Abstract
Background: MCT and MCTC types of human mast cells (MCs) are distinguished from one another on the basis of the protease compositions of their secretory granules, but their functional and developmental relationships have been uncertain.
Objective: These studies better define the functional properties and developmental relationship of MCT and MCTC cells.
Methods: Mast cells were dispersed from human skin and lung, purified with anti-Kit antibody, and separated into CD88+ and CD88- populations by cell sorting. These cells were evaluated by immunocytochemistry with antitryptase and antichymase mAbs; for chymase and tryptase mRNA by real-time RT-PCR; for conversion of MCT to MCTC cells during cell culture with recombinant human stem cell factor and recombinant human IL-6; and for degranulation and leukotriene C4 (LTC4) secretion when stimulated with anti-Fc∊RI, substance P, C5a, and compound 48/80.
Results: Mature MCT and MCTC cells were separated from one another on the basis of selective expression of CD88, the C5aR, on MCTC cells. Lung MCT cells had negligible levels of chymase mRNA and retained their MCT phenotype in culture. Mature MCTC cells from skin and lung degranulated in response to Fc∊RI cross-linking, C5a, compound 48/80, and substance P. Lung MCTC cells released LTC4 on activation, but no LTC4 was detected when skin-derived MCTC cells were activated. MCT cells from lung degranulated and released LTC4 in response to anti-Fc∊RI and substance P, but not to C5a and compound 48/80.
Conclusion: These observations functionally distinguish MCT from MCTC types of human mast cells and suggest important differences that may affect their participation in diseases such as asthma and urticaria.
Keywords: Mast cells, human, IL-6, complement, tryptase, chymase, C5a, substance P, CD88
The MCTC and MCT types of human mast cells (MCs) were initially recognized on the basis of the protease compositions of their secretory granules, with tryptase, chymase, carboxypeptidase A3, and cathepsin G in the former and only tryptase in the latter.1-3 MCTC cells are essentially the exclusive type of mast cell in normal skin but account for the minority of mast cells in normal lung. MCT cells predominate in the alveolar wall and epithelium of the lung, whereas MCTC cells favor the bronchial smooth muscle and glandular regions. Markedly elevated levels of the MCTC cell in the bronchial smooth muscle of patients with asthma correlate with their bronchial hyperreactivity.4 MCT cell numbers in respiratory epithelium increase during the pollen season in sensitive subjects.5-7 These distinct tissue distributions and disease associations suggest a purposeful presence for each type of mast cell, but little is known of their functional differences.
Skin-derived human mast cells (Sk-MCs) can be activated by C5a, whereas lung-derived human mast cells (Lu-MCs) do not respond, presumably because C5aR (CD88) is expressed on >80% of juvenile foreskin-derived mast cells but on, 10% of Lu-MC, tonsillar mast cells, and uterine mast cells.8 Compound 48/80 also stimulates Sk-MC but not Lu-MC. Various neuropeptides such as substance P, vasoactive intestinal peptide, somatostatin, and calcitonin gene-related protein fail to activate Lu-MC in some studies9,10 but stimulate such mast cells in others.11,12 One possibility to account for such discrepancies is the relative portions of MCT and MCTC cells in the tested preparations, a factor that typically is not considered in most studies. Whether these tissue-aligned functional differences in mast cell activation profiles reflect differences between MCTC or MCT types of mast cells or instead reflect the different microenvironments of these tissues cannot be precisely determined unless the 2 types of mast cell present in the lung-derived preparations are first purified and separated from one another.
Another unresolved issue is the developmental relationship between MCT and MCTC types of mast cells. This protease phenotype appears in vivo soon after granule formation can be detected by electron microscopy,13 suggesting parallel rather than sequential development. In contrast, observations with cord blood-derived human mast cells or bone marrow-derived mast cells suggest that tryptase+/chymase- cells become tryptase+/chymase+ over time,14-17 implying sequential development. Another interpretation is that MCTC cells expand whereas MCT cells expire under the culture conditions used. Indeed, Sk-MCTC cells proliferate in stem cell factor (SCF)-containing serum free medium more readily than MCT-enriched Lu-MC,18 and MCT but not MCTC cells undergo IL-4-mediated apoptosis.19 Alternatively, immature MCTC cells that produce little if any chymase protein, and thereby appear to be of the MCT type will become chymase+ as they mature. Again, purification and separation of MCT and MCTC cells will enable a more precise delineation of their developmental relationship.
The current study shows that mature MCTC cells from Lu-MC and Sk-MC express CD88 and can be separated from MCT cells by sorting with anti-CD88 antibody. Cultured MCT cells from lung remain deficient in CD88 and chymase protein and chymase mRNA. MCTC cells obtained from skin and from lung degranulate in response to anti-Fc∊RI, compound 48/80, substance P, and C5a. Although Lu-MCTC cells produce LTC4 in response to these agonists, Sk-MCTC cells do not. Lu-MCT cells only respond to anti-Fc∊RI and substance P by degranulating and secreting LTC4. Thus, MCT and MCTC cells appear to be functionally distinct.
