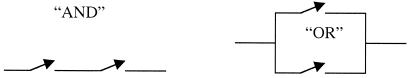Abstract
We propose a scheme for molecule-based information processing by combining well-studied spectroscopic techniques and recent results from chemical dynamics. Specifically it is discussed how optical transitions in single molecules can be used to rapidly perform classical (Boolean) logical operations. In the proposed way, a restricted number of states in a single molecule can act as a logical gate equivalent to at least two switches. It is argued that the four-level scheme can also be used to produce gain, because it allows an inversion, and not only a switching ability. The proposed scheme is quantum mechanical in that it takes advantage of the discrete nature of the energy levels but, we here discuss the temporal evolution, with the use of the populations only. On a longer time range we suggest that the same scheme could be extended to perform quantum logic, and a tentative suggestion, based on an available experiment, is discussed. We believe that the pumping can provide a partial proof of principle, although this and similar experiments were not interpreted thus far in our terms.
The need for increasing miniaturization of logical circuits is foreseen to soon reach the physical limit of MOSFET (Metal Oxide Semiconductor Field Effect) Transistors. There is therefore a worldwide search for alternatives. Single-molecule-based switches (1, 2) rectifiers (3, 4), and wires (5) have indeed been recently reported. The switch reported in ref. 2 allows an electrical current to flow or not, by causing a conformational change in [2]catanane type molecules. The molecule was incorporated in a solid state device and could be recycled many times. Other important recent work in this direction is reviewed in refs. 6 and 7. A different route, which has been studied for some time (8–10), is to combine chemical inputs with optical outputs. These are typically solution phase experiments, which have used to advantage the progress in supramolecular chemistry for the construction of molecular machines (11, 12).
We discuss a molecule-based scheme for logical operations, which is different in its features. One merit of our scheme is that it involves well-established and well-characterized photophysicochemical processes. This enables us to know that we could anticipate eventually an ultrafast processing and can expect a rather fast (picosecond time scale) response, even in preliminary studies. A point of chemical importance is that the scheme operates with the kind of molecules for which one has a great scope for organic synthesis so as to tailor the molecule to specific responses, both optical and kinetic. An important limitation, common to our scheme and to other proposals for computing with molecules (7), is the challenge of connecting the molecules. This paper provides an introduction to our proposal for molecular information processing, stressing the fundamental issues and avoiding mathematical formalism.
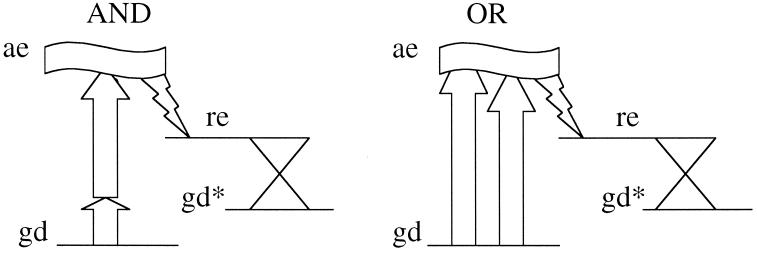
In the background to our discussion is the observation by Shannon (13) that there is an analogy between switches and Boolean logic operations. In fact, Shannon's proposal predates the invention of transistors and was first applied to electromechanical switches. The point is that it takes at least two switches to represent a logic operation. Fig. 1 shows a schematic representation of circuits that can perform the AND and OR operations. Transistors have replaced mechanical switches, but, in principle, the amplification ability of a transistor is not strictly essential for such applications. It is sufficient that the voltage applied to the gate can determine whether the transistor does or does not allow a current to flow. The output of existing gates can be used as input to other gates, thereby allowing concatenation. It has not escaped our attention that a molecular coordinate, typically the reactive one, cf. figure 2 below, could be used as a bus and so several gates could be accommodated on one molecule. But we here restrict the actual discussion to one single gate per molecule.
Figure 1.
