Abstract
Background
We identified an interleukin-1 receptor family member, ST2, as a gene markedly induced by mechanical strain in cardiac myocytes and hypothesized that ST2 participates in the acute myocardial response to stress and injury.
Methods and Results
ST2 mRNA was induced in cardiac myocytes by mechanical strain (4.7 ± 0.9-fold) and interleukin-1β(2.0 ± 0.2-fold). Promoter analysis revealed that the proximal and not the distal promoter of ST2 is responsible for transcriptional activation in cardiac myocytes by strain and interleukin-1β. In mice subjected to coronary artery ligation, serum ST2 was transiently increased compared with unoperated controls (20.8 ± 4.4 versus 0.8 ± 0.8 ng/mL, P < 0.05). Soluble ST2 levels were increased in the serum of human patients (N = 69) 1 day after myocardial infarction and correlated positively with creatine kinase (r = 0.41, P = 0.001) and negatively with ejection fraction (P = 0.02).
Conclusions
These data identify ST2 release in response to myocardial infarction and suggest a role for this innate immune receptor in myocardial injury.
Keywords: stress, receptors, creatine kinase, interleukins, infarction
The pathways that lead from biomechanical overload to cardiac remodeling are of paramount clinical importance, yet this process remains incompletely defined. A major advantage of genomic technology is the discovery of new, previously unsuspected pathways and biomarkers. By applying genomic technology to well-characterized model systems, unique genes that would otherwise not be candidates for investigation can be identified. To identify novel pathways in cardiac myocyte mechanotransduction, we applied DNA microarray technology to cultured cardiac myocytes subjected to precisely controlled biomechanical overload. Among 7000 transcripts of known function, the gene for the interleukin-1 receptor family member ST2 was exceptionally upregulated in cardiac myocytes subjected to mechanical strain.
Soluble and membrane receptor forms of ST2 are produced by alternative promoter splicing and 3′ processing.1,2 Soluble ST2 is induced by serum stimulation of resting fibroblasts,3 whereas membrane receptor ST2 has immunomodulary functions as a cell-surface marker of T helper type 2 (Th2) lymphocytes.4,5 Membrane receptor ST2 is also expressed on hematopoietic and cancer cell lines, although its function in these contexts is incompletely understood.6 Although the extracellular immunoglobulin domain of ST2 has high homology to this region of the interleukin-1 receptor, it does not bind interleukin-1 with high affinity.7,8
We report the inducible expression and regulation of ST2 in cultured cardiac myocytes and transient expression of serum soluble ST2 after myocardial infarction (MI) in mice. Soluble ST2 is increased in the circulation of patients 1 day after MI. These findings suggest that ST2 participates in the cardiovascular response to injury and that serum ST2 may be a useful biomarker.
Methods
Nomenclature
IL1RL1 (interleukin-1 receptor-like-1) is the approved symbol for ST2 from the Human Gene Nomenclature Database.9 IL1RL1-a is the designated symbol for the soluble ST2 receptor, and IL1RL1-b is the designated symbol for the ST2L membrane-bound receptor. This gene has been named Fit-1S (soluble) and Fit-1M (membrane receptor) in rat.
Culture and Biomechanical Strain of Myocytes
Neonatal rat cardiac myocytes (NRCMs) from 1-day-old Harlan Sprague-Dawley rats were isolated and plated on silicone dishes as described.10 Twenty-four hours after plating, media was changed to DMEM containing 1% ITS supplement (Sigma) for 48 hours before mechanical deformation.10
RNA Analysis
Northern analyses were performed as described.10 Hybridization was performed with a 32P-dCTP–labeled cDNA probe corresponding to rat Fit-1 generated by polymerase chain reaction (PCR). Autoradiograms were quantified by densitometry (Scion Image for Windows). mRNA levels are expressed relative to densitometry of 18S ribosomal RNA ethidium bromide staining. Fold changes were calculated relative to control.
Nuclease protection assay was performed with a 71-mer oligonucleotide spanning the splice junction of soluble Fit-1S and membrane Fit-1M, which was 3′ -end labeled with 32Pdd[α-ATP] (Amersham) using terminal deoxynucleotidyl transferase. Nuclease-digested hybridized products were electrophoresed through a 5% polyacrylamide gel followed by autoradiography.
