1 vector alone (TOP10 pCR2.1) was fluorescently labeled and incubated in microplate wells coated with type I collagen. Approximately 106 TOP10 pncaA CFU bound to collagen following incubation with 8 × 106 CFU, whereas fewer than 100 TOP10 pCR2.1 CFU remained after incubation with 3.2 × 107 CFU (Fig. 4). TOP10 pncaA did not bind wells coated with plasma fibronectin (data not shown). These data indicate that NcaA is both necessary and sufficient to elicit specific association with type I collagen.
FIG. 4.
Impact of NcaA expression on E. coli adherence to type I collagen. Fluorescently labeled E. coli TOP10 containing empty vector or ncaA plasmids was incubated in microplate wells coated with 40 μg/ml bovine type I collagen. Data presented are mean E. coli CFU bound (error bars show standard errors of the means) following incubation with 1.6 × 107 and 8.0 × 106 vector- or ncaA-containing CFU, respectively.
Effect of NcaA expression on H. ducreyi serum resistance and adherence to keratinocytes and foreskin fibroblasts.
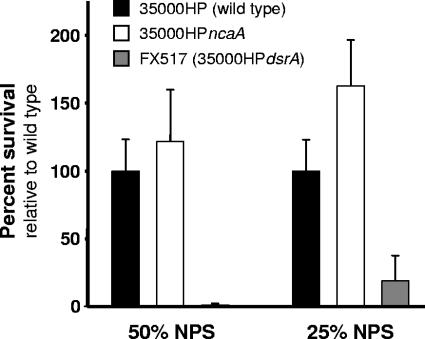
The Oca proteins UspA2, (2) and YadA (7, 35) and the Eib proteins A, C, D, E, and F (50, 51) impart resistance in their respective organisms to complement-mediated killing in the absence of opsonizing antibody and have also been shown to mediate binding to host receptors. NcaA has significant amino acid sequence similarity to DsrA, an H. ducreyi outer membrane protein and Oca protein that confers resistance to killing by normal human serum (19) as well as attachment to keratinocytes (15). We therefore tested the sensitivity of 35000HPncaA to normal human serum. The serum-sensitive strain H. ducreyi FX517 (35000HPdsrA) was effectively killed by 25% and 50% naïve pig serum with complement, whereas 35000HP and 35000HPncaA were both resistant to complement-mediated killing (Fig. 5). We also determined if NcaA mediates adhesion to keratinocytes and fibroblasts, which are the predominant cell types in skin. Both 35000HP and 35000HPncaA adhered to HaCaT cells, a human keratinocyte cell line (13), and HS27 cells, a human foreskin fibroblast cell line (1634-C; American Type Culture Collection, Manassas, VA) (data not shown). From these findings we concluded that NcaA neither contributes to serum resistance nor promotes adhesion to cultured skin fibroblasts or keratinocytes.
FIG. 5.
Serum sensitivity of H. ducreyi mutants. Survival of wild-type (black bars), ncaA mutant (white bars), and dsrA mutant (gray bars) H. ducreyi after incubation in 25% or 50% normal pig serum (NPS) is shown. Serum survival was assayed in triplicate in three independent experiments (error bars show standard errors of the means).
NcaA expression is necessary for virulence in the swine model of chancroid infection.
To determine if NcaA is important in skin lesion formation in vivo, we inoculated pigs with 35000HP or 35000HPncaA and assessed bacterial recovery from biopsies taken 2 or 7 days postinoculation (p.i.). These data were generated from 14 animals across six experimental iterations. The percentages of biopsies containing viable H. ducreyi at day 2 p.i. were not significantly different for sites inoculated with 35000HP or 35000HPncaA (60.3% versus 56.4% culture positive, respectively) (Table 1). However, there was a statistically significant difference (P < 0.0001) between the proportions of culture-positive biopsies taken at day 7 p.i. from sites inoculated with 35000HP (72.9%) or 35000HPncaA (20.0%). Despite similar numbers of culture-positive biopsies at day 2 p.i., the average number of CFU recovered from 35000HP-inoculated sites was over fivefold higher than the number recovered from mutant-inoculated sites (161.0 versus 30.4 CFU per biopsy). This span increased to over 24-fold at day 7 p.i. (146.8 versus 6.1). Differences in the number of CFU recovered per biopsy were statistically significant (P < 0.0001) at both days of biopsy. We also inoculated pigs with the cointegrate merodiploid strain generated during construction of 35000HPncaA in order to determine if the phenotype of 35000HPncaA was due to a spurious attenuating mutation rather than the targeted inactivation of the ncaA locus. Unlike 35000HPncaA, the cointegrate was recovered from pig ear lesions with similar frequency and in similar amounts as wild-type 35000HP at days 2 and 7 p.i. (Table 1), demonstrating that this strain is not attenuated.