The analogy between electrical circuits of two switches each and the two Boolean operations as pointed out by Shannon (13). The proposal of this paper, as summarized in Fig. 6, is to implement such gates in polyatomic molecules undergoing ultrafast changes associated with large, although reversible, reorganization in the electronic configuration, making use of optical as well as chemically reactive coupling phenomena. As we discuss, such couplings are well known and controllable.
In our scheme, we use a single molecule not as a switch but as a complete logical gate. In other words, a single molecule can replace at least two transistors. This is possible because, unlike ordinary transistors, molecules have more strict resonance conditions. We say “more strict” and not simply “strict” because there are always broadening mechanisms that limit how resonant a transition is. Instead of a voltage we propose to address and to read the system by laser pulses. This method avoids any need for electrical contacts. Among the serious limitations of laser pulses will be power broadening and the frequency width of short laser pulses, and so we propose to begin with somewhat longer pulses. Resonant tunneling junctions also have resonance conditions, but these are tuned by varying the voltage, and this method allows less control than is possible by optical means.
The discrete nature of molecular energy levels is, of course, a quantum mechanical phenomenon. Otherwise, however, our discussion will be classical. We are aware that the scheme we propose can be readily refined so as to make further use of quantum mechanics. There are two immediate aspects that come to mind. First is that not only the population but also the phase is at our disposal, so that one can perform quantum logical operations. The second is that one can operate the scheme so that it exhibits gain.
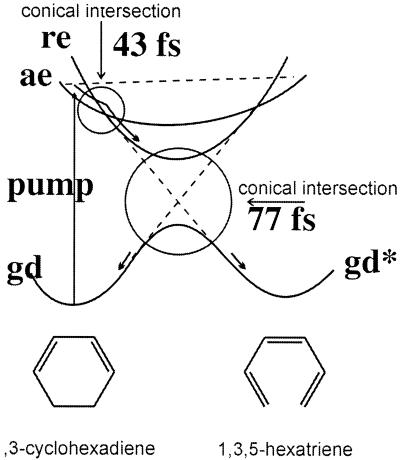
The immediate photochemical background to our proposal is the series of experiments (14, 15) on optically pumped electrocyclic ring-opening rearrangements (16) proceeding via conical intersections between different electronic potentials, as shown schematically in Fig. 2. The rearrangement is associated with corresponding changes in the absorption wavelengths and other electronic properties that can be used as a probe (≡ readout). The essential point for us is that ultrashort laser excitation experiments have firmly established that the entire ring opening occurs on a time scale shorter than (typically) 100 fs. It is this ultrafast rearrangement that allows us to claim that the gates that we discuss could be operated with unprecedentedly short time scales. However, even quite longer times are a worthwhile goal.
Figure 2.
A schematic drawing of the potentials for an ultrafast rearrangement that goes via a conical intersection. The time scales are experimental (14) from the kinetic simulation for the cyclohexadiene ring opening to hexatriene. The cyclohexadiene/hexatriene electrocyclic ring opening, occurring in less than 100 fs, may be considered as a prototype of a molecular ultrafast switch. The optical excitation goes via the direct access of a bright state, ae, which reacts by going first to a dark (photoreactive) excited state, re, and then to the product electronic ground state, gd*. For the cyclohexadiene system one can readily convert about 0.1% of all molecules in a sample to the excited state. The required molecular bistability in the ground state mediated by electronically excited states can be found also in related molecular systems (electrocyclic reactions, cycloadditions, sigmatropic shifts, tautomers, change in spin systems, electron transfer/redox photochemistry, etc.) (see also Figs. 4 and 5 and ref. 16).
Any fast reactive change resulting in a change in the electronic structure of a molecule is interesting for our proposal because such a change can be probed by light absorption. It is not restricted to conical intersections, but these provide us with measured ultrafast conversion rates and with absorption cross sections, etc., that are needed for a numerical simulation.
