Abstract
Inactivation of the von Hippel Lindau tumor suppressor (VHL) is an early event in greater than 60% of sporadic clear cell renal cell carcinoma (RCC). Loss of VHL E3 ubiquitin ligase function results in accumulation of the alpha subunit of the hypoxia-inducible heterodimeric transcription factor (HIF-α) and transcription of an array of genes including VEGF, TGF-α and erythropoietin. Studies have shown that HIF-α can be alternatively activated by reactive oxygen species (ROS). Nox4 is an NADP(H) oxidase that generates signalling levels of superoxide and is found in greatest abundance in the distal renal tubules. To determine if Nox4 contributes to HIF activity in RCC, we examined the impact of Nox4 expression on HIF-α expression and transactivation. We report here that siRNA knockdown of Nox4 in 786-0 human renal tumor cells expressing empty vector (PRC) or wild type VHL (WT) results in 50% decrease in intracellular ROS as measured by a fluorescent 2′,7′-dichlorofluorescin diacetate assay, and greater than 85% reduction in HIF2-α mRNA and protein levels by quantitative RT-PCR and Western blot analysis. Further, expression of the HIF target genes, VEGF, TGF-α and Glut-1 was abrogated by 93%, 74% and 99%, respectively after stable transfection with Nox4 siRNA relative to non-targeting siRNA, as determined by quantitative RT-PCR. Thus, renal Nox4 expression is essential for full HIF2-α expression and activity in 786-0 renal tumor cells, even in the absence of functional VHL. We propose the use of Nox4 as a target in the treatment of clear cell RCC.
Keywords: Renal cell carcinoma, HIF-alpha, Nox4, VHL, reactive oxygen species
Introduction
Advanced renal cell carcinoma (RCC) is responsible for over 12,000 deaths per year in the United States. Although tumors localized to the kidney can potentially be cured by surgical resection, one third of patients present with metastases at the time of diagnosis and half of the remaining patients will ultimately relapse. Unfortunately, these tumors are both radio- and chemo-resistant, and standard immunotherapies demonstrate complete response in fewer than 15% of patients (1). Thus, new molecularly targeted therapies are desperately needed for RCC. Greater than 60% of clear cell RCCs harbor biallelic loss of the von Hippel Lindau (VHL) tumor suppressor gene (2). VHL is the substrate-recognition subunit of an SCF-like E3 ubiquitin ligase complex that targets two alpha subunits of the heterodimer transcription factor, hypoxia inducible factor (HIF) for degradation (3). HIF regulates hypoxic expression of a number of genes involved in erythropoeisis, angiogenesis and anaerobic metabolism, including erythropoeitin, vascular endothelial growth factor (VEGF), glucose transporter 1 (Glut-1) and transforming growth factor-alpha (TGF-α). Under conditions of normal oxygen tension, HIF-α subunits are rapidly degraded by VHL, and loss of VHL results in HIF-α stabilization and increased expression of HIF target genes. Inhibition of HIF2-α was sufficient to suppress VHL-deficient renal tumor growth (4). Although VHL is widely expressed (5) and hypoxic HIF activation is a ubiquitous process, familial VHL patients, who carry a germline “first hit” VHL mutation, demonstrate marked tissue specificity for malignant transformation. There is a rapidly growing body of evidence to support a role for redox signaling by reactive oxygen species (ROS) in HIF transcription regulation (6). A novel NAD(P)H oxidase highly expressed in renal tubules, Nox4, has been implicated in oxygen sensing for regulation of erythropoietin, a HIF-regulated hormone produced solely by the kidney in adult humans (7, 8). To determine if Nox4 contributes to the tumorigenic phenotype of VHL loss in the kidney, we examined HIF-α expression and transcriptional activity in human renal cell lines after Nox4 knockdown by small inhibitory RNA (siRNA). Here we present the first report that Nox4 is essential for the full expression and transcriptional activity of HIF2-α.
