Size does matter. While endlessly debated in social circles, this is unquestionably true for the normal function of the epithelial and endothelial tubes that comprise such organs as the vascular system, lung and kidney. The enlarged tubules in a polycystic kidney, for instance, literally crush the surrounding normal tubules, while narrowed blood vessels can cause ischemic tissue injuries. Surprisingly, the molecular and cellular mechanisms of tube-size regulation are poorly understood, and therapeutics to intervene in conditions such as polycystic kidney disease are non-existent. However, a flurry of papers, including those by Luschnig et al. [1] and Wang et al. [2] in this issue of Current Biology, have now defined a new mechanistic framework for understanding epithelial tube-size regulation in one of the best studied models for tubulogenesis, the Drosophila tracheal system [3]. Importantly, the combined results have implications for understanding not only tube-size control, but also the mechanisms of specialized apical secretion, the role of extracellular matrix (ECM) in controlling cell shape, and possibly conserved signaling or morphogenic roles for what has been considered an invertebrate specific oligosaccharide.
The stage was set for the Wang and Luschnig papers [1,2] by recent papers from the Samakovlis, Uv, Krasnow, Casanova and Nüsslein-Volhard groups that showed that the genetically programmed tripling of the diameter of the tracheal tubes requires the formation of a transient lumenal chitin-based matrix [4-7]. Although this matrix could provide an internal scaffold that defines the shape of the tube surrounding it, the lumenal filling does not simply force the radial expansion or lengthening of the tracheal tubes because without chitin synthesis regions of the tubes actually become overly expanded in both diameter and length. Furthermore, Tonning et al. [6] show that the organization of subapical βH-spectrin is disrupted in the absence of chitin synthesis, suggesting that — rather than serving as a rigid template upon which cells are wrapped — the fibrillar chitin matrix signals the tracheal cells to reorganize their cytoskeletons in order to adjust cell shape. Regardless of the details of the mechanism, these data show that the tracheal lumenal matrix has the ‘inside job’ of orchestrating the cell shape changes that create tubes of particular sizes.
The work of Luschnig et al. [1] and Wang et al. [2] extends the previous observations in three key ways: First, tracheal tube length and diameter are known to be regulated separately [8], and both groups show that a late modification of the lumenal chitin matrix is specifically required for length control. Loss of either of the lumenal chitin-binding proteins Vermiform (Verm) or Serpentine (Serp) causes the chitinous matrix to be somewhat disordered and tracheal tubes to become too long. Importantly, diameter control is largely unaffected by mutations in these genes. Second, their observations suggest that the formation of the lumenal matrix is highly regulated, because Verm and Serp both contain predicted chitin deacetylase domains and that abnormal amounts or activity of these proteins causes matrix assembly defects and elongation of the tracheal tubes. The third and most exciting discovery was made by Wang et al. [2] who show that Verm is delivered to the apical lumen via a specialized secretory pathway that requires septate junctions.
Septate junctions are invertebrate junctions that provide a claudin-based transepithelial barrier function, but — in contrast to vertebrate tight junctions — are located basal to the adherens junctions and contain the conserved basal cell polarity components Lethal Giant Larvae (Lgl), Scribble and Discs Large [9]. In flies lacking septate junctions, Verm is not secreted into the lumenal (apical) space and is instead retained cytoplasmically, while other apical and lumenal markers are properly localized. This explains previous observations that septate junction mutations cause elongated tracheal phenotypes, similar to those caused by serp and verm mutants [10] and also disrupt the tracheal chitin matrix [6]. This role for septate junctions in polarized secretion is consistent with Lgl having similarity to the S. cerevisiae secretory proteins sro7 and sro77, which interact with the polarized secretory exocyst complex [11]. Moreover, a mammalian LGL homologue interacts with the machinery for polarized secretion in MDCK cells [12].
However, despite precedence for the exocyst acting in apical secretion in Drosophila [13], it is disconcerting that septate junctions are required for apical secretion, as septate junctions are located in the opposing, basolateral membrane and septate junction components help specify the extent of the basolateral domain during epithelial polarization [9]. Furthermore, in vertebrate epithelial cells there is strong evidence that the exocyst and LGL are involved in basolateral secretion [11,14]. A potential resolution for this discrepancy may lie in the observation that the vertebrate apical secretion component rab11 [15] can interact with the exocyst component sec15 [16], which suggests that Lgl and the exocyst may have more complex roles than previously appreciated. Further investigations will be required, but the ability to follow the subcellular localization of Verm in the tracheal system will be likely to prove an invaluable assay in these efforts. But for now, the discovery that septate junctions are required for polarized secretion of Verm is a key advance because it allows placement of septate junctions in a mechanistic pathway for tracheal tube-size control (Figure 1).