The other aspect, one that is central to our scheme, is the method of pumping the first optical transition, the transition labeled “pump” in Fig. 2. We discuss two options, which correspond to the classical OR and AND logical operations. However, one can easily generalize the scheme that we discuss to more elaborate gates. There are two motivations for examining more sophisticated schemes. One (still in the realm of classical logic) is that by using Raman processes and more wave mixing, one can develop a single-molecule gate that performs more complicated logical operations while still using a single molecule. We regard such extensions of the pumping scheme, where the optical excitation and the molecule are acting in concert to perform the required logical operation, as an important part of our proposal. The second extension is to use pulse shaping and other means of coherent control (17) so as to build coherence into the molecular level scheme. This method allows a single-molecule quantum mechanical logical gate operation.
In short, we argue that by bringing together tools and techniques that are already available one can build something new. From laser physics we need the pulses and their manipulation, from laser spectroscopy we take the pumping scheme, from femtochemistry we take the dynamics of the ultrafast molecular rearrangements, and from chemical synthesis we require the ability to tailor the molecule to the pumping scheme. Eventually, we can also use control strategies (18) for feedback. In the final analysis, it takes the synergism between the pump and the molecule to perform the logical operation.
Scheme
The essence of our proposal can be summarized in terms of a four-level scheme as shown in Fig. 3. We discuss first the photophysical systems that can be used to realize the required level scheme and then suggest how to use such a level scheme as a logic gate.
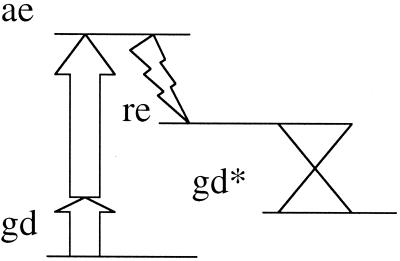
Figure 3.
A four-level scheme for a single-molecule-based logic gate. The ground state, gd, is pumped (shown as a two-photon transition) to an optically accessible excited state, ae. An ultrafast radiationless process leads the molecule to a rearranged state, re, which goes down, via a conical intersection, to an isomer of the ground state, gd*. Probing is done most conveniently by change of optical transmission.
Systems
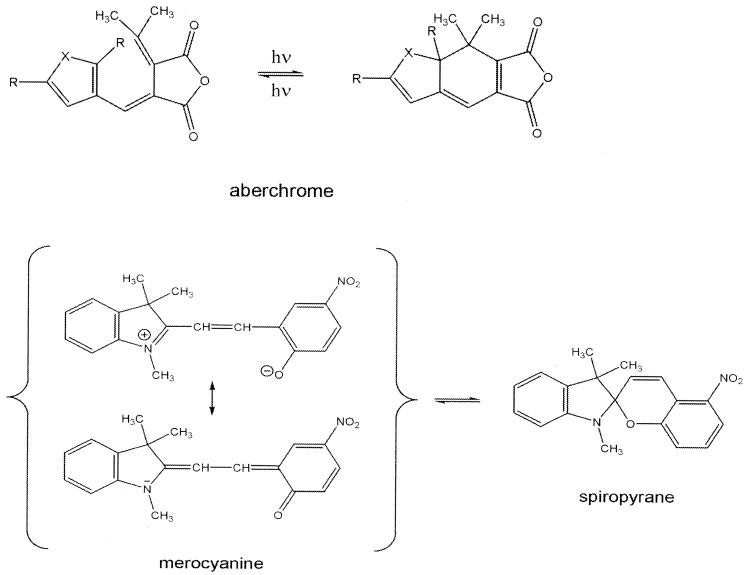
In the proposed four-level scheme, a molecule is first pumped from its ground state, gd, to an optically accessible excited state, ae. As shown in Fig. 2, the optically accessible excited state need not be the lowest excited state. Both Franck Condon and electronic selection rules typically are the cause for a lower excited state, identified as re in Fig. 3, being inaccessible from the ground state but which can be reached from the directly pumped state by a radiationless transition. The ultrafast intramolecular transition from the pumped state to the rearranged state is the key to the ultrafast switching. Such an ultrafast rearrangement is not unique to the photophysics of the polyenes. Fig. 4 shows an example for another class of compounds, the keto-enol isomerization (16, 19), that also has such an ultrafast response. Another class of potential candidates is the spyropyranes, which are shown in Fig. 5. Also shown in Fig. 5 are the aberchromes, which are a variant of the ultrafast ring opening shown in Fig. 2. There are therefore quite a number of prototypical systems that exhibit the ultrafast rearrangement ae → re that is necessary for an immediate response to the optical excitation.