TABLE 1.
Response to experimental inoculation of H. ducreyi 35000HP, 35000HPncaA, and 35000HPncaA merodiploid in pigs
| Strain |
H. ducreyi CFU per lesion biopsya at day p.i.:
|
% H. ducreyi-positive biopsiesb at day p.i.:
|
||
|---|---|---|---|---|
| 2 | 7 | 2 | 7 | |
| 35000HP | 161.0 (90.5-497.4) | 146.8 (111.2-320.8) | 60.3 (30.9-75.7) | 72.9 (57.5-96.8) |
| 35000HPncaA | 30.4 (19.5-82.3)c | 6.1 (2.2-28.6)c | 56.4 (31.6-68.7) | 20.0 (17.1-61.7)c |
| 35000HPncaA merodiploid | 280.9 (181.0-1390.2) | 320.3 (148.3-1080.7) | 70.0 (24.9-85.4) | 75.0 (64.3-95.7) |
Values indicate the observed mean CFU per biopsy, with 95% confidence intervals in parentheses, as modeled by poisson regression and GEE adjustment to account for variability between pigs.
Values indicate the observed mean proportion of biopsies harboring viable H. ducreyi, with 95% confidence intervals in parentheses, as modeled by logistic regression and GEE adjustment.
Significantly different (P < 0.0001) from values for wild-type or merodiploid lesions on same day postinoculation.
Human inoculation experiments.
The human challenge model allowed us to assess the importance of NcaA in establishing infection in the natural host of H. ducreyi. An escalating dose-response study was used to compare the virulences of 35000HP and 35000HPncaA. Eleven healthy adults (seven females and four males; age range, 20 to 53 years; mean age ± standard deviation, 38.6 ± 11.3 years) volunteered for the study. One subject (no. 245) withdrew prior to inoculation. In the first iteration, one subject was inoculated at three sites with an EDD of 34 CFU of the parent strain and at three sites with EDDs of 23, 46, and 92 CFU of the mutant. Papules developed at two of three sites inoculated with the parent and at two of three sites inoculated with the mutant, but all the papules resolved (Table 2). In the second iteration, three subjects were inoculated at three sites with an EDD of 85 CFU of the parent and at three sites with EDDs of 31, 62, and 124 CFU of the mutant. Papules developed at four of nine sites inoculated with the parent strain and at five of nine sites inoculated with the mutant (Table 2). A pustule developed at one parent site, while all mutant sites resolved (Table 2). In the third iteration, the subjects were inoculated with an EDD of 92 CFU of the parent and with EDDs of 74, 147, and 294 CFU of the mutant. Papules developed at all nine parent sites and at seven of nine mutant sites (Table 2). Pustules developed at six of nine parent sites, while all mutant papules resolved. In the fourth iteration, the subjects were inoculated with an EDD of 140 CFU of the parent and EDDs of 490, 960, and 1,920 CFU of the mutant. Papules developed at eight of nine sites inoculated with the parent and at eight of nine sites inoculated with the mutant (Table 2). A pustule developed at one parent site, while all mutant sites resolved (Table 2).
TABLE 2.