METHODS
Culture of Lu-MC and Sk-MC
All experimental protocols involving human tissues were approved by the Human Studies Committee at Virginia Commonwealth University. Surgical lung or skin tissue samples were obtained from consented patients through the Cooperative Human Tissue Network (Columbus, Ohio), the National Disease Research Interchange Center (Philadelphia, Pa), or the Departments of Surgery or Pathology at Virginia Commonwealth University. Lung specimens were typically lobectomies from patients with lung cancer, whereas skin specimens were from either breast reductions or abdominoplasties. Lu-MC20 and Sk-MC18 were prepared as described. Lu-MC, after enrichment by Percol (Amersham-Pharmacia Biotec, Uppsala, Sweden)-dependent sedimentation, ranged in purity from 5% to 45% (mean ± SD, 21% ± 12%; n = 9), and these mast cells consisted of, 5% (n = 2) to 13% MCTC cells (6% ± 3%; n = 9). They were cultured in RPMI 1640 supplemented with 10% heat-inactivated controlled process serum replacement medium 3 (Sigma-Aldrich, St Louis, Mo), 2 mmol/L L-glutamine, 0.1 mmol/L nonessential amino acids, 10 mmol/L HEPES, 50 mmol/L 2-β-mercaptoethanol, 200 U/mL penicillin, 100 mg/mL streptomycin, and recombinant human (rh) SCF (100 ng/mL; a gift from Amgen, Thousand Oaks, Calif) alone or with rhIL-6 (50 ng/mL; R & D Systems, Minneapolis, Minn), whereas Sk-MC was cultured in AIM-V medium (Life Technologies, Rockville, Md) containing rhSCF (100 ng/mL). All cells were cultured in a Nuaire incubator (Plymouth, Minn) at 37°C in 6% CO2, harvested and counted (viability by trypan blue dye exclusion), and then subjected to cytocentrifugation for immunocytochemistry, apoptosis determination, or cell sorting.
Immunocytochemistry
Cytocentrifuge preparations of cells were fixed in methanol containing 0.6% H2O2 for 30 minutes at room temperature, rinsed with H2O, and stored at 4°C until used. Slides were labeled with biotin-conjugated B7, a mouse IgG1 antichymase mAb, alkaline phosphatase-conjugated G3, a mouse IgG1 antitryptase, or nonimmune negative controls to identify chymase+ MCTC cells and tryptase+ mast cells as described.21
Flow cytometry, enrichment of mast cells, and cell sorting
Cells were analyzed for expression of surface Kit by using a phycoerythrin-labeled purified mouse antihuman CD117 mAb or a phycoerythrin-labeled isotype-matched mAb as the negative control (5 mg/mL for each mAb; BD Biosciences Pharmingen, San Diego, Calif). In the case of lung cell preparations, alveolar macrophages (about 30% of initially collected cells) were depleted with antihuman CD14 mAb bound to Dynabeads (4 beads per target cell) during a 1 hour of incubation at 4°C (Dynal, Oslo, Norway). A rabbit antihuman CD88 mAb (10 mg/mL) or a nonimmune rabbit IgG (10 mg/mL) as the negative control was used with an Alexa Fluor 488-labeled goat antirabbit IgG (5 mg/106 cells; Molecular Probes, Eugene, Ore) to identify CD88+ cells.
In some cases, mast cells were further purified with mouse IgG anti-Kit mAb (BD Biosciences Pharmingen) and magnetic beads coupled with sheep IgG antimouse IgG (Dynal, Oslo, Norway) as described.22 Purities of Lu-MC typically exceeded 95%. In other cases, a MoFlo high-performance cell sorter (Cytomation, Fort Collins, Colo) was used to purify Lu-MC and separate them into 2 groups, Kit+/CD88- and Kit+/CD88+, by using the antibodies described. Each mast cell group consisted of >99% mast cells on the basis of staining with acidic toluidine blue (0.5% in 0.5 moles/L HCl) and were further characterized for the MCT and MCTC phenotype by immunocytochemistry.
Quantitative real-time PCR
Total RNA was isolated by guanidinium-phenol extraction, and RT was performed with 1 mgto5 mg total RNA, 200 U of Moloneymurine leukemia virus RT, a 3′-primer mix (containing the 3′ primers for tryptase, chymase, and glyceraldehyde phosphate dehydrogenase (GAPDH) at 25 nmol/L each), 10 mmol/L dithiothreitol, 13 first strand buffer, and 1 mmol/L deoxynucleotide triphosphate mix at 37°C for 2 hours, as recommended by Invitrogen Corp (Carlsbad, Calif). Real-time PCR was performed by the VCU Nucleic Acids Research Facility with an ABI Prism 7900 Sequence Detection System (Applied Biosystems, Foster City, Calif) by using the TaqMan One Step PCR Mast Mix Reagents Kit (Applied Biosystems, P/N: 4309169). All of the samples were tested in triplicate under the conditions recommended by the fabricant. Cycling conditions were 48°C for 30 minutes, 95°C for 10 minutes, and 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. The cycle threshold was determined to provide the optimal standard curve values (0.98-1.0). The probes and primers were designed by using the Primer Express 2.0 version. Probes were labeled on the 5′ end with 6-carboxyfluoresceine and on the 3′ end with 6-carboxytetramethylrhodamine. Ribosomal RNA (18S) from the Pre-Developed TaqMan Assay Reagents (P/N: 4310893E) was used as endogenous control. The following primers and probes were used:
Tryptase. Sense (nucleotides 498-517) 5′-GGCGATGTGGACAATGATGA-3′, antisense (nucleotides 610-591) 5′-GTGTAGGCGCCAAGGTGGTA-3′, probe (nucleotides 556-584) 5′-TCCCCATAATGGAAAACCACATTTGTGAC-3′ for which the sense primer spans exons 4 and 5, whereas the antisense primer and probe recognizes sequences in exon 5.