Figure 1.
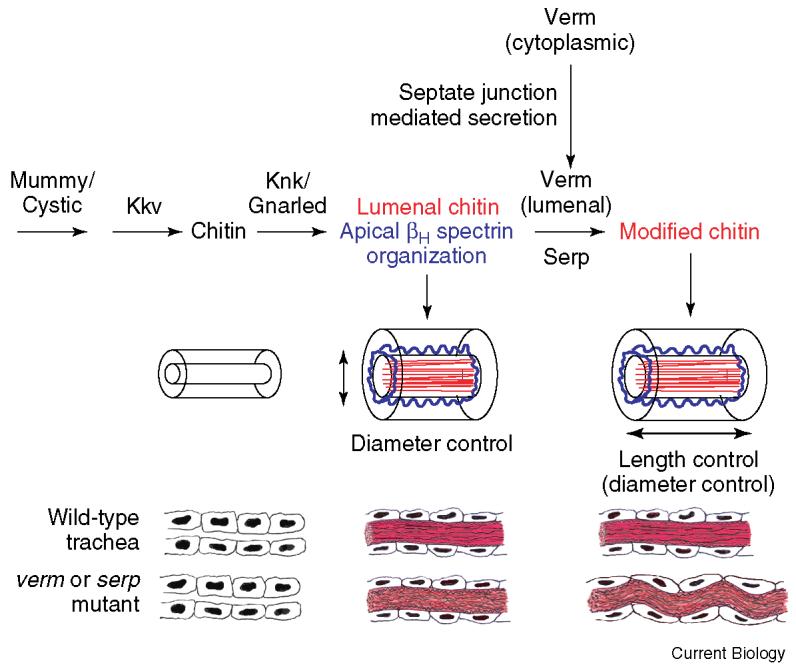
A model for tracheal tube-size control in Drosophila.
Expansion of the tracheal tubes from narrow to larger diameters during late embryogenesis is regulated by a transient fibrillar chitin-based matrix (red) that is also required for normal organization of the apical βH spectrin cytoskeleton (blue). Septate junctions appear to mediate the apical secretion of Verm, which along with Serp is required for subsequent modification of this matrix to prevent the tracheal cells and tubes from becoming too long and, to a lesser extent, too large in diameter. Adapted from [1,2].
Previous studies of the Drosophila tracheal system have provided important general insights into epithelial tube morphogenesis, including the roles of FGF and the discovery of the Sprouty family of FGF regulators [3]. Will lumenal-matrixbased mechanisms of tube-size control also be conserved in other systems? Several observations suggest that the answer may well be yes. First, consistent with the essentially universal presence of chitin in invertebrates ranging from fungi to lobsters, vertebrates also have enzymes that make short chitin oligosaccharides [17] and express chitinase-like proteins in tubular epithelia and tissue undergoing remodeling [18]. Thus, chitin-containing ECM may play important roles in vertebrate tube morphogenesis. Second, if chitin itself does not have a role in vertebrate tube-size control, the pathways responding to a lumenal ECM could well be conserved between vertebrates and invertebrates. Consistent with this possibility, a fibrillar material forms within the lumen of developing capillaries in vitro [19], and mature blood vessels and lung epithelia are lined with an oligosaccharide-based ‘glycocalyx’ [20]. However, beyond acting as surfactants in pulmonary development, the role of apical ECM in vertebrate tubule morphogenesis remains to be determined.
Whether or not the exact mechanisms of tracheal tube-size control turn out to be conserved, the results of Luschnig et al. [1] and Wang et al. [2] are exciting advances because they give Drosophila researchers a detailed framework and important tools for further investigations of tracheal morphogenesis, and provide vertebrate researchers with models and testable hypotheses for investigating tubulogenesis in a diverse array of organs.