Materials and Methods
Cell Culture. HeLa, HEK293 and 786-0, were maintained in DMEM supplemented with 10% fetal bovine serum, glutamine, penicillin and streptomycin. 786-0 is a sporadic human renal cell carcinoma (RCC) cell line with loss of one VHL allele and truncation of the second at amino acid 104. The sub-lines 786-0 (WT) and 786-0 (PRC) were created by stable transfection of wild-type VHL or empty vector, respectively, and were a gift from W. Kaelin. Transient transfections of HeLa and HEK293 were performed using a 3:1 ratio of Fugene6 (Roche Molecular Biochemicals, Branchburg, NJ) as previously described (9). For luciferase assays, cells were co-transfected with 1 μg VEGF reporter plasmid DNA (described previously, (9)) and 0.5 μg Nox4 small inhibitory RNA (siRNA), scramble siRNA or buffer and analyzed at 48 hours. Nucleofection (Amaxa, Cologne, Germany) was used for transfection of siRNA and vector into 786-0. Briefly, 2 million cells were suspended in 100 μl reagent VCA-1103 (Amaxa, Cologne, Germany) with 0.5μg of vector or siRNA duplex and subjected to nucleofection program G-16. Untransfected controls were treated identically using an equal volume of buffer in place of vector or siRNA. Cells were plated onto six-well plates and harvested at 24 hours. For stable expression, selection with 1μg/ml puromycin (Sigma-Aldrich, St. Louis, MO) was started at 48 hours and clones were screened after 3 weeks.
Inhibition of Nox4 by RNA interference. For the anti-Nox4 RNA interference experiments, we designed an siRNA specific to human Nox4, 5′-GAGAACAGACCUGACUAUG – 3′ from nucleotide 1695 – 1713 and its complementary sequence. For the non-specific siRNA (scramble), we employed the commercially available non-targeting siRNA: 5′ - UAGCGACUAAACACAUCAAUU - 3′ and its complement (Dharmacon, Lafayette, CO). For stable Nox4 knockdown, the siRNA was cloned into a pSilencer™ 4.1-CMV puro (Ambion, Austin, TX) as a 54 nucleotide hairpin loop, between restriction sites Bam HI and Hind III using the oligos: sense, 5′-GATCCGAGAACAGACCTGACTATGTTCAAGAGACATAGTCAGGTCTGTTCTC TTA 3′, and antisense 5′-AGCTTAAGAGAACAGACCTGACTATGTCTCTTGAACATAGTCAGGTCTGTTCT CG-3′. Insert sequence was confirmed by DNA sequencing analysis.
Protein extraction and Western blot analysis. An affinity purified rabbit anti-human Nox4 antibody was raised against the 139 CVELNAARYRDEDPRKL154 and 564NSYGTRFEYNKESFS578 peptides synthesized and purified by Biosource (Hopkinton, MA). Adherent cells were washed once with phosphate-buffered saline then lysed in 250 μl of 100mM NaCl, 20mM Tris-HCL (pH 7.6), Igepal CA630 (Sigma), 5uM MgCl2, 1mM sodium orthovanadate, aprotinin (1 μg/ml), “complete” protease inhibitor (Boehringer Mannheim) and 1mM AEBSF. Protein concentration was determined by the Dc Protein Assay system (Bio-Rad), using bovine serum albumin (Pierce, Rockford, IL) as a standard. 20 μg aliquots of total protein were resolved by SDS-PAGE and transferred to PVDF (Immobilon-P; Millipore, Bedford, MA) on a semi-dry transfer apparatus (BioRad, Hercules, CA). The Western blots were then incubated with the rabbit anti-Nox4 antibody or a mouse anti-HIF2-α (Abcam, Cambridge, MA). Antigen-antibody complexes were detected using the appropriate secondary antibody linked to horseradish peroxidase and visualized using the Western Lightening™ Chemiluminescent Reagent Plus (PerkinElmer, Wellesley, MA).
Gene expression assays. In HeLa and HEK293 cells, luciferase assays were performed using the Steady-Glo® luciferase assay system (Promega, Madison, WI) according to the manufacturer's instructions. Total RNA was extracted from cells using RNeasy (QIAGEN, Valencia, CA), and quantitative reverse transcription-PCR was done as described (10). Primers for Nox4, HIF2-α, VHL, TGF-α and GAPDH are as follows. Nox4 sense 5′-ACAGGGGTCTGCATGGTGGT-3′ and Nox4 antisense, 5′-GCAGCCCTCCTGAAACATGC-3′. HIF2-α sense 5′-TGGCCGCTCAGCCTATGAAT-3′ and HIF2-α antisense 5′-TGGGTCTCCAGCCACACGTA-3′. VHL sense 5′-GTCACCTTTGGCTCTTCAGAGATGC-3′ and VHL antisense 5′-CTGGCAGTGTGATATTGGCAAAAATAG-3′. TGF-α sense 5′-CAGGTCCGAAAACACTGTGA-3′ and TGF-α antisense 5′-GTGTTTCTGAGTGGCAGCAA-3′. GAPDH sense 5′-GAAGGTGAAGGTCGGAGTCA-3′ and GAPDH antisense 5′-GACAAGCTTCCCGTTCTCAG-3. Primers for VEGF and Glut-1 were described previously (9). Reactions were performed in triplicate, normalized to GAPDH value. Standard error of the mean and t-tests were calculated using SigmaStat 3.1 (Systat Software Inc, Point Richmond, CA).