Human volunteer response to inoculation of live H. ducreyi strainsa
| Volunteer | Genderb | Days of observation | Strain | No. of initial papules | Final outcome of initial papule
|
|
|---|---|---|---|---|---|---|
| No. of pustules | No. resolved | |||||
| 244 | F | 5 | 35000HP | 2 | 2 | |
| 35000HPncaA | 2 | 2 | ||||
| 243 | F | 9 | 35000HP | 3 | 1 | 2 |
| 35000HPncaA | 2 | 2 | ||||
| 247 | M | 3 | 35000HP | 1 | 1 | |
| 35000HPncaA | 0 | |||||
| 248 | F | 5 | 35000HP | 0 | ||
| 35000HPncaA | 2 | 2 | ||||
| 249 | M | 8 | 35000HP | 3 | 1 | 2 |
| 35000HPncaA | 2 | 2 | ||||
| 250 | F | 7 | 35000HP | 3 | 3 | |
| 35000HPncaA | 3 | 3 | ||||
| 251 | M | 8 | 35000HP | 3 | 2 | 1 |
| 35000HPncaA | 3 | 3 | ||||
| 242 | F | 6 | 35000HP | 3 | 3 | |
| 35000HPncaA | 3 | 3 | ||||
| 252 | F | 9 | 35000HP | 3 | 1c | 1 |
| 35000HPncaA | 3 | 3 | ||||
| 253 | F | 5 | 35000HP | 2 | 2 | |
| 35000HPncaA | 2 | 2 | ||||
Volunteer 244 was inoculated in iteration 1. Volunteers 243, 247, and 248 were inoculated in iteration 2. Volunteers 249, 250, and 251 were inoculated in iteration 3. Volunteers 242, 252, and 253 were inoculated in iteration 4.
M, male; F, female.
One site remained a papule at the time the subject reached the end point.
The cumulative results for the four iterations showed that papules developed at 76.7% (95% confidence interval [CI], 55.9% to 97.4%) of sites inoculated with 35000HP and at 74.3% (95% CI, 55.3% to 91.3%) of sites inoculated with 35000HPncaA. Overall, pustules formed at 8/30 (26.7%; 95% CI, 6.4% to 46.9%) sites inoculated with 35000HP, compared to 0 of 30 (0%; 95% CI, 0% to 25.9%) sites inoculated with 35000HPncaA (P = 0.005). Thus, the mutant was impaired in its ability to form pustules compared to the parent.
For the four parent and four mutant broth cultures used to prepare the inocula, all 192 parent colonies and all 192 mutant colonies tested were phenotypically correct (mutant, chloramphenicol resistant [Cmr]; parent, Cms). Of the 30 sites that were inoculated with the parent, 6 (20%) yielded at least one positive surface culture, while 0 of 18 mutant sites yielded a positive surface culture. All colonies obtained from surface cultures (n = 346) and biopsy specimens (n = 9) from parent sites were phenotypically correct (Cms). Thus, all tested colonies from the inocula, surface cultures, and biopsy specimens had the expected phenotype. We were unable to test the merodiploid strain for complementation of the 35000HPncaA phenotype because inoculation of human volunteers with strains that harbor plasmids is not permitted.
DISCUSSION
During the course of identifying targets of immune serum that conferred protection against H. ducreyi infection in the swine chancroid model, we found that at least one of the antibodies bound a previously uncharacterized outer membrane protein. We named this protein NcaA because our findings have shown it to be necessary for collagen association in H. ducreyi. The sequence of the C-terminal 79 amino acids of NcaA is similar to that of the oligomeric coiled adhesin (Oca) subfamily of autotransporter proteins. Oca proteins form oligomeric lollipop-shaped surface projections on the bacterial outer membrane, consisting of N-terminal head and neck domains followed by a coiled-coil stalk and a YadA-like C-terminal domain that anchors the protein in the outer membrane (28, 48). The amino acid sequence of NcaA is consistent with this proposed structure, but its three-dimensional structure has not been determined. Oca proteins also migrate as heat-stable oligomers during SDS-PAGE. This peculiarity is thought to reflect the avid oligomerization property of these proteins on the bacterial surface (3, 19, 36, 48, 51). YadA exists as a trimer both when anchored to the bacterial outer membrane and during migration through SDS-PAGE (48). Monomeric NcaA has a predicted molecular mass of 33 kDa but shows an apparent size of >100 kDa in SDS-PAGE (Fig. 2), suggesting that it also oligomerizes, though it is not known how many NcaA monomers assemble to form a functional complex.