Chymase. Sense (nucleotides 198-216) 5′-GCTGACGGCTGCTCATTGT-3′, antisense (nucleotides 290-269) 5′-TCAAGCTTCTGCCATGTGTCTT-3′, probe (nucleotides 223-254) 5′-AGGTCTATAACAGTCACCCTTGGAGCCCATAA-3′ for which the sense primer recognizes an exon 2 sequence, the probe recognizes a sequence that spans exons 2 and 3, and the antisense primer recognizes an exon 3 sequence.
18S rRNA. Sense and antisense primers and probes were obtained from the Predeveloped TaqManAssay Reagents (P/N:4310893E) and used as directed (Applied Biosystems).
Quantification of chymase and tryptase staining intensities of Lu-MC
Photomicrographs of immunocytochemically stained cytocentrifuged mast cells were taken with a digital camera by using a 40× objective (Olympus MagnaFire; Olympus, Melville, NY). A fixed area was drawn within the cytoplasm of each cell to be analyzed. Gray value intensities were displayed as pixels on a scale of 0 to 255. Staining intensities were quantified within each area, and data were analyzed by using the analySIS software package (Soft Imaging System, Lakewood, Colo). Each region of interest provided a given intensity profile, with a mean intensity calculated by the software. Background values were determined with isotype-matched negative control mAbs. Mast cells from 3 separate lung cell preparations were analyzed by a blinded observer.
Human mast cell activation and mediator assays
Mast cells were adjusted to a concentration of 106 cells/mL, washed twice in Tyrodes buffer (without magnesium and calcium) containing 0.05% gelatin, and resuspended in Tyrodes-gelatin buffer supplemented with 2.5 mmol/L CaCl2 and 1 mmol/L MgCl2. After cells were preincubated for 5 minutes at 37°C in microcentrifuge tubes, activation experiments were performed for 2 hours at 37°C with 3 mg/mL anti-Fc∊RIa (22E7) mAb, which was generously provided by Jarema P. Kochan, PhD (Hoffman-LaRoche, Inc, Nutley, NJ),23,24 C5a (1 mg/mL), compound 48/80 (1 μg/mL), and substance P (1 mmol/L; Sigma-Aldrich). These doses were determined previously to be optimal for degranulation without toxicity. Ice-cold Tyrodes-gelatin buffer without calcium and magnesium was used to stop the reaction. After centrifugation, cell releasates in the supernatants were collected in separate tubes and brought to a final NaCl concentration of 1 mol/L. Cell pellets were resuspended in Tyrodes-gelatin buffer containing 1 mol/L NaCl and lysed by sonication on ice. Cell releasates and lysates were kept at 280°C until assayed. β-Hexosaminidase was measured in cell releasates and lysates and used to calculate percent degranulation: releasate/(lysate + releasate) × 100. Net percent release values were calculated by subtracting the percent release values for unstimulated cells from those of the stimulated cells. Cysteinyl-leukotriene (LT) levels in releasates were measured with an ELISA for LTC4/D4/E4 (Amersham Pharmacia Biotech, Piscataway, NJ). For this ELISA, the cross-reactivity between LTC4 and LTD4 is 100%; LTC4 and LTE4 is 70%; and LTC4 and LTB4 is 0.3%. Concentrations were determined according to the manufacturer’s instructions, with a lower limit of detection of 10 pg/mL.
RESULTS
CD88-dependent sorting of MCT and MCTC cells from lung
Because MCTC cells from skin express the C5aR, CD88, experiments were conducted to see whether CD88 also was expressed on MCTC cells from lung and could enable MCTC cells to be separated from MCT cells by cell sorting. As shown in Fig 1, after sorting Lu-MC, 4% of the cells in the Kit+/CD88- gate were chymase+, whereas greater than 98% of the cells in the Kit+/CD88+ gate were chymase+. This result was representative of 3 independent experiments. By real-time/RT-PCR, the 18S-rRNA-normalized ratios of mRNAs from chymase- Lu-MC to those of Sk-MC are shown in Table I. Chymase mRNA levels in Lu-MCT cells were negligible (undetected in 2 of 3 preparations). In contrast, tryptase mRNA levels, although not as high as in Sk-MCTC cells, were still substantial in all 3 preparations of Lu-MCT cells.
FIG 1.
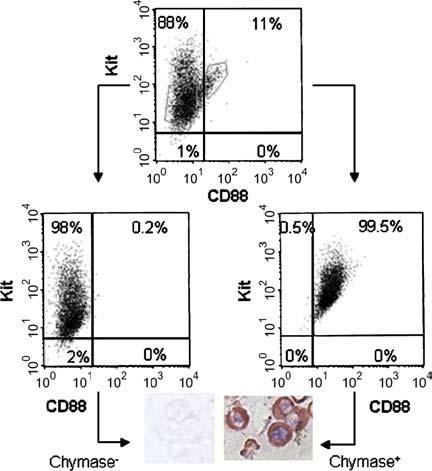
Purification and separation of MCTC and MCT cells from Lu-MC. Mast cells were labeled with rabbit anti-CD88/Alexa 488-goat antirabbit IgG and phycoerythrin-mouse anti-Kit mAb and sorted by flow cytometry. Sorting gates are shown by the gray polygons. Sorted cells were cultured overnight and subjected to analytical flow cytometry (lower panels) or cytocentrifugation and immunocytochemistry with antichymase mAb.