ROS production assay
Intracelluar generation of reactive oxygen species was measured using 2′,7′-dichlorofluorescin diacetate (Sigma-Aldrich, St. Louis, MO) as described (11). Fluorescence at 530 nm was measured using a Wallac Victor2 1420 multilabel counter (Wallac Oy, Turku, Finland).
Results
Nox4 knockdown resulted in decreased transcription of VEGF and TGF-α.
To determine if superoxide generation by Nox4 impacted stabilization or function of HIF-α, we knocked-down Nox4 using specific siRNA. A series of candidate siRNA were transfected into human epithelial kidney cells (HEK-293), and Nox4 protein was evaluated by Western blot using a rabbit antibody raised against Nox4 (Figure 1A). siRNA2 (lane 4) consistently demonstrated a 75-85% decrease in Nox4 protein expression and was selected for use in all subsequent experiments.
Figure 1.
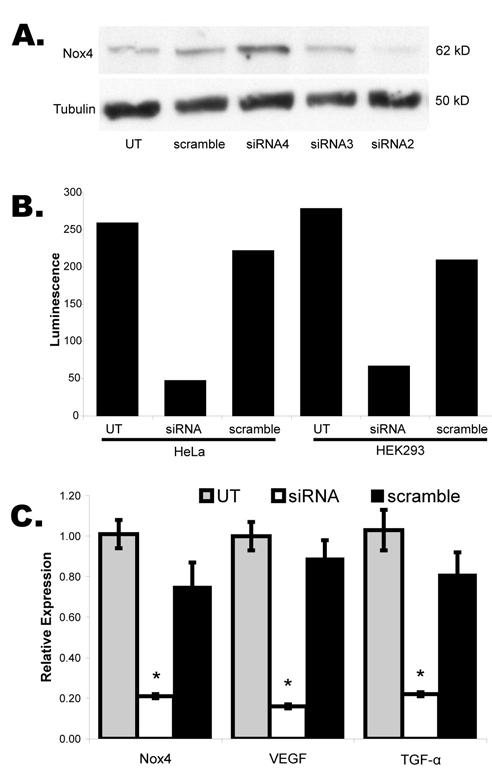
Transient siRNA knockdown of Nox4. A. Western blot of whole cell lysates from transiently transfected HEK293 cells with an array of siRNA candidates demonstrated greatest protein knockdown for siRNA2 relative to untransfected (UT) or non-targeting siRNA (scramble). B. Luciferase assays following co-transfection of siRNA2 (siRNA) and VEGF-luciferase reporter in HEK293 and Hela cells show dramatic reduction in transcription from the reporter plasmid. C. Quantitative RT-PCR for Nox4 and the HIF transcription targets, VEGF and TGF-α, in 786-0 WT cells transiently transfected with siRNA or scramble. Error bars represent mean +/- standard error. Asterisks: p<0.01.
We next examined the effect of Nox4 knockdown on expression of VEGF, a gene known to be transcriptionally induced by HIF. HEK-293 and HeLa cells were transiently co-transfected with a luciferase reporter containing the minimal promoter and hypoxia recognition element of VEGF, and either Nox4 siRNA or a non-targeting (scramble) siRNA (Figure 1B). A greater than 70% decrease in transcription from the VEGF luciferase reporter was seen for Nox4 siRNA relative to scramble in both cell lines, suggesting that Nox4 activity is necessary in these cells for full transcriptional regulation of VEGF under conditions of normal oxygen tension.