Chancroid transmission occurs when H. ducreyi penetrates the keratinized skin surface layer and colonizes the dermis or epidermis of the skin. Type I collagen is the predominant structural component of skin (6), and H. ducreyi is found associated with type I collagen in the dermis of biopsy samples collected from human volunteers 48 h postinoculation (8). Other ECM attachment studies also demonstrated that H. ducreyi binds to immobilized type I collagen in vitro (1, 9). Our results indicate that specific association of H. ducreyi with type I collagen is mediated by NcaA. Collagen binding is a crucial factor in the pathogenesis of other organisms that inhabit skin during infection. For example, loss of YadA-mediated collagen binding is associated with loss of Yersinia enterocolitica virulence in a mouse model of infection (56). In addition, the Staphylococcus aureus collagen adhesin Cna is required for virulence in multiple animal models of infection (18, 26, 44, 46). Our finding that NcaA binds collagen is consistent with the nature of H. ducreyi as a dermal pathogen.
Our in vitro experiments showed that NcaA neither mediates adherence to cultured skin cells nor confers resistance to complement-mediated killing. We also demonstrated that NcaA is necessary and sufficient for bacterial association with type I collagen. Bacterial adherence to host tissues is generally considered to be an essential step in establishing infection (10, 43). Our studies here illustrate that adhesins mediating association of bacteria with structural ECM proteins in contrast to adherence to host cells can also be important virulence determinants. NcaA appears to have such a role in H. ducreyi pathogenesis. In pigs, the ncaA mutant was recovered from significantly fewer lesions than the wild type. In human volunteers, the ncaA mutant was unable to form pustules, making NcaA the seventh H. ducreyi protein shown to be essential for virulence in the native human host (4, 12, 21, 29, 53). Whereas these findings demonstrate that NcaA is required for infection of humans and pigs, we are conducting further studies to elucidate the collagen binding property of NcaA and to determine whether it is directly responsible for the contribution of NcaA to H. ducreyi pathogenesis. We are also determining if an antibody response against NcaA can confer protection against H. ducreyi infection.
Acknowledgments
This work was supported by NIH NIAID grants AI42824 (to T.H.K.) and AI31494 and AI27863 (to S.M.S.), NSF predoctoral fellowship and UNC-Chapel Hill Division of Infectious Diseases training grant NIH 5-T32 AI07001 (to L.E.C.), and a UNC-Chapel Hill Graduate School Royster Fellowship (to R.A.F.). D.M.J. was supported by NIAID training grant T32 AI07367. The human challenge trials were also supported by grant MO1RR00750 to the GCRC at Indiana University.
We are grateful for the technical expertise of Patty Routh and Drew Reinbold, as well as the generous assistance provided by Chris Elkins and colleagues in performing the OMP radioimmunoprecipitation studies. We also thank Martha Greenwald and Beth Zwickl for enrolling the volunteers, and we thank the volunteers who participated in the trial.
Editor: D. L. Burns
REFERENCES
- 1.Abeck, D., A. P. Johnson, and H. Mensing. 1992. Binding of Haemophilus ducreyi to extracellular matrix proteins. Microb. Pathog. 13:81-84. [DOI] [PubMed] [Google Scholar]
- 2.Aebi, C., E. R. Lafontaine, L. D. Cope, J. L. Latimer, S. L. Lumbley, G. H. McCracken, Jr., and E. J. Hansen. 1998. Phenotypic effect of isogenic uspA1 and uspA2 mutations on Moraxella catarrhalis 035E. Infect. Immun. 66:3113-3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aebi, C., I. Maciver, J. L. Latimer, L. D. Cope, M. K. Stevens, S. E. Thomas, G. H. McCracken, Jr., and E. J. Hansen. 1997. A protective epitope of Moraxella catarrhalis is encoded by two different genes. Infect. Immun. 65:4367-4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Tawfiq, J. A., K. R. Fortney, B. P. Katz, A. F. Hood, C. Elkins, and S. M. Spinola. 2000. An isogenic hemoglobin receptor-deficient mutant of Haemophilus ducreyi is attenuated in the human model of experimental infection. J. Infect. Dis. 181:1049-1054. [DOI] [PubMed] [Google Scholar]
- 5.Al-Tawfiq, J. A., A. C. Thornton, B. P. Katz, K. R. Fortney, K. D. Todd, A. F. Hood, and S. M. Spinola. 1998. Standardization of the experimental model of Haemophilus ducreyi infection in human subjects. J. Infect. Dis. 178:1684-1687. [DOI] [PubMed] [Google Scholar]
- 6.Ayad, S. 1998. The extracellular matrix factsbook, 2nd ed. Academic Press/Harcourt Brace, San Diego, Calif.