TABLE I.
Chymase and tryptase mRNA levels in MCT cells from cord blood and lung
| 18S rRNA-normalized ratio to that in Sk-MC |
||
|---|---|---|
| Cell source | Tryptase | Chymase |
| Lung MCT cells | 0.48 ± 0.44 | 4.0 × 10-5 ± 1.8 × 10-5 |
The lower limit of mRNA detection after 40 cycles of PCR ranged from a ratio of 2 to 20 × 10-7. Values shown are means ± SEs for 3 preparations of lung mast cells with 1.3% ± 0.3% MCTC cells, each performed in triplicate.
Activation profiles for MCT and MCTC cells from skin and lung for degranulation and LTC4 release
To define further the functional phenotypes of MCT and MCTC cells purified from Lu-MC and of MCTC cells from skin, each was challenged with anti-Fc∊RI mAb, C5a, compound 48/80, and substance P. MCT and MCTC cells from lung were purified and sorted on the basis of surface Kit and CD88 as in Fig 1, whereas Sk-MCTC cells were used after 4 to 8 weeks of culture. As shown in Fig 2 (left panels), both types of mast cell from lung as well as MCTC cells from skin degranulated (β-hexosaminidase release) when exposed to anti-Fc∊RI mAb and substance P. MCTC cells from each source degranulated in response to C5a and compound 48/80, with those from lung showing similar levels of β-hexosaminidase release compared with those from skin. In contrast, MCT cells from lung failed to degranulate when challenged with C5a and compound 48/80. Secretion of the newly generated lipid mediator, LTC4, also was assessed. As shown in Fig 2 (right panels), the activation profiles for Lu-MCT and Lu-MCTC cells to produce LTC4 were similar to those for degranulation. In contrast, Sk-MCTC cells failed to secrete LTC4 in response to all stimuli.
FIG 2.
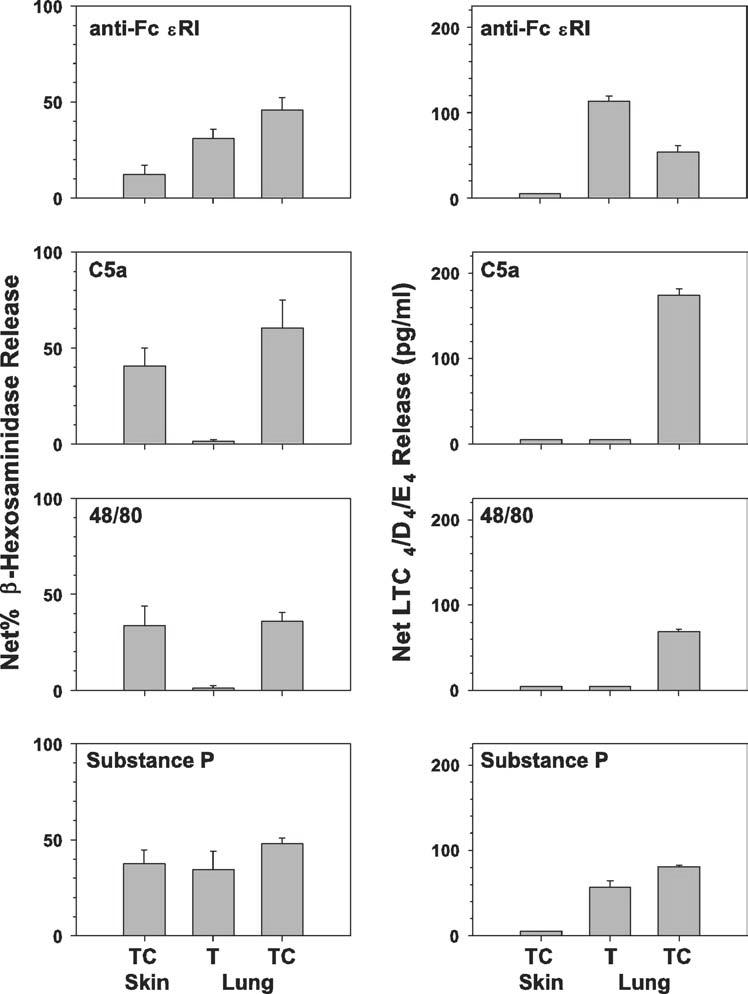
Activation profiles of purified MCT and MCTC cells from different sources. Cells (106/mL) were exposed to anti-Fc∊RI, C5a, compound 48/80, and substance P for 2 hours at 37°C. Net percent release of β-hexosaminidase (left panels) and net release of sulfidopeptide LTs (right panels) were determined. Overall, spontaneous release values for β-hexosaminidase were ≤6.5% and for LTC4/D4/E4 were undetectable.
