Abstract
Understanding of the chemical nature of the dioxygen moiety of oxyhemoglobin is crucial for elucidation of its physiological function. In the present work, direct Raman spectroscopic observation of both the Fe—O2 and O—O stretching modes unambiguously establishes the vibrational characteristics of the oxygen-bound heme moiety in the hemoglobins of Chlamydomonas eugametos and Synechocystis PCC6803. In addition to providing the resonance Raman assignment of the O—O stretching mode (1136 cm−1 for Chlamydomonas, 1133 cm−1 for Synechocystis) in an oxyhemoglobin with an iron-porphyrin, this study also reports unusually low frequencies for the Fe—O2 stretching modes (554 cm−1). The effect of strong hydrogen bonding to the bound oxygen is confirmed by changes in the frequency of the Fe—O2 stretching mode on mutation of distal residues. These findings suggest an enzymatic function rather than an oxygen transport role for these hemoglobins.
There has been a renewed interest in understanding the function of hemoglobins in recent years. Vertebrate hemoglobins, which are established as oxygen carrier proteins, have been proposed to have additional cellular activities (See for example, refs. 1 and 2). In the nonvertebrates, hemoglobins are expressed in a wide range of phyla (3–5). Although the nonvertebrate hemoglobins bind oxygen and other ligands just as the vertebrate hemoglobins do, the kinetic and structural properties of the oxygen complexes differ significantly, suggesting functional diversity. However, identification of the physiological functions remains elusive. One of the most vital clues to discerning hemoglobin function lies in the structure and stability of its oxygen complex. Despite the fact that hemoglobin is one of the most studied biological molecules, the energetics and structure of the oxy complexes of the many different hemoglobins that have been recently discovered remain poorly understood. For example, whereas the interactions of the distal residues with the bound oxygen play a central role in determining the stability and reactivity of the oxy complex, thus critically influencing the function of the protein, it is not established how the distal factors affect the orientation and strength of the Fe—O2 bond.
Resonance Raman spectroscopy is a powerful tool for studying hemeproteins. Assignments of several useful heme marker bands that are sensitive to oxidation, coordination, and spin states are known (6). Furthermore, assignments of the iron-ligand vibrational modes have been established for several intrinsic as well as exogenous ligands (see ref. 7). Determination of the frequency of the iron-ligand stretching mode not only gives a direct estimate of the bond strength, but also allows assessment of how the surrounding environment may affect the stability of such a bond. In hemeproteins, such information is very crucial to understand their function because the heme prosthetic group can display a variety of biological activities depending on the nature of its surroundings.
In the present work, we have studied the oxy complex of two novel hemoglobins by resonance Raman spectroscopy with the aim of assigning the Fe—O2 as well as the O—O stretching modes and determining their sensitivity to the environment in the heme pocket. There is no consensus on the assignment of the O—O stretching mode in oxyhemoglobins largely because the mode displays substantial vibrational coupling with other modes, resulting in very complex spectra. In addition, this mode has not been reported in the resonance Raman spectra of globins with an iron-containing heme coordinated by a proximal histidine, so indirect methods, such as replacing the heme iron by cobalt, were used (8) to measure the O—O stretching frequency. In independent studies, the intrinsic O—O stretching mode in cobalt-porphyrin-substituted hemoglobin was assigned by one group at 1122 and 1153 cm−1, representing two different species (8), by another group of investigators as a single species at 1134 cm−1 (9), and by a third group as a single conformation at 1139 cm−1 (10). The O—O stretching mode of iron-containing heme in hemoglobins is present in their infrared spectra, but the spectrum is also complicated by vibrational coupling. Caughey and coworkers (11) carried out a detailed and careful study of the multiple oxygen-sensitive lines in various hemoglobins and myoglobin and interpreted the infrared spectra as arising from two conformers of FeO2 with O—O stretching frequencies at 1155 and 1125 cm−1 (for HbA). However, Bruha and Kincaid (12), on the basis of elaborate studies on model complexes, provided an alternative interpretation, arguing that the multiple frequencies arose from vibrational coupling of internal modes of the proximal histidine, with a single O—O stretching mode having an inherent frequency of ≈1135 cm−1.