Relative reverse transcription–polymerase chain reaction (RT-PCR) for soluble ST2 (ST2) and membrane receptor ST2 (ST2L) was performed as described11 with modifications. First strand cDNA was synthesized from total RNA and used as template with the following primers: mouse ST2 forward, 5′-ACGCTCGACTTATCCTGTGG-3′; reverse, 5′-CAGGTCAATTGTTGGACACG-3′; mouse ST2L forward, 5′-GTGATAGTCTTAAAAGTGTTCTGG-3′; reverse, 5′-TCAAAAGTGTTTCAGGTCTAAGCA-3′); and β-actin forward, 5′-TGTTTGA GACCTTCAACACC-3′; reverse, 5′-CGCTCATTGCCGATAGTGAT-3′. The PCR reaction mix containing template and 32P-dCTP was divided, and specific primers were added. PCR products were electrophoresed on agarose gels. The single band for ST2, ST2L, and β-actin was excised, and Cherenkov counts were determined. Slopes of linear amplification plots over a range of cycles were similar in MI and sham samples. The final cycle number used for relative quantification was in the linear range of amplification by cycle number. ST2 and ST2L levels are expressed relative to β-actin levels.
Promoter-Reporter Assays
A 2.0-kb region surrounding IL1RL1 exon 1a and a 3.4-kb region surrounding IL1RL1 exon 1b (Genbank Accession No. AC007248.4) were amplified from human genomic DNA with the following primers: Exon 1a forward, 5′-ATCGACGCGTAGATAGGCCATCTCGGGCAT G-3′; reverse, 5′-ATATCTCGAGCAGGCACCCGCAACAAACTTG-3′(nucleotides 83,340 to 85,303) and exon 1b forward, 5′-ATCGACGCGTCGCCTGCAGAATTTCATCATTATG-3′; reverse, 5′-ATATCTCGAGAGCTGGTAGAAACTTCAGAAGTT-3′(nucleotides 108,365 to 111,749). Underlines represent added MluI and XhoI restriction sites to facilitate subcloning. PCR products for exon 1a and exon 1b were cloned into the pGL3 luciferase reporter vector (Promega).
Myocytes were transfected with pGL3 plasmid containing exon 1a or exon 1b and β-galactosidase plasmid using FuGENE 6 Transfection Reagent (Roche). Six hours after transfection, mechanical strain (8%), interleukin-1β(R and D Systems) (rat, 10 ng/mL), or phorbol ester (Sigma) (phorbol myristate acetate, 200 nmol/L) was applied, and cells were harvested 24 hours later. Luciferase and β-galactosidase were detected using chemiluminescent reporter assay (Applied Biosystems).
Experimental MI in Mice
Procedures were approved by the Harvard Medical School Standing Committee on Animals. MI was created by coronary artery ligation in mice of C57/BL6J strain, anesthetized with intraperitoneal ketamine (50 mg/kg) and xylazine (2.5 mg/kg) as described.12 Additional mice underwent the identical surgical procedure without ligation of the coronary artery (sham). Tissues were harvested for mRNA analysis 4 hours after ligation (N = 7 per group, MI; N = 8 per group, sham). Blood was collected for serum ST2 levels 1 day and 3 days after ligation (N = 4 per group, MI and sham). Unoperated mice (N = 4) were included for RNA analysis and serum ST2 levels.
Patient Studies and ELISA for ST2
The Healing and Early Afterload Reducing Therapy (HEART) study enrolled 352 patients with acute MI from 36 centers in the United States and Canada. Details of the trial have been described.13–15 Serum samples from days 1, 14, and 90 after MI from 69 randomly chosen patients in the HEART trial were assayed for soluble ST2 with a sandwich ELISA.16 Serum ST2 levels in mice were measured with a sandwich ELISA using a rat monoclonal anti-mouse ST2 antibody (Morwell Diagnostics) and a rabbit polyclonal anti-mouse ST2 antibody.17
Statistics
In vitro experiments were performed a minimum of 3 times. Values are mean ± SEM. Data were analyzed by one-way ANOVA with post hoc Bonferroni multiple comparison analyses and with linear regression analysis. P < 0.05 was considered significant.