To evaluate the impact of Nox4 on endogenous transcription of HIF regulated genes, we used quantitative RT-PCR to determine relative expression levels of Nox4, VEGF and TGF-α (Figure 1C). Nox4 siRNA or scramble were transiently transfected into 786-0 human renal tumor cells with stable expression of wild type VHL, and total RNA was harvested after 24 hours. The first three lanes of Figure 1C demonstrate a 72% reduction of Nox4 mRNA after siRNA transfection (p=0.01 when compared to scramble), consistent with the observed reduction in Nox4 protein expression. Further, expression of VEGF and TGF-α, known targets of HIF transcription regulation, decreased by 82% (p=0.001 relative to scramble) and 72% (p=0.007 relative to scramble) respectively, suggesting that Nox4 expression is required for transcription of HIF regulated genes at normal oxygen tension, in the presence of intact VHL. Nox4 knockdown had no impact on expression of the housekeeping gene, GAPDH, used as an internal control.
Nox4 knockdown resulted in decreased intracellular ROS.
To evaluate the impact of Nox4 expression on the HIF pathway in VHL-deficient clear cell renal cell carcinoma, we next established stable Nox4 knockdown in the paired 786-0 renal carcinoma cell lines containing wild type VHL (WT) or empty vector (PRC). The Nox4 siRNA or scramble was cloned as a hairpin loop into the pSilencer™ 4.1-CMV puro DNA expression vector, and stable transfectants were selected using puromycin. Single cell clones were screened for reduced production of ROS using a fluorescent 2′,7′-dichlorofluorescin diacetate assay. Figure 2 shows three candidate 786-0 PRC clones with 50-70% reduction in ROS, demonstrating that loss of Nox4 activity has a significant impact on the oxidative cellular environment. ROS levels correlated with Nox4 protein expression as determined by Western (data not shown).
Figure 2.
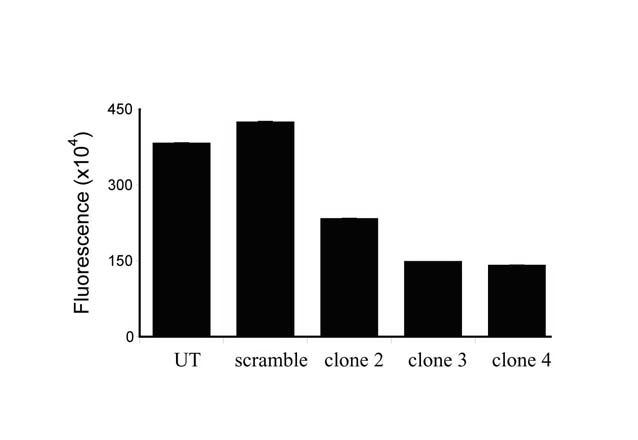
Production of intracellular reactive oxygen species by stable 786-0 PRC Nox4 knockdown clones as measured by the 2′,7′-dichlorofluorescin diacetate florescent assay. UT: untransfected. Scramble: stable expression of non-targeting siRNA vector. Error bars representing mean +/- standard error are indiscernible.
Nox4 activity is critical for HIF-regulated gene expression in the absence of VHL.
Stable 786-0 PRC and WT Nox4 knockdown clones demonstrating the lowest level of intracellular ROS production were selected for evaluation of HIF regulated gene expression by quantitative RT-PCR (Figure 3). Nox4 knockdown in 786-0 WT again resulted in dramatic reductions in mRNA expression of VEGF (94%, p<0.0001) and TGF-α (96%, p<0.0001) as well as Glut-1 (65%, p=0.0018), another HIF-regulated gene (Figure 3B). Of even greater importance, however, was the finding that increased HIF-regulated gene expression associated with loss of VHL was also dependent upon Nox4 activity. In the VHL-deficient 786-0 PRC clones (Figure 3A), VEGF, TGF-α and Glut-1 demonstrated reduced expression relative to scramble of 93% (p=0.0001), 74% (p<0.0001) and 99% (p<0.0001), respectively. No significant change was seen in the expression of Lamin, a non HIF-regulated gene, following Nox4 knockdown in either cell line. These findings indicate that Nox4 activity is necessary for activation of the HIF pathway even when HIF-α has accumulated due to loss of the VHL tumor suppressor and suggest a potential role for Nox4 as a therapeutic target in clear cell renal carcinoma.
Figure 3.