Lu-MCT cells fail to convert to MCTC cells in culture
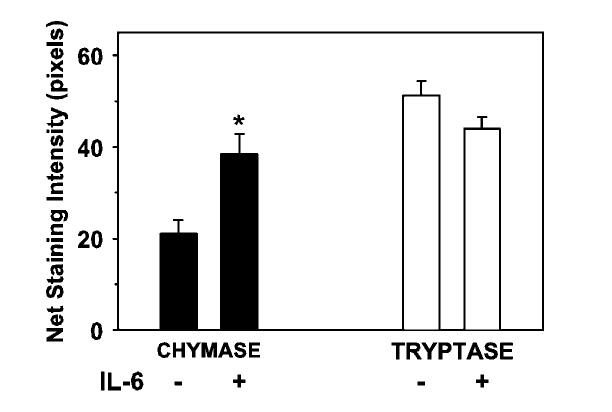
Kit-purified lung-derived mast cells were cultured for 1 week with rhSCF (100 ng/mL) in the presence or the absence of rhIL-6 (50 ng/mL; n = 3 lungs). rhIL-6 was used because it enhances chymase and CD88 expression by cord blood-derived human mast cells (Oskeritzian CA and Schwartz LB, unpublished data, 2004). Cells were counted, cytocentrifuged, stained separately with antichymase and antitryptase mAbs, and analyzed for staining intensity as described in Methods. The mean percentages of chymase+ mast cells in the group treated with rhIL-6 for 6 days (8.7% ± 1.2%) were not significantly different from those for the group not treated with rhIL-6 (9.7% ± 2.9%; P = .67; 2-tailed t test; n = 3). Thus, new chymase-expressing cells were not evident in these mixed cultures. Nevertheless, Fig 3 shows that rhIL-6 significantly increased the mean staining intensity for chymase, presumably among those cells that already had declared themselves to be of the MCTC type by day 0. In contrast, no significant difference in the mean intensities of staining for tryptase between rhIL-6-treated and rhIL-6-untreated cells was observed.
FIG 3.
Effect of IL-6 on chymase protein expression in Lu-MC. Cytospins of purified lung-derived mast cells were stained with antichymase or antitryptase mAbs. The staining intensities of positive cells from 3 different lung mast cell preparations were then measured by image analysis. For each preparation, 10 to 15 chymase+ and 12 to 20 tryptase+ mast cells were examined. *P < .05 compared to no IL-6.
When Kit+/CD88- mast cells were placed into culture for 6 days with and without rhIL-6, the percentages of chymase+ cells among the untreated (1.8 ± 1.1) and treated (2.0 ± 2.0) groups were not significantly different from one another (P = .9; n = 3), nor from the starting percentages. This result indicates that mature Lu-MCT cells do not convert to MCTC cells when placed in culture with rhSCF alone or together with rhIL-6 under the experimental conditions used. In none of the lung mast cell experiments was an appreciable difference detected in cell numbers between the treated and untreated groups after the 6-day cultures. Thus, Lu-MCT cells do not express chymase mRNA and are not converted into MCTC cells under our culture conditions.
DISCUSSION
The current study makes 3 important observations. First, the mature MCTC type of mast cell in lung and skin expresses CD88, the receptor for C5a, whereas mature MCT cells in lung do not. Accordingly, cell sorting of Kithi lung-derived mast cells with anti-CD88 antibody separates CD88- MCT from CD88+ MCTC cells. Thus, surface expression of CD88 corresponds to the MCTC phenotype, regardless whether the MCTC cell originates from the skin or lung. Lu-MCT cells express negligible levels, if any, of chymase mRNA, similar to a previous report.20 This extends the definition of MCT cells to include the absence of chymase mRNA as well as protein. How these criteria will apply to immature, primed, mastocytosis, or leukemic mast cells and to mast cells at other sites remains to be determined. For example, HMC1 leukemic mast cells express small amounts of chymase mRNA in the absence of detectable chymase protein,20 but also exhibit functional CD88 on their surface.8,25,26
Second, Lu-MCT cells do not convert to MCTC cells under the culture conditions used, which include rhSCF in the presence and absence of rhIL-6 for 6 days. RhIL-6 enhances chymase and CD88 expression by cord blood-derived human mast cells (Oskeritzian CA and Schwartz LB, unpublished data, 2004). Indeed, Lu-MCTC cells cultured with rhIL-6 express somewhat higher levels of chymase based on immunocytochemical staining intensity, whereas tryptase staining intensities do not change. In contrast, when Lu-MCT cells are treated with rhIL-6, they become resistant to the apoptosis-mediating effect of IL-4.19 MCTC cells at baseline are not susceptible to IL-4-mediated apoptosis.
A third important finding is that lung MCTC cells, like those from skin, are activated by C5a and compound 48/80. Previous reports that Lu-MC fail to degranulate in response to C5a or compound 48/80 were probably a result of MCTC cells accounting for a minor portion of the total mast cells,8 their activation obscured by the spontaneous release of mediators from the more numerous MCT cells. Previously, the mean percentage of MCT cells among the mast cells dispersed from lung were reported as 90,21 although rarely a preparation appears with almost all MCT cells.20 In certain patients with chronic urticaria, activation of MCTC cells in skin by anti-Fc∊RI autoantibody and locally generated C5a has been proposed.27 In patients with asthma, C′ anaphylatoxin levels are elevated in bronchoalveolar lavage fluid28 and MCTC cell numbers are elevated in bronchial smooth muscle in relation to bronchial hyperreactivity,4 raising the possibility of C5a-facilitated activation of these mast cells.