- 7.Balligand, G., Y. Laroche, and G. Cornelis. 1985. Genetic analysis of virulence plasmid from a serogroup 9 Yersinia enterocolitica strain: role of outer membrane protein P1 in resistance to human serum and autoagglutination. Infect. Immun. 48:782-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bauer, M. E., M. P. Goheen, C. A. Townsend, and S. M. Spinola. 2001. Haemophilus ducreyi associates with phagocytes, collagen, and fibrin and remains extracellular throughout infection of human volunteers. Infect. Immun. 69:2549-2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bauer, M. E., and S. M. Spinola. 1999. Binding of Haemophilus ducreyi to extracellular matrix proteins. Infect. Immun. 67:2649-2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beachey, E. H. 1980. Bacterial adherence. Chapman and Hall, London, United Kingdom.
- 11.Behets, F. M., J. Andriamiadana, D. Randrianasolo, R. Randriamanga, D. Rasamilalao, C. Y. Chen, J. B. Weiss, S. A. Morse, G. Dallabetta, and M. S. Cohen. 1999. Chancroid, primary syphilis, genital herpes, and lymphogranuloma venereum in Antananarivo, Madagascar. J. Infect. Dis. 180:1382-1385. [DOI] [PubMed] [Google Scholar]
- 12.Bong, C. T., R. E. Throm, K. R. Fortney, B. P. Katz, A. F. Hood, C. Elkins, and S. M. Spinola. 2001. DsrA-deficient mutant of Haemophilus ducreyi is impaired in its ability to infect human volunteers. Infect. Immun. 69:1488-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boukamp, P., R. T. Petrussevska, D. Breitkreutz, J. Hornung, A. Markham, and N. E. Fusenig. 1988. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 106:761-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bozue, J. A., L. Tarantino, and R. S. Munson, Jr. 1998. Facile construction of mutations in Haemophilus ducreyi using lacZ as a counter-selectable marker. FEMS Microbiol. Lett. 164:269-273. [DOI] [PubMed] [Google Scholar]
- 15.Cole, L. E., T. H. Kawula, K. L. Toffer, and C. Elkins. 2002. The Haemophilus ducreyi serum resistance antigen DsrA confers attachment to human keratinocytes. Infect. Immun. 70:6158-6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cole, L. E., K. L. Toffer, R. A. Fulcher, L. R. San Mateo, P. E. Orndorff, and T. H. Kawula. 2003. A humoral response confers protection against Haemophilus ducreyi infection. Infect. Immun. 71:6971-6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dada, A. J., A. O. Ajayi, L. Diamondstone, T. C. Quinn, W. A. Blattner, and R. J. Biggar. 1998. A serosurvey of Haemophilus ducreyi, syphilis, and herpes simplex virus type 2 and their association with human immunodeficiency virus among female sex workers in Lagos, Nigeria. Sex. Transm. Dis. 25:237-242. [DOI] [PubMed] [Google Scholar]
- 18.Elasri, M. O., J. R. Thomas, R. A. Skinner, J. S. Blevins, K. E. Beenken, C. L. Nelson, and M. S. Smelter. 2002. Staphylococcus aureus collagen adhesin contributes to the pathogenesis of osteomyelitis. Bone 30:275-280. [DOI] [PubMed] [Google Scholar]
- 19.Elkins, C., K. J. Morrow, Jr., and B. Olsen. 2000. Serum resistance in Haemophilus ducreyi requires outer membrane protein DsrA. Infect. Immun. 68:1608-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleming, D. T., and J. N. Wasserheit. 1999. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex. Transm. Infect. 75:3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fortney, K. R., R. S. Young, M. E. Bauer, B. P. Katz, A. F. Hood, R. S. Munson, Jr., and S. M. Spinola. 2000. Expression of peptidoglycan-associated lipoprotein is required for virulence in the human model of Haemophilus ducreyi infection. Infect. Immun. 68:6441-6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gray, R. H., M. J. Wawer, R. Brookmeyer, N. K. Sewankambo, D. Serwadda, F. Wabwire-Mangen, T. Lutalo, X. Li, T. vanCott, T. C. Quinn, and R. P. Team. 2001. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet 357:1149-1153. [DOI] [PubMed] [Google Scholar]