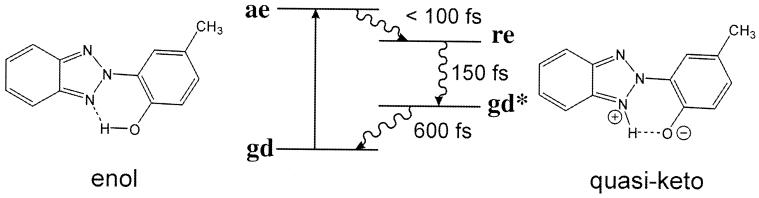
Figure 4.
A four-level scheme that represents the ultrafast rearrangement during a enol-keto isomerization. The transfer times are experimental (19). The Tinuvin molecule shown (2-(2′-hydroxy-5′ methylphenyl)benzotriazole) is special among the systems exhibiting proton transfer in the excited state in that it undergoes the complete cycle within less than a picosecond.
Figure 5.
The aberchromes (I), fulgides, and merocyanines (II) can also exhibit ultrafast intramolecular dynamics. The photochromism of spiropyranes was long considered as a potential candidate for computer memories, high-resolution photography, and photovoltaic and holographic systems.
So far we have discussed three levels. The fourth level is a rearranged (typically vibrationally hot but on the ground state) conformation, gd*, which is very rapidly accessed via a conical intersection. The re → gd* transition is the major loss channel. Considerable energy is dissipated at this stage. It is typically ultrafast because it goes via a conical intersection, but the sliding down the funnel surrounding the intersection, cf. Fig. 2, requires that other molecular modes and the environment help to dissipate some of the energy. In the longer run, this transition could be controlled (see refs. 20 and 21 for concrete case studies). An even longer range prospect is to dispose altogether of the conical intersection and to bring the molecule down to a desired region of the ground-state potential by a suitable laser pulse as in stimulated emission pumping (22). In the shorter run, this transition can be tailored by the choice of the specific molecule.
In some systems the hot ground state, gd*, can slowly relax back to the starting ground state. In other systems this relaxation will not occur spontaneously and needs to be induced. This too allows one to tailor the system to a specific application.
Logic
The ability to make a response to a classical (Boolean) operation is introduced through the optical pumping scheme, which is of the pump-probe type. We emphasize that the scheme that we here describe is the simplest one that we can think of and that it, as has already been mentioned, can be refined in several ways.
Consider first making an AND gate. This gate has to respond in one way when both signals are on and in another way if one or both signals are off. To achieve this discriminating response we pump the molecule, with a two-color two-photon transition, with one photon of each color being absorbed. Only if laser signals of both colors are switched on will the molecule go from the ground to the optically accessible state and within about 100 fs or less one can have a response. For the systems we considered, the response is the change in transmission due to the isomerization of the molecule.
Two photon transitions in the kind of molecules that we discuss are well studied and documented (23, 24). Of course, to really perform an AND operation, the molecule should not absorb two photons of the same color. This means that the pumping laser intensity must be low enough. Furthermore, the pulse duration must be long enough that its frequency bandwidth is narrow, so that the resonance absorption by the molecule can discriminate between two photons of different colors and two photons of the same color. This discrimination could be achieved even with femtosecond laser pulses, but we prefer to try it out with picosecond pulses, where the problem is not serious.