Results
Induction of ST2 mRNA in Neonatal Rat Cardiac Myocytes
Northern analyses of RNA from neonatal rat cardiac myocytes (NRCMs) subjected to mechanical strain or no strain are shown in Figure 1a. The major inducible transcript was soluble ST2 at ≈2.7 kb. The 4.7 ± 0.9-fold (P < 0.0001, N = 6) maximal induction occurred at 2 hours and was sustained for 15 hours. ST2 was induced 2.0 ± 0.2-fold (P < 0.02, N = 6) by interleukin-1β and >25 fold (P < 0.0001, N = 6) by phorbol ester (Figure 1b). There was no additive or synergistic effect of strain and strain plus interleukin-1β, suggesting common pathways for induction of ST2 by these stimuli (Figure 1b).
Figure 1.

Induction of soluble ST2 mRNA in cardiac myocytes. a, Induction of soluble ST2 mRNA by mechanical strain in NRCM. Northern analyses showing early (left) and later (right) time course. Maximal induction occurs at 2 hours, is sustained for 9 hours, and declines by 15 hours. b, Induction of soluble ST2 mRNA by mechanical strain, interleukin-1β (IL-1, 10 ng/mL), combined strain and interleukin-1β, and phorbol ester (PMA, 200 nmol/L) at 1 and 3 hours. Top, ST2 mRNA. Bottom, Ethidium bromide staining of 18S ribosomal RNA. No Str indicates no strain.
Treatment with angiotensin II (100 nmol/L) did not induce, nor did angiotensin II type 1 receptor blockade (CP-191,166, 100 nmol/L) block, the biomechanical induction of ST2 (data not shown), suggesting that angiotensin II does not mediate the induction of ST2.18 Antioxidants (TIRON, 10 mmol/L and catalase, 500 U/mL) did not block biomechanical induction of ST2, and hydrogen peroxide (100 μmol/L) did not induce ST2 (data not shown), suggesting that oxidant signaling pathways do not mediate induction of ST2.
Interleukin-4 (0.2 to 10 ng/mL), which activates naive CD4+ T cells to express membrane ST2,19 did not induce ST2 mRNA in NRCM. Lipopolysaccharide (10 μmol/L), a ligand for the related toll-like receptor 4 (TLR4), failed to induce ST2, as did tumor necrosis factor-α(10 ng/mL) (data not shown). These results suggest that the induction of ST2 is not indiscriminate for multiple pathways. The rank order potency for the induction of ST2 mRNA in NRCM was phorbol ester > mechanical strain > interleukin-1β.
Both Soluble and Membrane Forms of ST2 Are Induced by Mechanical Strain in NRCM
A 32P end-labeled 71 oligonucleotide spanning the splicing junction of the soluble (Fit-1S) and membrane (Fit-1M) ST2 was used as a probe in a nuclease protection assay to determine whether both isoforms of ST2 are induced in response to strain. The 5′ sequences of Fit-1S and Fit-1M are identical; on nuclease digestion, Fit-1M yields a larger protected fragment. Both soluble Fit-1S and membrane Fit-1M were induced by mechanical strain in NRCM, although the more abundant transcript was the soluble Fit-1S isoform (Figure 2).
Figure 2.
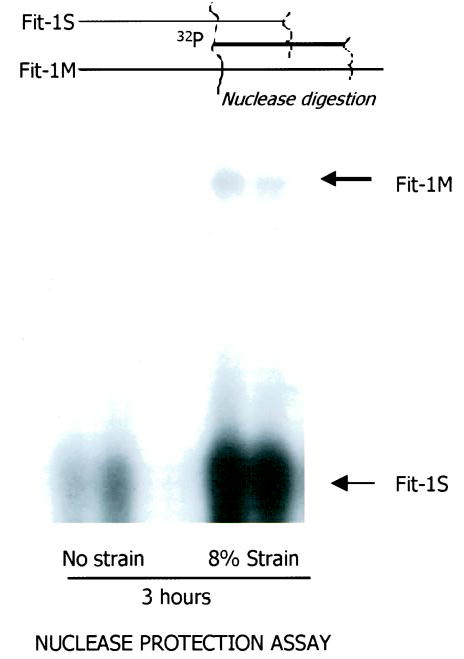
Nuclease protection assay demonstrating mRNA induction of soluble (Fit-1S) and membrane (Fit-1M) forms of ST2 in cardiac myocytes by mechanical strain. The expression of soluble ST2 mRNA is more abundant than membrane ST2 mRNA.