- 23.Greenblatt, R. M., S. A. Lukehart, F. A. Plummer, T. C. Quinn, C. W. Critchlow, R. L. Ashley, L. J. D'Costa, J. O. Ndinya-Achola, L. Corey, A. R. Ronald, et al. 1988. Genital ulceration as a risk factor for human immunodeficiency virus infection. AIDS 2:47-50. [DOI] [PubMed] [Google Scholar]
- 24.Hayes, R. J., K. F. Schulz, and F. A. Plummer. 1995. The cofactor effect of genital ulcers on the per-exposure risk of HIV transmission in sub-Saharan Africa. J. Trop. Med. Hyg. 98:1-8. [PubMed] [Google Scholar]
- 25.Henderson, I. R., F. Navarro-Garcia, M. Desvaux, R. C. Fernandez, and D. Ala'Aldeen. 2004. Type V protein secretion pathway: the autotransporter story. Microbiol. Mol. Biol Rev. 68:692-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hienz, S. A., T. Schennings, A. Heimdahl, and J. I. Flock. 1996. Collagen binding of Staphylococcus aureus is a virulence factor in experimental endocarditis. J. Infect. Dis. 174:83-88. [DOI] [PubMed] [Google Scholar]
- 27.Hobbs, M. M., L. R. San Mateo, P. E. Orndorff, G. Almond, and T. H. Kawula. 1995. Swine model of Haemophilus ducreyi infection. Infect. Immun. 63:3094-3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoiczyk, E., A. Roggenkamp, M. Reichenbecher, A. Lupas, and J. Heesemann. 2000. Structure and sequence analysis of Yersinia YadA and Moraxella UspAs reveal a novel class of adhesins. EMBO J. 19:5989-5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janowicz, D., K. Fortney, B. Katz, J. L. Latimer, K. Deng, E. J. Hansen, and S. M. Spinola. 2004. Expression of the LspA1 and LspA2 proteins by Haemophilus ducreyi is required for virulence in human volunteers. Infect. Immun. 72:4528-4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamali, A., A. J. Nunn, D. W. Mulder, E. Van Dyck, J. G. Dobbins, and J. A. Whitworth. 1999. Seroprevalence and incidence of genital ulcer infections in a rural Ugandan population. Sex. Transm. Infect. 75:98-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.King, R., S. H. Choudhri, J. Nasio, J. Gough, N. J. Nagelkerke, F. A. Plummer, J. O. Ndinya-Achola, and A. R. Ronald. 1998. Clinical and in situ cellular responses to Haemophilus ducreyi in the presence or absence of HIV infection. Int. J. Sex. Transm. Dis. AIDS 9:531-536. [DOI] [PubMed] [Google Scholar]
- 32.King, R., J. Gough, A. Ronald, J. Nasio, J. O. Ndinya-Achola, F. Plummer, and J. A. Wilkins. 1996. An immunohistochemical analysis of naturally occurring chancroid. J. Infect. Dis. 174:427-430. [DOI] [PubMed] [Google Scholar]
- 33.Korenromp, E. L., S. J. de Vlass, N. J. Nagelkerke, and J. D. Habbema. 2001. Estimating the magnitude of STD cofactor effects on HIV transmission: how well can it be done? Sex. Transm. Dis. 28:613-621. [DOI] [PubMed] [Google Scholar]