There are many variations on the two-photon pumping scheme that will allow us, for example, to do a Not–AND single-molecule gate, often referred to as a NAND gate, etc. Nor have we used any property of the laser light except its color. Polarization comes immediately to mind, and it can be used in a variety of ways, including making the molecule a two-qubit gate. Instead we here turn to the other basic gate, OR.
An OR gate is simpler. For the molecules we consider the absorption band is fairly broad, and detailed studies have verified that over a fairly wide frequency range, the photophysical response is the same (14, 15). Therefore, an OR gate uses two laser pumps of comparable frequencies, each one of which can pump the molecule to level 2. If either laser is switched on, the transmission of the probe signal will change.
Our proposal for the two types of gates is shown schematically in Fig. 6.
Figure 6.
A schematic representation of the four level AND and OR single-molecule gates. The optically accessible excited state, ae, is shown as a level of finite width. It can be accessed by a two-photon transition (AND) of quite different colors or by either one-photon transition (OR) of similar color. The ultrafast radiationless transition ae → re is shown, as well as the conical intersection leading to the ground state isomer, gd*. Compare with Figs. 2 and 4. Fig. 5 shows other molecular types that can be used in this manner. Our proposal is based on the experimentally easier task of detecting the photoinduced change. If instead, or in addition, one can spectroscopically monitor the depletion of the ground state, the logical repertoire becomes richer. For example, a Not“AND” (≡“NAND”) gate can be so operated.
Discussion
It is our intention to implement the two-photon one-molecule logical gate first in the gas phase with an all-optical data transfer. One of the colors can be in the infrared because we can thereby shift the absorption to a region where a single visible or UV photon is not resonant. This kind of pumping, monitored by dissociation of the excited molecule, is used in IR-visible double-resonance photodissociation (25). The second stage has to do with fading. One wants the gate to be operable over many cycles. The first step is therefore the need to regenerate the ground state. In some systems, e.g., the keto-enol tautomerism shown in Fig. 4, this will be inherently satisfied. In other systems such as the electrocyclic ring openings, this will require either a more rigid molecular frame than cyclodiene itself or the use of another mode to regenerate the initial state. Either way, the readout is done, optically, before the return to the starting state.
It is worthwhile to point out that the available knowledge base in molecular spectroscopy allows the two-photon-pump one-photon-probe scheme to be generalized in a number of interesting ways. Here we mention just a few of these possibilities, concluding in Fig. 7, with an experiment that already has been realized, which allows us an entry into the quantum computing regime. In addition to the options that we already mentioned, including the possibility of amplification, a two-photon-pump two-photon-probe scheme comes obviously to mind. Indeed, by combining the resonance requirements of the molecule with the tailoring of laser pulses, there is much that can be achieved, even on an otherwise classical level. The molecule can be used as more than simply a pass filter. When one considers the density matrix and not just the populations, then a four-level system has a very rich structure. One could think that such experiments should be performed for atoms, but how this would be done is not necessarily obvious. Molecules going through conical intersections and other narrow bottlenecks show a remarkable retention of their phases (19, 26–30). Then the pump (or probe) can also be tailored up to and including interference between the pump (or probe) pulses. Such spectroscopies include the three-pulse four-wave mixing technique (31).
Figure 7.
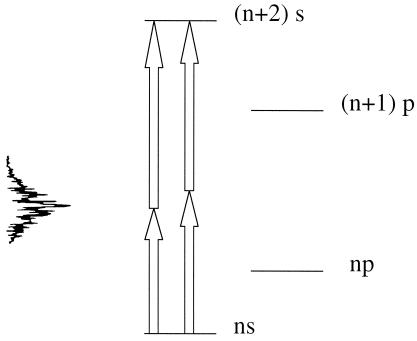
Schematic four-level diagram for an alkali atom [Cs (28), Na (13)] with a two photon resonance. Pumping is allowed for all pairs of photons whose frequencies add up. The power spectrum of the excitation pulse is shown to indicate that different pairs of photons are possible. Interference between the two pathways allows a coherent control of the population transfer.