References
- 1.Luschnig S, Baetz T, Armbruster K, Krasnow MA. serpentine and vermiform encode matrix proteins with chitin binding and deacetylation domains that limit tracheal tube length in Drosophila. Curr. Biol. 2006;16 doi: 10.1016/j.cub.2005.11.072. this issue. [DOI] [PubMed] [Google Scholar]
- 2.Wang S, Jayaram SA, Hemphala J, Senti KA, Tsarouhas V, Jin H, Samakovlis C. Septate junction dependent luminal deposition of chitin deacetylases restricts tube elongation in the Drosophila trachea. Curr. Biol. 2006;16 doi: 10.1016/j.cub.2005.11.074. this issue. [DOI] [PubMed] [Google Scholar]
- 3.Uv A, Cantera R, Samakovlis C. Drosophila tracheal morphogenesis: intricate cellular solutions to basic plumbing problems. Trends Cell Biol. 2003;13:301–309. doi: 10.1016/s0962-8924(03)00083-7. [DOI] [PubMed] [Google Scholar]
- 4.Araujo SJ, Aslam H, Tear G, Casanova J. mummy/cystic encodes an enzyme required for chitin and glycan synthesis, involved in trachea, embryonic cuticle and CNS development-Analysis of its role in Drosophila tracheal morphogenesis. Dev. Biol. 2005;288:179–193. doi: 10.1016/j.ydbio.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 5.Moussian B, Schwarz H, Bartoszewski S, Nusslein-Volhard C. Involvement of chitin in exoskeleton morphogenesis in Drosophila melanogaster. J. Morphol. 2005;264:117–130. doi: 10.1002/jmor.10324. [DOI] [PubMed] [Google Scholar]
- 6.Tonning A, Hemphala J, Tang E, Nannmark U, Samakovlis C, Uv A. A transient luminal chitinous matrix is required to model epithelial tube diameter in the Drosophila trachea. Dev. Cell. 2005;9:423–430. doi: 10.1016/j.devcel.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Devine WP, Lubarsky B, Shaw K, Luschnig S, Messina L, Krasnow MA. Requirement for chitin biosynthesis in epithelial tube morphogenesis. Proc. Natl. Acad. Sci. USA. 2005;102:17014–17019. doi: 10.1073/pnas.0506676102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beitel GJ, Krasnow MA. Genetic control of epithelial tube size in the Drosophila tracheal system. Development. 2000;127:3271–3282. doi: 10.1242/dev.127.15.3271. [DOI] [PubMed] [Google Scholar]
- 9.Knust E, Bossinger O. Composition and formation of intercellular junctions in epithelial cells. Science. 2002;298:1955–1959. doi: 10.1126/science.1072161. [DOI] [PubMed] [Google Scholar]
- 10.Wu VM, Beitel GJ. A junctional problem of apical proportions: epithelial tube-size control by septate junctions in the Drosophila tracheal system. Curr. Opin. Cell Biol. 2004;16:493–499. doi: 10.1016/j.ceb.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X, Wang P, Gangar A, Zhang J, Brennwald P, TerBush D, Guo W. Lethal giant larvae proteins interact with the exocyst complex and are involved in polarized exocytosis. J. Cell Biol. 2005;170:273–283. doi: 10.1083/jcb.200502055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Musch A, Cohen D, Yeaman C, Nelson WJ, Rodriguez-Boulan E, Brennwald PJ. Mammalian homolog of Drosophila tumor suppressor lethal (2) giant larvae interacts with basolateral exocytic machinery in Madin-Darby canine kidney cells. Mol. Biol. Cell. 2002;13:158–168. doi: 10.1091/mbc.01-10-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beronja S, Laprise P, Papoulas O, Pellikka M, Sisson J, Tepass U. Essential function of Drosophila Sec6 in apical exocytosis of epithelial photoreceptor cells. J. Cell Biol. 2005;169:635–646. doi: 10.1083/jcb.200410081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Folsch H. The building blocks for basolateral vesicles in polarized epithelial cells. Trends Cell Biol. 2005;15:222–228. doi: 10.1016/j.tcb.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Prekeris R, Klumperman J, Scheller RH. A Rab11/Rip11 protein complex regulates apical membrane trafficking via recycling endosomes. Mol. Cell. 2000;6:1437–1448. doi: 10.1016/s1097-2765(00)00140-4. [DOI] [PubMed] [Google Scholar]
- 16.Zhang XM, Ellis S, Sriratana A, Mitchell CA, Rowe T. Sec15 is an effector for the Rab11 GTPase in mammalian cells. J. Biol. Chem. 2004;279:43027–43034. doi: 10.1074/jbc.M402264200. [DOI] [PubMed] [Google Scholar]
- 17.Semino CE, Robbins PW. Synthesis of “Nod”-like chitin oligosaccharides by the Xenopus developmental protein DG42. Proc. Natl. Acad. Sci. USA. 1995;92:3498–3501. doi: 10.1073/pnas.92.8.3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bleau G, Massicotte F, Merlen Y, Boisvert C. Mammalian chitinase-like proteins. EXS. 1999;87:211–221. doi: 10.1007/978-3-0348-8757-1_15. [DOI] [PubMed] [Google Scholar]
- 19.Folkman J, Haudenschild C. Angiogenesis in vitro. Nature. 1980;288:551–556. doi: 10.1038/288551a0. [DOI] [PubMed] [Google Scholar]
- 20.Pries AR, Secomb TW, Gaehtgens P. The endothelial surface layer. Pflugers Arch. 2000;440:653–666. doi: 10.1007/s004240000307. [DOI] [PubMed] [Google Scholar]