- 34.Lewis, D. A. 2003. Chancroid: clinical manifestations, diagnosis, and management. Sex. Transm. Infect. 79:68-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez, R. J. 1989. Thermoregulation-dependent expression of Yersinia enterocolitica protein 1 imparts serum resistance to Escherichia coli K-12. J. Bacteriol. 171:3732-3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McMichael, J. C., M. J. Fiske, R. A. Fredenburg, D. N. Chakravarti, K. R. VanDerMeid, V. Barniak, J. Caplan, E. Bortell, S. Baker, R. Arumugham, and D. Chen. 1998. Isolation and characterization of two proteins from Moraxella catarrhalis that bear a common epitope. Infect. Immun. 66:4374-4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mertz, K. J., J. B. Weiss, R. M. Webb, W. C. Levine, J. S. Lewis, K. A. Orle, P. A. Totten, J. Overbaugh, S. A. Morse, M. M. Currier, M. Fishbein, and M. E. St Louis. 1998. An investigation of genital ulcers in Jackson, Mississippi, with use of a multiplex polymerase chain reaction assay: high prevalence of chancroid and human immunodeficiency virus infection. J. Infect. Dis. 178:1060-1066. [DOI] [PubMed] [Google Scholar]
- 38.Mroczkowski, T. F., and D. H. Martin. 1994. Genital ulcer disease. Dermatol. Clin. 12:753-764. [PubMed] [Google Scholar]
- 39.Ndinya-Achola, J. O., A. N. Kihara, L. D. Fisher, M. R. Krone, F. A. Plummer, A. Ronald, and K. K. Holmes. 1996. Presumptive specific clinical diagnosis of genital ulcer disease (GUD) in a primary health care setting in Nairobi. Int. J. Sex. Transm. Dis. AIDS 7:201-205. [DOI] [PubMed] [Google Scholar]
- 40.Nelson, K. E., S. Eiumtrakul, D. Celentano, I. Maclean, A. Ronald, S. Suprasert, D. R. Hoover, S. Kuntolbutra, and J. M. Zenilman. 1997. The association of herpes simplex virus type 2 (HSV-2), Haemophilus ducreyi, and syphilis with HIV infection in young men in northern Thailand. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 16:293-300. [DOI] [PubMed] [Google Scholar]
- 41.Nika, J. R., J. L. Latimer, C. K. Ward, R. J. Blick, N. J. Wagner, L. D. Cope, G. G. Mahairas, R. S. Munson, Jr., and E. J. Hansen. 2002. Haemophilus ducreyi requires the flp gene cluster for microcolony formation in vitro. Infect. Immun. 70:2965-2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Farrell, N. 2001. Targeted interventions required against genital ulcers in African countries worst affected by HIV infection. Bull. W. H. O. 79:569-577. [PMC free article] [PubMed] [Google Scholar]
- 43.Ofek, I., and E. H. Beachey. 1980. Bacterial adherence. Adv. Intern. Med. 25:503-532. [PubMed] [Google Scholar]
- 44.Patti, J. M., T. Bremell, D. Krajewska-Pietrasik, A. Abdelnour, A. Tarkowski, C. Ryden, and M. Hook. 1994. The Staphylococcus aureus collagen adhesin is a virulence determinant in experimental septic arthritis. Infect. Immun. 62:152-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quale, J., E. Teplitz, and M. Augenbraun. 1990. Atypical presentation of chancroid in a patient infected with the human immunodeficiency virus. Am. J. Med. 88:43N-44N. [PubMed] [Google Scholar]
- 46.Rhem, M. N., E. M. Lech, J. M. Patti, D. McDevitt, M. Hook, D. B. Jones, and K. R. Wilhelmus. 2000. The collagen-binding adhesin is a virulence factor in Staphylococcus aureus keratitis. Infect. Immun. 68:3776-3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Risbud, A., K. Chan-Tack, D. Gadkari, R. R. Gangakhedkar, M. E. Shepherd, R. Bollinger, S. Mehendale, C. Gaydos, A. Divekar, A. Rompalo, and T. C. Quinn. 1999. The etiology of genital ulcer disease by multiplex polymerase chain reaction and relationship to HIV infection among patients attending sexually transmitted disease clinics in Pune, India. Sex. Transm. Dis. 26:55-62. [DOI] [PubMed] [Google Scholar]