The Proximal Promoter Region Surrounding ST2 Exon 1b Is Responsive to Interleukin-1β, Mechanical Strain, and Phorbol Ester in NRCM
Transcription and translation of soluble and membrane ST2 are determined by alternative promoter usage and are cell-type specific.2,19,20 The ability of interleukin-1β, strain, and phorbol ester to activate exon 1b proximal promoter region and exon 1a distal promoter region was examined (Figure 3). All 3 stimuli activated the ST2 exon 1b proximal promoter region (ANOVA, P < 0.01). Mechanical strain caused a 2.6 ± 0.4-fold induction (P < 0.001, N = 8), interleukin-1β caused a 1.8 ± 0.2-fold induction (P < 0.05, N = 4), and phorbol ester caused a 7.8 ± 1.0-fold induction (P < 0.001, N = 4) over control. These results show that the proximal promoter region is active in NRCM with the same rank order potency as for induction of ST2 mRNA by Northern analysis. A less robust, but significant, activation of ST2 exon 1a distal promoter region was observed in response to phorbol ester only (1.3 ± 0.2-fold change, P < 0.05; N = 4 per group). Interleukin-1βwas weakly repressive (0.7 ± 0.1-fold change, P < 0.05), and strain showed no effect (1.1 ± 0.2-fold change, P = NS). These results suggest that the ST2 exon 1a distal promoter region is less responsive in NRCM for induction of ST2.
Figure 3.

Activation of human ST2 promoter-luciferase constructs. a, Schematic representation of proximal and distal ST2 promoter region usage that gives rise to soluble and membrane ST2. Promoter usage is cell-type specific to produce a predominant transcript (indicated by thick lines) and a minor transcript (thin lines). b, 3.4-kb DNA flanking ST2 exon 1b (proximal promoter region) fused to luciferase reporter was responsive to interleukin-1β, mechanical strain, and phorbol ester. 2.1-kb DNA flanking ST2 exon 1a (distal promoter region) fused to luciferase reporter was slightly repressed by interleukin-1β, nonresponsive to mechanical strain, and weakly responsive to phorbol ester. *P < 0.05.
ST2 Is Induced In Vivo After MI in Mice
To test the hypothesis that ST2 is induced in vivo, we assayed serum for soluble ST2 in mice after experimental MI (Figure 4). Serum ST2 levels in unoperated mice were 0.84 ± 0.78 ng/mL. One day after MI, serum ST2 levels in MI mice were 20.8 ± 4.4 ng/mL (P < 0.01 versus unoperated controls). In sham mice, serum ST2 levels were 10.5 ± 2.2 ng/mL (P < 0.05 versus control and versus MI), indicating that surgical stress induces serum ST2 in the absence of MI. Three days after MI, ST2 serum levels were similar among unoperated, sham (1.6 ± 0.8 ng/mL), and MI mice (2.5 ± 1.5 ng/mL) (ANOVA = NS).
Figure 4.
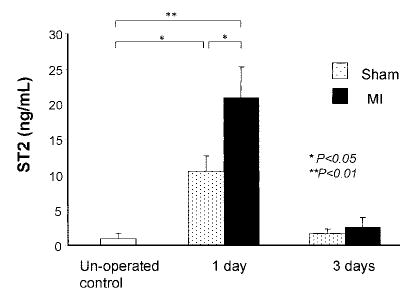
Serum ST2 levels in mice after MI. Serum ST2 levels were significantly increased 1 day after MI compared with sham-operated mice. Three days after MI, serum ST2 levels were similar among unoperated, sham-operated, and MI mice.
To determine whether ST2 is transcriptionally regulated in the heart after MI, we performed relative RT-PCR for ST2. In left ventricular (LV) tissue, ST2 mRNA levels were significantly increased 4 hours after MI (1.44 ± 0.06 U) compared with sham (1.21± 0.06 U, P < 0.05), which were slightly, but not significantly, higher than in unoperated mice (1.00± 0.10 U). We observed a trend for an increase in ST2L mRNA in LV from MI versus sham (1.28 ± 0.07 versus 1.00 ± 0.09 U, P = 0.06). These results demonstrate that ST2 is transcriptionally regulated in vivo in the mouse heart after MI, although transcriptional regulation of ST2 is notably less in vivo than in NRCM. We explored transcriptional regulation of ST2 in other tissues. ST2 mRNA was significantly increased in thymus in MI mice compared with sham mice (1.42 ± 0.10 versus 1.10 ± 0.07 U, P < 0.05), which was not different from unoperated mice (1.00 ± 0.14 U). ST2L mRNA levels in the thymus were similar in all groups. In spleen, lung, atrium, and liver, ST2 mRNA was not different among the 3 experimental groups. Of note, ST2 mRNA levels were 10-fold lower in liver compared with all other tissues examined (data not shown).