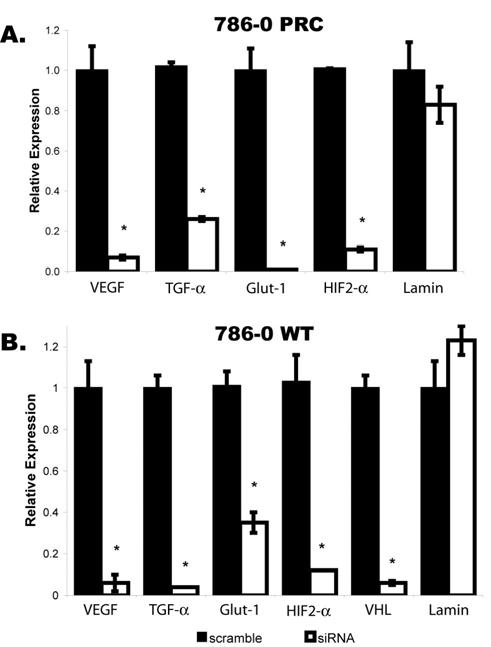
Quantitative RT-PCR in Nox4 siRNA stable 786-0 clones. A. 786-0 PRC, lacking functional VHL product, and B. 786-0 WT VHL-expressing cells demonstrate decreased relative expression of HIF2-α and HIF transcription targets VEGF, Glut-1 and TGF-α and VHL with no significant change in Lamin expression. Error bars represent mean +/- standard error. Asterisks: p < 0.005.
Nox4 knockdown results in decreased transcription of HIF2-α.
To determine at what level Nox4 impacts HIF activity, we examined HIF2-α mRNA levels by quantitative RT-PCR (Figure 3) and were surprised to find that HIF2-α itself was transcriptionally regulated by Nox4 activity. HIF2-α expression was decreased by 89% (p<0.0001) and 88% (p=0.0001) in 786-0 PRC and WT, respectively. Consistent with prior reports, we found no detectable expression of HIF1-α in the 786-0 cells. Western blot confirmed significantly decreased protein expression of HIF2-α in the Nox4 stable knockdown clones relative to stable scramble clones (Figure 4).
Figure 4.
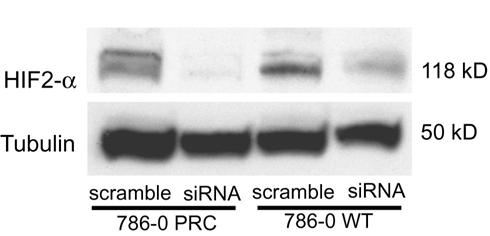
Western blot demonstrating decreased protein levels of HIF2-α in the human renal cancer cell line 786-0 containing empty vector (PRC) or wild type VHL (WT) after stable expression of Nox4 siRNA vs. scramble.
Nox4 knockdown results in decreased transcription of VHL.
Because VHL has been implicated in negative transcriptional regulation of HIF2-α (12), we also examined VHL mRNA levels by quantitative PCR in the 786-0 WT stable Nox4 knockdown clone relative to scramble (Figure 3B). VHL demonstrated 94% (p=0.0005) reduction in expression, suggesting the existence of an oxidative regulatory circuit whereby VHL and HIF2-α are both positively regulated by ROS, but HIF2-α activity is attenuated by VHL at both the transcriptional and post-translational levels.
Discussion
This represents the first report that Nox4 expression directly influences HIF2-α expression and activity. We show a dramatic abrogation of HIF activity even in the absence of functional VHL, suggesting a role for Nox4 as a target for treatment of clear cell renal carcinoma.
Despite early contradictory results, there is a rapidly growing and compelling body of evidence supporting a role for oxidative signaling in both hypoxic and normoxic regulation of the HIF pathway, which is well summarized in a recent review (6). Much of this work has been performed in vascular smooth muscle cells, where ROS have been implicated in HIF-mediated angiogenesis and vascular remodeling due to injury.