Mast cells dispersed from synovium of patients with rheumatoid arthritis, when examined by flow cytometry, were almost all CD88+, even though only about half were chymase+ by immunocytochemistry; and they released histamine in response to C5a.29 In contrast, mast cells obtained from osteoarthritis synovium, which also were about 50% chymase+, showed negligible release of histamine to C5a. Whether only CD88+ mast cells expressed chymase in this study was not directly examined. Another study reported that CD88 was expressed on mast cells dispersed from juvenile foreskin, but not from adult mammary skin.30 This latter finding contrasts with the current study in which essentially all Sk-MCs, most of which were from breast skin, were CD88+. Nevertheless, these older reports raise the possibility that not all MCTC cells are CD88+.
In contrast with C5a, the neuropeptide substance P, like Fc∊RI cross-linking, activates MCT and MCTC cells to degranulate, regardless of whether their source was skin or lung. Modest variations in the magnitude of degranulation among the different types of mast cells was observed. Whether this reflects differences in the surface expression of Fc∊RI or the substance P receptor or in the activation status of signal transduction pathways inside these cells is uncertain. Activation of Sk-MC and Lu-MC by substance P has been previously reported,11,12,31,32 whereas mast cells from the intestine33 and heart34 fail to respond to substance P. These data suggest that the tissue source or local microenvironment of mast cells might be an important determinant of substance P responsiveness. Whether neuropeptides such as substance P can be released in sufficient quantity in the lung or skin to activate mast cells remains to be clarified but poses a potentially important connection of the nervous system to bronchospastic and urticarial reactions associated with stress or neurogenic stimulation.
The activation profiles of MCT and MCTC cells leading to secretion of LTC4 follow a pattern similar to those for degranulation with the exception that Sk-MCTC cells failed to secrete LTC4 in response to any of the 4 stimuli tested, in spite of their ability to degranulate. Low to negligible levels of LTC4 production by Sk-MC activated by Fc∊RI cross-linking have been reported previously.35,36 This may explain why chronic urticaria typically fails to respond to LT antagonists.37 However, the current study shows that MCTC cells from lung produce amounts of LTC4 that are comparable with MCT cells from lung, suggesting the possibility that MCTC cells can acquire the ability to produce this lipid mediator. In fact, mast cells derived from intestinal mucosa38 and those from cord blood under the influence of both SCF and IL-6,39 when challenged with antigen to cross-link Fc∊RI, release small quantities of LTC4 unless first primed with IL-4, in which case LTC synthase is induced and LTC4 production is substantially amplified. Whether LTC4 production by activated Sk-MC can be induced was not addressed by the current study. Our results provide compelling evidence that MCT and MCTC cells are functionally distinct types of human mast cells.
Acknowledgments
We thank Donald Purkall for assistance with cell sorting, Dr Margaret Grimes for cooperation in obtaining postsurgical specimens, and Aileen I. Velezcabassa, Maureen L. Baran, George D. Dalton, and Larry Okumoto for their excellent technical assistance.
Abbreviations used
- LT
Leukotriene
- Lu-MC
Lung-derived human mast cell
- MC
Mast cell
- rh
Recombinant human
- SCF
Stem cell factor
- Sk-MC
Skin-derived human mast cell
Footnotes
Supported by grants R01-AI27517 and R01-AI20487 to Dr. Schwartz and K08-AI057357 to Dr Zhao from the National Institutes of Health.
Disclosure of potential conflict of interest: C. A. Oskertizian: none disclosed; W. Zhao: none disclosed; H.-K. Min: none disclosed; H.-Z. Xia: none disclosed; A. Pozez: none disclosed; J. Kiev: none disclosed; L. B. Schwartz: none disclosed.
REFERENCES
- 1.Irani A-MA, Goldstein SM, Wintroub BU, Bradford T, Schwartz LB. Human mast cell carboxypeptidase: selective localization to MCTC cells. J Immunol. 1991;147:247–53. [PubMed] [Google Scholar]
- 2.Irani AA, Schechter NM, Craig SS, DeBlois G, Schwartz LB. Two types of human mast cells that have distinct neutral protease compositions. Proc Natl Acad Sci U S A. 1986;83:4464–8. doi: 10.1073/pnas.83.12.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schechter NM, Irani A-MA, Sprows JL, Abernethy J, Wintroub B, Schwartz LB. Identification of a cathepsin G-like proteinase in the MCTC type of human mast cell. J Immunol. 1990;145:2652–61. [PubMed] [Google Scholar]
- 4.Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med. 2002;346:1699–705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]