- 48.Roggenkamp, A., N. Ackermann, C. A. Jacobi, K. Truelzsch, H. Hoffmann, and J. Heesemann. 2003. Molecular analysis of transport and oligomerization of the Yersinia enterocolitica adhesin YadA. J. Bacteriol. 185:3735-3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Royce, R. A., A. Sena, W. Cates, Jr., and M. S. Cohen. 1997. Sexual transmission of HIV. N. Engl. J. Med. 336:1072-1078. [DOI] [PubMed] [Google Scholar]
- 50.Sandt, C. H., and C. W. Hill. 2000. Four different genes responsible for nonimmune immunoglobulin-binding activities within a single strain of Escherichia coli. Infect. Immun. 68:2205-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sandt, C. H., and C. W. Hill. 2001. Nonimmune binding of human immunoglobulin A (IgA) and IgG Fc by distinct sequence segments of the EibF cell surface protein of Escherichia coli. Infect. Immun. 69:7293-7303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.San Mateo, L. R., K. L. Toffer, P. E. Orndorff, and T. H. Kawula. 1999. Immune cells are required for cutaneous ulceration in a swine model of chancroid. Infect. Immun. 67:4963-4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spinola, S. M., K. R. Fortney, B. P. Katz, J. L. Latimer, J. R. Mock, M. Vakevainen, and E. J. Hansen. 2003. Haemophilus ducreyi requires an intact flp gene cluster for virulence in humans. Infect. Immun. 71:7178-7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spinola, S. M., A. Orazi, J. N. Arno, K. Fortney, P. Kotylo, C. Y. Chen, A. A. Campagnari, and A. F. Hood. 1996. Haemophilus ducreyi elicits a cutaneous infiltrate of CD4 cells during experimental human infection. J. Infect. Dis. 173:394-402. [DOI] [PubMed] [Google Scholar]
- 55.Spinola, S. M., L. M. Wild, M. A. Apicella, A. A. Gaspari, and A. A. Campagnari. 1994. Experimental human infection with Haemophilus ducreyi. J. Infect. Dis. 169:1146-1150. [DOI] [PubMed] [Google Scholar]
- 56.Tamm, A., A. M. Tarkkanen, T. K. Korhonen, P. Kuusela, P. Toivanen, and M. Skurnik. 1993. Hydrophobic domains affect the collagen-binding specificity and surface polymerization as well as the virulence potential of the YadA protein of Yersinia enterocolitica. Mol. Microbiol. 10:995-1011. [DOI] [PubMed] [Google Scholar]
- 57.Thomas, C. E., N. H. Carbonetti, and P. F. Sparling. 1996. Pseudo-transposition of a Tn5 derivative in Neisseria gonorrhoeae. FEMS Microbiol. Lett. 145:371-376. [DOI] [PubMed] [Google Scholar]
- 58.Throm, R. E., J. A. Al-Tawfiq, K. R. Fortney, B. P. Katz, A. F. Hood, C. A. Slaughter, E. J. Hansen, and S. M. Spinola. 2000. Evaluation of an isogenic major outer membrane protein-deficient mutant in the human model of Haemophilus ducreyi infection. Infect. Immun. 68:2602-2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trees, D. L., and S. A. Morse. 1995. Chancroid and Haemophilus ducreyi: an update. Clin. Microbiol. Rev. 8:357-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wasserheit, J. N. 1992. Epidemiological synergy. Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex. Transm. Dis. 19:61-77. [PubMed] [Google Scholar]
- 61.Wawer, M. J., R. H. Gray, N. K. Sewankambo, D. Serwadda, X. Li, O. Laeyendecker, N. Kiwanuka, G. Kigozi, M. Kiddugavu, T. Lutalo, F. Nalugoda, F. Wabwire-Mangen, M. P. Meehan, and T. C. Quinn. 2005. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J. Infect. Dis. 191:1403-1409. [DOI] [PubMed] [Google Scholar]
- 62.Zaretzky, F. R., and T. H. Kawula. 1999. Examination of early interactions between Haemophilus ducreyi and host cells by using cocultured HaCaT keratinocytes and foreskin fibroblasts. Infect. Immun. 67:5352-5360. [DOI] [PMC free article] [PubMed] [Google Scholar]