The concrete proposal is to couple the two-photon absorption to access a state that is the gateway to an ultrafast molecular rearrangement. But there are other two-photon processes that can be promptly probed. We already mentioned stimulated emission pumping (22). Then there is STIRAP (32), which requires two laser pulses to access the level of interest. The newer version of tripod-STIRAP will also allow the creation of robust quantal superpositions of states (33). Nor need the probe operate by an absorption. Fluorescence is an acceptable probe, particularly if it is stimulated. Intramolecular electronic energy transfer (34) between a two-photon pumped donor part of the molecule and fluorescence from an acceptor part is the obvious possibility. A concrete example of such a scheme, but in the ultrafast domain, is provided by recent experiments (21, 35) demonstrating coherent control (17) of the population transfer from 3 s to 5 s in sodium pumped by a pair of photons, the energies of which add up to the resonant energy gap. The subsequent fluorescence to the 3p state and collisional relaxation to the 4p state served as the output. There are many pairs of photons whose energies will add up to the required electronic energy gap. Interference between any two such pairs allows constructive or destructive interference, leading to enhanced or suppressed population transfer (17). By using a femtosecond laser pulse, the spectral width of which is broad, many distinct photon pairs are available. Adaptive control (18) was used to optimize the pulse to achieve either target (21, 35, 36).
By operating a single molecule as a gate one avoids the need to connect two switches. However, this is only the beginning. The power of organic synthesis to design molecules for any required purpose should not be overlooked. It is also not out of the question to integrate more than one logical gate in the same molecular framework. In our approach this is a poor person's way of making a molecule the equivalent not just of two transistors but of a more extended integrated circuit.
Concluding Remarks
There is, of course, a considerable difference between a proof-of-principle experiment and a device. For the latter many additional and critical considerations come into play. The simple pumping scheme that we propose to begin with is not very efficient. We process information at a high cost of free energy. One can see ways around this cost, such as the use of Raman pumping schemes. But at the moment we are content with just the principle.
Acknowledgments
We thank Profs. E. E. B. Campbell, J. L. Kinsey, and F. Remacle for their comments on the manuscript. This work was supported by the James Franck Program for Laser–Matter Interaction.
References
- 1.Collier C P, Wong E W, Belohradsk M, Raymo F M, Stoddart J F, Kuekes P J, Williams R S, Heath J R. Science. 1999;285:391–394. doi: 10.1126/science.285.5426.391. [DOI] [PubMed] [Google Scholar]
- 2.Collier C P, Mattersteig G, Wong E W, Luo Y, Beverly K, Sampaio J, Raymo F M, Stoddart J F, Heath J R. Science. 2000;289:1172–1175. doi: 10.1126/science.289.5482.1172. [DOI] [PubMed] [Google Scholar]
- 3.Metzger R M. J Mater Chem. 2000;10:55–62. [Google Scholar]
- 4.Metzger R M. Acc Chem Res. 1999;32:950–957. [Google Scholar]