Soluble ST2 Is Increased in the Systemic Circulation of Patients One Day After MI
To test whether ST2 is released into the circulation in humans after MI, we assayed serum samples for ST2 from 69 participants of the HEART study on days 1, 14, and 90 after MI (Figure 5). Circulating ST2 was increased on day 1 (3.8 ± 0.4 ng/mL, P < 0.001; range, 0.32 to 17.42 ng/mL) compared with day 14 (0.98± 0.06 ng/mL; range, 0.25 to 3.42 ng/mL) and day 90 (0.79± 0.07 ng/mL; range, 0.02 to 3.53 ng/mL; day 14 versus day 90, P = NS; panel a). Circulating ST2 correlated positively with peak creatine kinase (r = 0.41, P < 0.001; panel b) and negatively with LV ejection fraction (r = −0.32, P = 0.02; panel c). These results suggest an association between the extent of myocardial injury or biomechanical load and soluble ST2 in the circulation.
Figure 5.
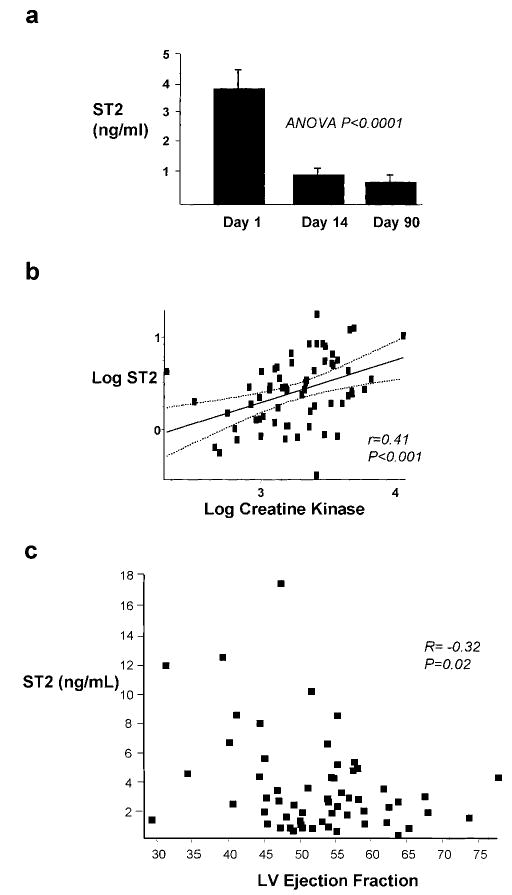
ST2 protein levels in the circulation of human patients after MI. Serial blood samples from 69 patients from the HEART study were analyzed for ST2 by ELISA. a, ST2 was significantly increased 1 day after MI compared with day 14 and day 90. b, Linear regression analysis demonstrating a significant positive relationship (P < 0.001) between circulating ST2 and creatine kinase 1 day after MI. Log ST2(ng/mL)=0.454[log CK(U/L)]−1.07. c, Linear regression analysis demonstrating a significant negative relationship (P = 0.02) between circulating ST2 and LV ejection fraction 1 day after MI.
Discussion
We present the induction and regulation of the interleukin-1 receptor family member ST2 in cardiac myocytes, a transient increase in soluble ST2 in the serum of mice after MI, and increased levels of soluble ST2 in the systemic circulation of human patients 1 day after MI. The expression of ST2 has not previously been described in the cardiovascular system, and therefore this represents a novel cytokine receptor pathway in myocardial pathophysiology.
Soluble ST2 was identified by its serum induction in resting fibroblasts3 as a transcript regulated by AP-1/Fos-1 transcription factor activity.2 We found that ST2 is induced in cardiac myocytes by mechanical strain, interleukin-1β, and phorbol ester but not lipopolysaccharide, a ligand for toll-like receptors, or tumor necrosis factor-α. The interleukin-1 receptor, toll-like receptors, and membrane ST2 receptor all belong to the TIR (toll interleukin receptor) family. Our findings that lipopolysaccharide and tumor necrosis factor-αdid not induce ST2 mRNA suggest that among the TIR family and tumor necrosis factor receptor, there is specificity for interleukin-1β/interleukin-1 receptor signaling for ST2 induction.