- 5.Gibson PG, Allen CJ, Yang JP, Wong BJO, Dolovich J, Denburg J, et al. Intraepithelial mast cells in allergic and nonallergic asthma: assessment using bronchial brushings. Am Rev Respir Dis. 1993;148:80–6. doi: 10.1164/ajrccm/148.1.80. [DOI] [PubMed] [Google Scholar]
- 6.Bentley AM, Jacobson MR, Cumberworth V, Barkans JR, Moqbel R, Schwartz LB, et al. Immunohistology of the nasal mucosa in seasonal allergic rhinitis: increases in activated eosinophils and epithelial mast cells. J Allergy Clin Immunol. 1992;89:877–83. doi: 10.1016/0091-6749(92)90444-7. [DOI] [PubMed] [Google Scholar]
- 7.Juliusson S, Pipkorn U, Karlsson G, Enerbäck L. Mast cells and eosinophils in the allergic mucosal response to allergen challenge: changes in distribution and signs of activation in relation to symptoms. J Allergy Clin Immunol. 1992;90:898–909. doi: 10.1016/0091-6749(92)90462-b. [DOI] [PubMed] [Google Scholar]
- 8.Füreder W, Agis H, Willheim M, Bankl HC, Maier U, Kishi K, et al. Differential expression of complement receptors on human basophils and mast cells: evidence for mast cell heterogeneity and CD88/C5aR expression on skin mast cells. J Immunol. 1995;155:3152–60. [PubMed] [Google Scholar]
- 9.Lowman MA, Rees PH, Benyon RC, Church MK. Human mast cell heterogeneity: histamine release from mast cells dispersed from skin, lung, adenoids, tonsils, and colon in response to IgE-dependent and nonimmunologic stimuli. J Allergy Clin Immunol. 1988;81:590–7. [PubMed] [Google Scholar]
- 10.Nissen D, Petersen LJ, Nolte H, Permin H, Melchior N, Skov PS. Measurement of histamine release from human lung tissue ex vivo by microdialysis technique. Inflamm Res. 1998;47:501–5. doi: 10.1007/s000110050365. [DOI] [PubMed] [Google Scholar]
- 11.Heaney LG, Cross LJM, Stanford CF, Ennis M. Differential reactivity of human bronchoalveolar lavage mast cells to substance P. Agents Actions. 1994;41(suppl C):C19–21. doi: 10.1007/BF02007748. [DOI] [PubMed] [Google Scholar]
- 12.Louis RE, Radermecker MF. Substance P-induced histamine release from human basophils, skin and lung fragments: effect of nedocromil sodium and theophylline. Int Arch Allergy Appl Immunol. 1990;92:329–33. doi: 10.1159/000235160. [DOI] [PubMed] [Google Scholar]
- 13.Craig SS, Schechter NM, Schwartz LB. Ultrastructural analysis of maturing human T and TC mast cells in situ. Lab Invest. 1989;60:147–57. [PubMed] [Google Scholar]
- 14.Ahn K, Takai S, Pawankar R, Kuramasu A, Ohtsu H, Kempuraj D, et al. Regulation of chymase production in human mast cell progenitors. J Allergy Clin Immunol. 2000;106:321–8. doi: 10.1067/mai.2000.108107. [DOI] [PubMed] [Google Scholar]
- 15.Dvorak AM, Furitsu T, Kissell-Rainville S, Ishizaka T. Ultrastructural identification of human mast cells resembling skin mast cells stimulated to develop in long-term human cord blood mononuclear cells cultured with 3T3 murine skin fibroblasts. J Leukoc Biol. 1992;51:557–69. doi: 10.1002/jlb.51.6.557. [DOI] [PubMed] [Google Scholar]
- 16.Shimizu Y, Sakai K, Miura T, Narita T, Tsukagoshi H, Satoh Y, et al. Characterization of “adult-type” mast cells derived from human bone marrow CD34(+) cells cultured in the presence of stem cell factor and interleukin-6: interleukin-4 is not required for constitutive expression of CD54, Fc epsilon RI alpha and chymase, and CD13 expression is reduced during differentiation. Clin Exp Allergy. 2002;32:872–80. doi: 10.1046/j.1365-2222.2002.01373.x. [DOI] [PubMed] [Google Scholar]
- 17.Toru H, Eguchi M, Matsumoto R, Yanagida M, Yata J, Nakahata T. Interleukin-4 promotes the development of tryptase and chymase double-positive human mast cells accompanied by cell maturation. Blood. 1998;91:187–95. [PubMed] [Google Scholar]
- 18.Kambe N, Kambe M, Kochan JP, Schwartz LB. Human skin-derived mast cells can proliferate while retaining their characteristic functional and protease phenotypes. Blood. 2001;97:2045–52. doi: 10.1182/blood.v97.7.2045. [DOI] [PubMed] [Google Scholar]
- 19.Oskeritzian CA, Zhao W, Pozez AL, Cohen NM, Grimes M, Schwartz LB. Neutralizing endogenous IL-6 renders mast cells of the MCT type from lung, but not the MCTC type from skin and lung, susceptible to human recombinant IL-4-induced apoptosis. J Immunol. 2004;172:593–600. doi: 10.4049/jimmunol.172.1.593. [DOI] [PubMed] [Google Scholar]
- 20.Xia H-Z, Kepley CL, Sakai K, Chelliah J, Irani A-MA, Schwartz LB. Quantitation of tryptase, chymase, Fc∊RIa, and Fc∊RIgamma mRNAs in human mast cells and basophils by competitive reverse transcription-polymerase chain reaction. J Immunol. 1995;154:5472–80. [PubMed] [Google Scholar]
- 21.Irani A-MA, Bradford TR, Kepley CL, Schechter NM, Schwartz LB. Detection of MCT and MCTC types of human mast cells by immunohistochemistry using new monoclonal anti-tryptase and anti-chymase antibodies. J Histochem Cytochem. 1989;37:1509–15. doi: 10.1177/37.10.2674273. [DOI] [PubMed] [Google Scholar]