Nox4, first described as a kidney-specific NADP(H) oxidase, is most abundant in the kidney (7, 8) where it constitutively produces intracellular superoxide in the distal renal tubules adjacent to the region of erythropoietin production. It is now known to be widely expressed at lower levels in vascular endothelium, heart, pancreas, ovary, testis, osteoclasts, placenta and astrocytes (13) in a pattern reminiscent of the organ distribution of the clinical manifestations of VHL familial syndrome (14). Nox4 activity elevates intracellular ROS via rapid conversion of superoxide to other ROS including hydrogen peroxide, hydroxyl radicals, peroxynitrite, hypochlorous acid and singlet oxygen. Superoxide dismutase (SOD), glutathione peroxidases, catalase, peroxiredoxins and thioredoxin function as endogenous antioxidants to maintain physiologic equilibrium (6). Interestingly, although the kidney of an affected familial VHL patient demonstrates hundreds of unicellular sites of HIF-α stabilization consistent with loss of the second VHL allele, the only multi-cellular sites are found in the distal renal tubule where Nox4 expression is located in the human kidney (15).
In contrast to the HIF1-α knockout, which results in embryonic lethality, HIF2-α double knockout mice are viable but demonstrate multiple-organ pathology, biochemical abnormalities and altered gene expression patterns (16). Specifically, they show enhanced generation of ROS and reduced expression of genes encoding the primary antioxidant enzymes due to loss of HIF2-α regulation of antioxidant promoters. Indeed, many aspects of the HIF2-α null phenotype are reversed by administration of an SOD mimetic, leading to the proposal of a rheostat role for HIF2-α in the maintenance of intracellular ROS (16).
A number of studies support a role for Nox4 superoxide production in regulation of HIF. Extracellular SOD, the primary superoxide scavenger, has greatest expression in the kidney where it co-localizes with erythropoietin. Homozygous knock-outs demonstrate that SOD functions as a major repressor of erythropoietin expression (17) consistent with an essential role for superoxide in HIF transcriptional activity. Homozygous knockout of JunD also results in increased intracellular ROS, decreased PDH2 activity, accumulation of HIF-α and increased VEGF mRNA. Gene expression profiling revealed a three-fold up-regulation of Nox4 expression in JunD-/- cells vs wild type (18) suggesting that JunD might affect HIF-α via increased expression of Nox4. Further, siRNA knock-down of Rac1, as essential subunit of the functional NADP(H) complex, results in decreased expression of HIF1-α and VEGF(19). Finally, Goyal et al demonstrated that exogenous expression of the related Nox1 NADP(H) oxidase in human lung adenocarcinoma cells resulted in accumulation and transactivation of HIF1-α that could be reversed by the flavoprotein inhibitor diphenylene iodonium (DPI) or catalase (11), again supporting a role for superoxide production in HIF regulation.
We conclude that Nox4 is directly responsible for HIF2-α expression and activity and is critical to HIF2-α activity even in VHL-deficient RCC. As the predominant renal NADP(H) oxidase, Nox4 has a number of qualities that make it a promising target for manipulation of HIF activity in RCC, including relative tissue specificity and cell membrane localization. Investigational drugs aimed at individual downstream components of the VHL-HIF pathway have demonstrated clinical activity in advanced RCC (20). We propose that specific inhibition of Nox4 may prove more effective by down-regulating the entire array of HIF-dependent genes that are over-expressed in RCC.
Footnotes
Supported in part by NIH award #DK064887 to JKM
References
- 1.Bukowski RM. Cytokine therapy for metastatic renal cell carcinoma. Semin Urol Oncol. 2001;19(2):148–54. May. [PubMed] [Google Scholar]
- 2.Gnarra JR, Tory K, Weng Y, et al. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Genet. 1994;7(1):85–90. doi: 10.1038/ng0594-85. [DOI] [PubMed] [Google Scholar]