No function for soluble or membrane receptor ST2 has yet been identified, in part because no activating ligand for ST2 is known. However, using chimeric receptors, it has been shown that membrane ST2 signaling includes nuclear factor (NF)-κB activation,21–23 but the downstream ST2 signaling targets are not known.
Soluble and membrane forms of ST2 are produced through cell- and context-dependent regulation of proximal and distal promoter regions surrounding ST2 exons 1b and 1a, respectively.1,2,19,20 In mast cells, soluble ST2 is induced by GATA-1 transcription factor mechanisms via activation of the distal exon 1a promoter region,20 whereas in fibroblasts, the proximal promoter region is activated.2 Both promoter regions used in the present study contain consensus sites for AP-1, NF-κB, Oct-1, GATA-1, and SP-1 transcription factors. In this study, the magnitude of induction of the proximal exon 1b region in response to interleukin-1β, strain, and phorbol ester was similar to the mRNA induction to these stimuli, supporting the conclusion that in cardiac myocytes, similar to fibroblasts, regulation of induced ST2 expression is via activation of the proximal promoter region surrounding exon 1b to generate abundant soluble ST2 and minor amounts of membrane ST2L transcript.
MI is associated with an early loss in myocardial function that leads to increased LV wall stress. Stress-activated cytokines including interleukin-1, interleukin-6, and tumor necrosis factor-αalso participate in the initial myocardial response to stress and injury.24,25 We speculate based on our studies that myocardial stress or stress-activated cytokine release may be one mechanism leading to induction and release of ST2. However, this study found a disparity between the level of transcriptional regulation (mRNA induction) of ST2 in neonatal cardiac myocytes in vitro and in the mouse heart in vivo, suggesting that mechanisms other than transcriptional regulation are operative in vivo. We cannot determine whether in humans after MI, serum ST2 is elevated in response to cell injury or in response to increased ventricular stress. We observed a significant increase in serum ST2 in mice subjected to sham surgery compared with unoperated mice. However, serum ST2 levels in mice with MI were increased 2-fold compared with sham-operated mice and increased 4-fold in patients after MI. Serum ST2 levels showed a weak but highly significant correlation to levels of creatine kinase, a marker of cellular injury that could have originated from noncardiac sources. We cannot determine the origin of circulating serum ST2 in patients. Serum ST2 may be synthesized and released from cell types remote from the site of cell injury or stress.
Membrane ST2 is homologous to the innate immunity toll-like receptors, which are early response mediators of immunity and inflammation.26 Membrane ST2 is a surface marker for Th2 lymphocytes that produce interleukin-4 and interleukin-10,5,27 Th2 cytokines that have been shown to be anti-inflammatory cytokines in cardiac disease secondary to interleukin-1 signaling.28,29 No study has demonstrated a functional link between secreted ST2 and Th2 immune function through membrane-anchored ST2.
Signals from stressed and necrotic cells can activate antigen-presenting cells residing in all tissues, a process aided by Th2 cells.30–32 Soluble ST2 released in response to stress or injury after MI may participate in these immunoregulatory functions, suggested by our finding of a correlation between systemic ST2 levels and creatine kinase, a marker of cell necrosis.
In summary, we identified a novel stress-activated signaling pathway: induction of ST2, an interleukin-1 receptor family member, after myocardial stress or injury. The potential importance of this is supported by the finding of increased levels of circulating ST2 in patients after MI, although the biological and clinical significance of this is presently unknown. Additional work is needed to clarify the role of ST2 in immune modulation of cardiovascular disease and to determine the usefulness of serum ST2 as a biomarker for myocardial injury.
Acknowledgments
This study was funded by National Institutes of Health grants HL69484, HL63927, and HL052320. We thank Suphi Muangman, Joe Gannon, and Merry Lindsey.