- 22.Oskeritzian CA, Wang Z, Kochan JP, Grimes M, Du Z, Chang HW, et al. Recombinant human (rh)IL-4-mediated apoptosis and recombinant human IL-6-mediated protection of recombinant human stem cell factor-dependent human mast cells derived from cord blood mononuclear cell progenitors. J Immunol. 1999;163:5105–15. [PubMed] [Google Scholar]
- 23.Riske F, Hakim J, Mallamaci M, Griffin M, Pilson B, Tobkes N, et al. High affinity human IgE receptor (Fc∊RI): analysis of functional domains of the α-subunit with monoclonal antibodies. J Biol Chem. 1991;266:11245–51. [PubMed] [Google Scholar]
- 24.Kambe N, Kambe M, Chang HW, Matsui A, Min HK, Hussein M, et al. An improved procedure for the development of human mast cells from dispersed fetal liver cells in serum-free culture medium. J Immunol Methods. 2000;240:101–10. doi: 10.1016/s0022-1759(00)00174-5. [DOI] [PubMed] [Google Scholar]
- 25.Wojta J, Kaun C, Zorn G, Ghannadan M, Hauswirth AW, Sperr WR, et al. C5a stimulates production of plasminogen activator inhibitor-1 in human mast cells and basophils. Blood. 2002;100:517–23. doi: 10.1182/blood.v100.2.517. [DOI] [PubMed] [Google Scholar]
- 26.Werfel T, Oppermann M, Butterfield JH, Begemann G, Elsner J, Gotze O, et al. The human mast cell line HMC-1 expresses C5a receptors and responds to C5a but not to C5a(desArg) Scand J Immunol. 1996;44:30–6. doi: 10.1046/j.1365-3083.1996.d01-272.x. [DOI] [PubMed] [Google Scholar]
- 27.Ferrer M, Nakazawa K, Kaplan AP. Complement dependence of histamine release in chronic urticaria. J Allergy Clin Immunol. 1999;104:169–72. doi: 10.1016/s0091-6749(99)70129-6. [DOI] [PubMed] [Google Scholar]
- 28.Krug N, Tschernig T, Erpenbeck VJ, Hohlfeld JM, Kohl J. Complement factors C3a and C5a are increased in bronchoalveolar lavage fluid after segmental allergen provocation in subjects with asthma. Am J Respir Crit Care Med. 2001;164:1841–3. doi: 10.1164/ajrccm.164.10.2010096. [DOI] [PubMed] [Google Scholar]
- 29.Kiener HP, Baghestanian M, Dominkus M, Walchshofer S, Ghannadan M, Willheim M, et al. Expression of the C5a receptor (CD88) on synovial mast cells in patients with rheumatoid arthritis. Arthritis Rheum. 1998;41:233–45. doi: 10.1002/1529-0131(199802)41:2<233::AID-ART7>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 30.Ghannadan M, Baghestanian M, Wimazal F, Eisenmenger M, Latal D, Kargül G, et al. Phenotypic characterization of human skin mast cells by combined staining with toluidine blue and CD antibodies. J Invest Dermatol. 1998;111:689–95. doi: 10.1046/j.1523-1747.1998.00359.x. [DOI] [PubMed] [Google Scholar]
- 31.Ebertz JM, Hirshman CA, Kettelkamp NS, Uno H, Hanifin JM. Substance P-induced histamine release in human cutaneous mast cells. J Invest Dermatol. 1987;88:682–5. doi: 10.1111/1523-1747.ep12470339. [DOI] [PubMed] [Google Scholar]
- 32.Church MK, El-Lati S, Caulfield JP. Neuropeptide-induced secretion from human skin mast cells. Int Arch Allergy Appl Immunol. 1991;94:310–8. doi: 10.1159/000235393. [DOI] [PubMed] [Google Scholar]
- 33.Bischoff SC, Schwengberg S, Lorentz A, Manns MP, Bektas H, Sann H, et al. Substance P and other neuropeptides do not induce mediator release in isolated human intestinal mast cells. Neurogastroenterol Motil. 2004;16:185–93. doi: 10.1111/j.1365-2982.2004.00502.x. [DOI] [PubMed] [Google Scholar]
- 34.Patella V, Marinò I, Lampärter B, Arbustini E, Adt M, Marone G. Human heart mast cells: isolation, purification, ultrastructure, and immunologic characterization. J Immunol. 1995;154:2855–65. [PubMed] [Google Scholar]
- 35.Lawrence ID, Warner JA, Cohan VL, Hubbard WC, Kagey-Sobotka A, Lichtenstein LM. Purification and characterization of human skin mast cells. J Immunol. 1987;139:3062–9. [PubMed] [Google Scholar]
- 36.Columbo M, Horowitz EM, Botana LM, MacGlashan DW, Jr, Bochner BS, Gillis S, et al. The human recombinant c-kit receptor ligand, rhSCF, induces mediator release from human cutaneous mast cells and enhances IgE-dependent mediator release from both skin mast cells and peripheral blood basophils. J Immunol. 1992;149:599–608. [PubMed] [Google Scholar]
- 37.Kaplan AP. Clinical practice: chronic urticaria and angioedema. N Engl J Med. 2002;346:175–9. doi: 10.1056/NEJMcp011186. [DOI] [PubMed] [Google Scholar]
- 38.Bischoff SC, Sellge G, Lorentz A, Sebald W, Raab R, Manns MP. IL-4 enhances proliferation and mediator release in mature human mast cells. Proc Natl Acad Sci U S A. 1999;96:8080–5. doi: 10.1073/pnas.96.14.8080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsieh FH, Lam BK, Penrose JF, Austen KF, Boyce JA. T helper cell type 2 cytokines coordinately regulate immunoglobulin E-dependent cysteinyl leukotriene production by human cord blood-derived mast cells: profound induction of leukotriene C-4 synthase expression by interleukin 4. J Exp Med. 2001;193:123–33. doi: 10.1084/jem.193.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]