- 5.Tour J M. Acc Chem Res. 2000;33:791–804. doi: 10.1021/ar0000612. [DOI] [PubMed] [Google Scholar]
- 6.Reed M A. Proc IEEE. 1999;87:652–658. [Google Scholar]
- 7.Reed M A, Tour J M. Sci Am. 2000;282:86–89. doi: 10.1038/scientificamerican0600-86. [DOI] [PubMed] [Google Scholar]
- 8.Gunnlaugsson T, Donail D A M, Parker D. Chem. Comm. 1999. , 107–114. [Google Scholar]
- 9.Silva A P d, Dixon I M, Gunaratne H Q N, Gunnlaugsson T, Maxwell P R S, Rice T E. J Am Chem Soc. 1999;121:1393–1394. [Google Scholar]
- 10.Silva A P d, McClenaghan N D. J Am Chem Soc. 2000;122:3965–3966. [Google Scholar]
- 11.Balzani V, Credi A, Mattersteig G, Matthews O A, Raymo F M, Stoddart J F, Venturi M, White A J P, Williams D J. J Org Chem. 2000;65:1924–1936. doi: 10.1021/jo991781t. [DOI] [PubMed] [Google Scholar]
- 12.Credi A, Balzani V, Langford S J, Stoddart J F. J Am Chem Soc. 1997;119:2679–2681. [Google Scholar]
- 13.Shannon C E. Trans Am Inst Elec Eng. 1938;57:713–723. [Google Scholar]
- 14.Fuss W, Schmid W E, Trushin S A. J Chem Phys. 2000;112:8347–8362. [Google Scholar]
- 15.Lochbrunner S, Fuss W, Schmid W E, Kompa K L. J Phys Chem. 1998;102:9334–9342. [Google Scholar]
- 16.Klessinger M, Michl J. Excited States and Photochemistry of Organic Molecules. New York: VCH; 1995. [Google Scholar]
- 17.Shapiro M, Brumer P. Adv At Mol Opt Phys. 2000;42:287–320. [Google Scholar]
- 18.Rabitz H, Vivie-Riedle R D, Motzkus M, Kompa K L. Science. 2000;288:824–828. doi: 10.1126/science.288.5467.824. [DOI] [PubMed] [Google Scholar]
- 19.Chudoba C, Riedle E, Pfeiffer M, Elsaesser T. Chem Phys Lett. 1996;263:622–628. [Google Scholar]
- 20.Hornung T, Meier R, Motzkus M. Chem Phys Lett. 2000;326:445–451. [Google Scholar]
- 21.Hornung T, Meier R, Zeidler D, Kompa K L, Proch D, Motzkus M. Appl Phys B. 2000;71:277–284. [Google Scholar]
- 22.Hamilton C E, Kinsey J L, Field R W. Annu Rev Phys Chem. 1986;37:493–524. [Google Scholar]
- 23.Ashfold M N R, Howe J D. Annu Rev Phys Chem. 1994;45:57–82. [Google Scholar]
- 24.Friedrich D M, McClain W M. Annu Rev Phys Chem. 1980;31:559–577. [Google Scholar]
- 25.Reid S A, Reisler H. Annu Rev Phys Chem. 1996;47:495–525. [Google Scholar]
- 26.Lenderink E, Duppen K, Wiersma D A. J Phys Chem. 1995;99:8972–8980. [Google Scholar]
- 27.Szarka A Z, Pugliano N, Palit D K, Hochstrasser R M. Chem Phys Lett. 1995;240:25–30. [Google Scholar]
- 28.Ben-Nun M, Levine R D. Chem Phys Lett. 1993;203:450–455. [Google Scholar]
- 29.Khundkar L R, Zewail A H. Annu Rev Phys Chem. 1990;41:15–60. [Google Scholar]
- 30.Pollard W T, Mathies R A. Annu Rev Phys Chem. 1992;43:497–523. doi: 10.1146/annurev.pc.43.100192.002433. [DOI] [PubMed] [Google Scholar]
- 31.Pastirk I, Lozovoy V V, Grimberg B I, Brown E J, Dantus M. J Chem Phys A. 1999;103:10226–10236. [Google Scholar]
- 32.Bergmann K, Theuer H, Shore B W. Rev Mod Phys. 1998;70:1003–1025. [Google Scholar]
- 33.Theuer H, Unanyan R G, Habscheid C, Klein K, Bergmann K. Opt Express. 1999;4:77–79. doi: 10.1364/oe.4.000077. [DOI] [PubMed] [Google Scholar]
- 34.Speiser S. Chem Rev. 1996;96:1953–1976. doi: 10.1021/cr941193+. [DOI] [PubMed] [Google Scholar]
- 35.Zeidler, D., Frey, S., Kompa, K. L. & Motzkus, M. (2001) Chem. Phys., in press.
- 36.Meshulach D, Silberberg Y. Nature (London) 1998;396:239–242. [Google Scholar]