References
- 1.Iwahana H, Yanagisawa K, Ito-Kosaka A, et al. Different promoter usage and multiple transcription initiation sites of the interleukin-1 receptor-related human ST2 gene in UT-7 and TM12 cells. Eur J Biochem. 1999;264:397–406. doi: 10.1046/j.1432-1327.1999.00615.x. [DOI] [PubMed] [Google Scholar]
- 2.Bergers G, Reikerstorfer A, Braselmann S, et al. Alternative promoter usage of the fos-responsive gene Fit-1 generates RNA isoforms coding for either secreted or membrane-bound proteins related to the IL-1 receptor. EMBO J. 1994;13:1176–1188. doi: 10.1002/j.1460-2075.1994.tb06367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tominaga S. A putative protein of a growth specific cDNA from BALB/c-3T3 cells is highly similar to the extracellular portion of mouse interleukin-1 receptor. FEBS Lett. 1989;258:301–304. doi: 10.1016/0014-5793(89)81679-5. [DOI] [PubMed] [Google Scholar]
- 4.Lohning M, Stroehmann A, Coyle AJ, et al. T1/ST2 is preferentially expressed on murine Th2 cells, independent of interleukin 4, interleukin 5, and interleukin 10, and important for Th2 effector function. Proc Natl Acad Sci U S A. 1998;95:6930–6935. doi: 10.1073/pnas.95.12.6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coyle AJ, Lloyd C, Tian J, et al. Crucial role of the interleukin 1 receptor family member T1/ST2 in T helper cell type 2-mediated lung mucosal immune responses. J Exp Med. 1999;190:895–902. doi: 10.1084/jem.190.7.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prechtel D, Harbeck N, Berger U, et al. Clinical relevance of T1-S, an oncogene-inducible, secreted glycoprotein of the immunoglobulin superfamily, in node-negative breast cancer. Lab Invest. 2001;81:159–165. doi: 10.1038/labinvest.3780223. [DOI] [PubMed] [Google Scholar]
- 7.Kumar S, Minnich MD, Young PR. ST2/T1 protein functionally binds to two secreted proteins from Balb/c 3T3 and human umbilical vein endothelial cells but does not bind interleukin 1. J Biol Chem. 1995;270:27905–27913. doi: 10.1074/jbc.270.46.27905. [DOI] [PubMed] [Google Scholar]
- 8.Gayle MA, Slack JL, Bonnert TP, et al. Cloning of a putative ligand for the T1/ST2 receptor. J Biol Chem. 1996;271:5784–5789. doi: 10.1074/jbc.271.10.5784. [DOI] [PubMed] [Google Scholar]
- 9.Dale M, Nicklin MJH. Interleukin-1 receptor cluster: gene organization of IL1R2, IL1R1, IL1RL2 (IL-1Rrp2), IL1RL1 (T1/ST2), and IL18R1(IL-1Rrp) on human chromosome 2q. Genomics. 1999;57:177–179. doi: 10.1006/geno.1999.5767. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto K, Dang QN, Kennedy SP, et al. Induction of tenascin-C in cardiac myocytes by mechanical deformation. J Biol Chem. 1999;274:21840–21846. doi: 10.1074/jbc.274.31.21840. [DOI] [PubMed] [Google Scholar]
- 11.Feldman AM, Weinberg EO, Ray PE, et al. Selective changes in cardiac gene expression during compensated hypertrophy and the transition to cardiac decompensation in rats with chronic aortic banding. Circ Res. 1993;73:184–192. doi: 10.1161/01.res.73.1.184. [DOI] [PubMed] [Google Scholar]
- 12.Rohde LE, Ducharme A, Arroyo LH, et al. Matrix metalloproteinase inhibition attenuates early left ventricular enlargement after experimental myocardial infarction in mice. Circulation. 1999;23:3063–3070. doi: 10.1161/01.cir.99.23.3063. [DOI] [PubMed] [Google Scholar]
- 13.Pfeffer MA, Greaves SC, Arnold MO, et al. Early versus delayed angiotensin-converting enzyme inhibition therapy in acute myocardial infarction. Circulation. 1997;95:2643–2651. doi: 10.1161/01.cir.95.12.2643. [DOI] [PubMed] [Google Scholar]
- 14.Solomon SD, Glynn RJ, Greaves S, et al. Recovery of ventricular function after myocardial infarction in the reperfusion era: the healing and early afterload reducing therapy study. Ann Intern Med. 2001;134:451–458. doi: 10.7326/0003-4819-134-6-200103200-00009. [DOI] [PubMed] [Google Scholar]