- 3.Maxwell PH, Wiesener MS, Chang GW, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis [see comments] Nature. 1999;399(6733):271–5. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 4.Kondo K, Kim WY, Lechpammer M, Kaelin WG., Jr. Inhibition of HIF2alpha is sufficient to suppress pVHL-defective tumor growth. PLoS Biol. 2003;1(3):E83. doi: 10.1371/journal.pbio.0000083. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Los M, Jansen GH, Kaelin WG, Lips CJ, Blijham GH, Voest EE. Expression pattern of the von Hippel-Lindau protein in human tissues. Lab Invest. 1996;75(2):231–8. [PubMed] [Google Scholar]
- 6.Kietzmann T, Gorlach A. Reactive oxygen species in the control of hypoxia-inducible factor-mediated gene expression. Semin Cell Dev Biol. 2005 doi: 10.1016/j.semcdb.2005.03.010. May 16. [DOI] [PubMed] [Google Scholar]
- 7.Shiose A, Kuroda J, Tsuruya K, et al. A novel superoxide-producing NAD(P)H oxidase in kidney. J Biol Chem. 2001;276(2):1417–23. doi: 10.1074/jbc.M007597200. [DOI] [PubMed] [Google Scholar]
- 8.Geiszt M, Kopp JB, Varnai P, Leto TL. Identification of renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci U S A. 2000;97(14):8010–4. doi: 10.1073/pnas.130135897. Jul 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maranchie JK, Vasselli JR, Riss J, Bonifacino JS, Linehan WM, Klausner RD. The contribution of VHL substrate binding and HIF1-alpha to the phenotype of VHL loss in renal cell carcinoma. Cancer Cell. 2002;1(3):247–55. doi: 10.1016/s1535-6108(02)00044-2. Apr. [DOI] [PubMed] [Google Scholar]
- 10.Tam NN, Gao Y, Leung YK, Ho SM. Androgenic regulation of oxidative stress in the rat prostate: involvement of NAD(P)H oxidases and antioxidant defense machinery during prostatic involution and regrowth. Am J Pathol. 2003;163(6):2513–22. doi: 10.1016/S0002-9440(10)63606-1. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goyal P, Weissmann N, Grimminger F, et al. Upregulation of NAD(P)H oxidase 1 in hypoxia activates hypoxia-inducible factor 1 via increase in reactive oxygen species. Free Radic Biol Med. 2004;36(10):1279–88. doi: 10.1016/j.freeradbiomed.2004.02.071. May 15. [DOI] [PubMed] [Google Scholar]
- 12.Krieg M, Haas R, Brauch H, Acker T, Flamme I, Plate KH. Up-regulation of hypoxia-inducible factors HIF-1alpha and HIF-2alpha under normoxic conditions in renal carcinoma cells by von Hippel-Lindau tumor suppressor gene loss of function. Oncogene. 2000;19(48):5435–43. doi: 10.1038/sj.onc.1203938. [DOI] [PubMed] [Google Scholar]
- 13.Krause KH. Tissue distribution and putative physiological function of NOX family NADPH oxidases. Jpn J Infect Dis. 2004;57(5):S28–9. Oct. [PubMed] [Google Scholar]
- 14.Maranchie JK, Linehan WM. Genetic disorders and renal cell carcinoma. Urol Clin North Am. 2003;30(1):133–41. doi: 10.1016/s0094-0143(02)00120-9. Feb. [DOI] [PubMed] [Google Scholar]
- 15.Mandriota SJ, Turner KJ, Davies DR, et al. HIF activation identifies early lesions in VHL kidneys. Evidence for site-specific tumor suppressor function in the nephron. Cancer Cell. 2002;1(5):459–68. doi: 10.1016/s1535-6108(02)00071-5. Jun. [DOI] [PubMed] [Google Scholar]
- 16.Scortegagna M, Ding K, Oktay Y, et al. Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1-/- mice. Nat Genet. 2003;35(4):331–40. doi: 10.1038/ng1266. Dec. [DOI] [PubMed] [Google Scholar]
- 17.Zelko IN, Folz RJ. Extracellular superoxide dismutase functions as a major repressor of hypoxia-induced erythropoietin gene expression. Endocrinology. 2005;146(1):332–40. doi: 10.1210/en.2004-1007. Jan. [DOI] [PubMed] [Google Scholar]
- 18.Gerald D, Berra E, Frapart YM, et al. JunD reduces tumor angiogenesis by protecting cells from oxidative stress. Cell. 2004;118(6):781–94. doi: 10.1016/j.cell.2004.08.025. Sep 17. [DOI] [PubMed] [Google Scholar]
- 19.Xue Y, Bi F, Zhang X, et al. Inhibition of endothelial cell proliferation by targeting Rac1 GTPase with small interference RNA in tumor cells. Biochem Biophys Res Commun. 2004;320(4):1309–15. doi: 10.1016/j.bbrc.2004.06.088. Aug 6. [DOI] [PubMed] [Google Scholar]
- 20.Atkins MB, Avigan DE, Bukowski RM, et al. Innovations and challenges in renal cancer: consensus statement from the first international conference. Clin Cancer Res. 2004;10(18):6277S–81S. doi: 10.1158/1078-0432.CCR-040720. Sep 15 Pt2. [DOI] [PubMed] [Google Scholar]