- 15.Aikawa Y, Rohde L, Plehn J, et al. Regional wall stress predicts ventricular remodeling after anteroseptal myocardial infarction in the Healing and Early Afterload Reducing Trial (HEART): an echocardiography-based structural analysis. Am Heart J. 2001;141:234–242. doi: 10.1067/mhj.2001.112237. [DOI] [PubMed] [Google Scholar]
- 16.Kuroiwa K, Li H, Tago K, et al. Construction of ELISA system to quantify human ST2 protein in sera of patients. Hybridoma. 2000;19:151–159. doi: 10.1089/02724570050031194. [DOI] [PubMed] [Google Scholar]
- 17.Takagi T, Yanagisawa K, Tsukamoto T, et al. Identification of the product of the murine ST2 gene. Biochim Biophys Acta. 1993;1178:194–200. doi: 10.1016/0167-4889(93)90009-e. [DOI] [PubMed] [Google Scholar]
- 18.Sadoshima J, Xu Y, Slayter HS, et al. Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell. 1993;5:977–984. doi: 10.1016/0092-8674(93)90541-w. [DOI] [PubMed] [Google Scholar]
- 19.Carter RW, Sweet MJ, Xu D, et al. Regulation of ST2L expression on T helper (Th) type 2 cells. Eur J Immunol. 2001;31:2979–2985. doi: 10.1002/1521-4141(2001010)31:10<2979::aid-immu2979>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 20.Gachter T, Werenskiold AK, Klemenz R. Transcription of the interleukin-1 receptor-related T1 gene is initiated at different promoters in mast cells and fibroblasts. J Biol Chem. 1996;271:124–129. doi: 10.1074/jbc.271.1.124. [DOI] [PubMed] [Google Scholar]
- 21.Born TL, Smith DE, Garka KE, et al. Identification and characterization of two members of a novel class of the interleukin-1 receptor (IL-1R) family. J Biol Chem. 2000;275:29946–29954. doi: 10.1074/jbc.M004077200. [DOI] [PubMed] [Google Scholar]
- 22.Reikerstorfer A, Holz H, Stunnenberg HG, et al. Low affinity binding of interleukin-1 β and intracellular signaling via NF-κB identify Fit-1 as a distant member of the interleukin-1 receptor family. J Biol Chem. 1995;270:17645–17648. doi: 10.1074/jbc.270.30.17645. [DOI] [PubMed] [Google Scholar]
- 23.Mitcham JL, Parnett P, Bonnert TP, et al. T1/ST2 signaling establishes it as a member of an expanding interleukin-1 receptor family. J Biol Chem. 1996;271:5777–5783. doi: 10.1074/jbc.271.10.5777. [DOI] [PubMed] [Google Scholar]
- 24.Mann DL. Stress activated cytokines and the heart. Cytokine Growth Factor Rev. 1996;7:341–354. doi: 10.1016/s1359-6101(96)00043-3. [DOI] [PubMed] [Google Scholar]
- 25.St John Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101:2981–2988. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 26.O’Neill LAJ, Dinarello CA. The IL-1 receptor/toll-like receptor superfamily: crucial receptors for inflammation and host defense. Immunol Today. 2000;21:206–209. doi: 10.1016/s0167-5699(00)01611-x. [DOI] [PubMed] [Google Scholar]
- 27.Xu D, Chan WL, Leung BP, et al. Selective expression of a stable cell surface molecule on type 2 but not type 1 helper T cells. J Exp Med. 1998;187:787–794. doi: 10.1084/jem.187.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamaoka M, Yamaguchi S, Okuyama M, et al. Anti-inflammatory cytokine profile in human heart failure: behaviour of interleukin-10 in association with tumor necrosis factor-α. Jpn Circ J. 1999;63:951–954. doi: 10.1253/jcj.63.951. [DOI] [PubMed] [Google Scholar]
- 29.Ohtsuka T, Hamada M, Hiasa G, et al. Effect of β-blockers on circulating levels of inflammatory and anti-inflammatory cytokines in patients with dilated cardiomyopathy. J Am Coll Cardiol. 2001;37:412–417. doi: 10.1016/s0735-1097(00)01121-9. [DOI] [PubMed] [Google Scholar]
- 30.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 31.Delves PJ, Roitt IM. The immune system. New Engl J Med. 2000;343:37–49. doi: 10.1056/NEJM200007063430107. [DOI] [PubMed] [Google Scholar]
- 32.More SH, Breloer M, von Bonin A. Eukaryotic heat shock proteins as molecular links in innate and adaptive immune responses: Hsp60-mediated activation of cytotoxic T cells. Int Immunol. 2001;13:1121–1127. doi: 10.1093/intimm/13.9.1121. [DOI] [PubMed] [Google Scholar]