We report here the resonance Raman scattering spectrum of the O—O stretching mode in the oxygen complex of hemoglobins with an iron-containing heme. The mode was detected in the hemoglobins from both Chlamydomonas (13) and Synechocystis (14). We also report an unusually low frequency of the Fe—O2 stretching mode in these oxyhemoglobins and discuss the role of the heme pocket residues in modulating the vibrational frequencies to identify the hydrogen bond donors to the heme-bound oxygen. Confirmation of hydrogen bonding to the iron-bound oxygen is presented, judged by the shift of the frequency of the Fe—O2 stretching mode on single point mutations of the distal residues.
Materials and Methods
Preparations of the recombinant hemoglobins of Chlamydomonas eugametos (13) and Synechocystis PCC6803 (14) have been described elsewhere. The resonance Raman measurements were done as described previously (13). The oxy complex of Chlamydomonas and Synechocystis hemoglobins was prepared either by using a ferredoxin-based enzymatic reduction method or by exposing the desalted dithionite-reduced protein to air (13). OxyHb samples of either 16O2 or 18O2 (ICON, Mt. Marion, NY) isotopic composition were prepared in 100 mM sodium phosphate buffer, pH 7.4. All measurements reported here were carried out by using a laser excitation wavelength of 413.1 nm and a power of ≈2–5 mW at the sample. The sample cell was spun at 6000 rpm to avoid laser heating. Under these conditions, there was no evidence of any photodissociation of the Fe—O2 moiety.
Results
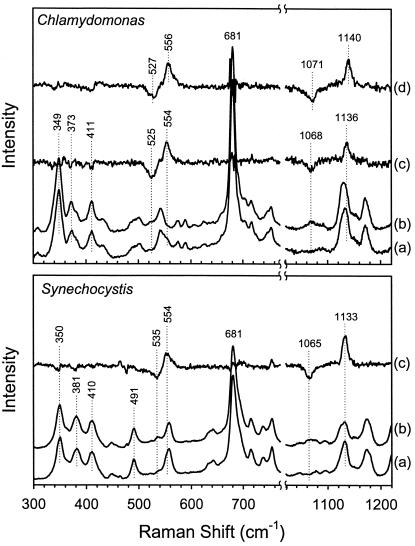
To assign the Fe—O2 (νFe—O2) and O—O (νO—O) stretching modes, the spectrum of the 16O2 adduct was compared with that of the 18O2 adduct as shown in Fig. 1. In Chlamydomonas hemoglobin (Fig. 1 Upper), the lines at 1136 and 554 cm−1 are assigned to the frequencies of the νFe—O2 and νO—O modes, respectively, determined from the difference spectrum (16O2 − 18O2, spectrum c). Similarly, in Synechocystis hemoglobin (Fig. 1 Lower), the lines at 1133 and 554 cm−1 are assigned to the νFe—O2 and νO—O modes, respectively, from the difference spectrum (16O2 − 18O2, spectrum c). The observation of isotope shifts of 68 cm−1 for the lines at 1136 cm−1 and 1133 cm−1 in Chlamydomonas and Synechocystis hemoglobins, respectively, agrees well with the calculated value (65 cm−1) for the stretching mode of an isolated O—O group. On the other hand, the isotope shift of 29 cm−1 for the Fe—O2 stretching mode in Chlamydomonas hemoglobin (Hb) is higher than the predicted value of 20 cm−1. Although there is no clear origin for this difference, it should be noted that, in heme oxygenase, a similarly large isotope shift has been attributed to a highly bent Fe—O—O conformation (15). In Synechocystis, the shift of 19 cm−1 is in agreement with the predicted value.
Figure 1.
Assignment of the frequencies of the O—O and Fe—O2 stretching modes in the resonance Raman spectra of Chlamydomonas and Synechocystis oxyhemoglobins. Spectra shown are (a) 16O2 in H2O, (b) 18O2 in H2O, and (c) the 16O2 − 18O2 difference spectrum in H2O. The 16O2 − 18O2 difference spectrum for the oxy complex of K(E10)A mutant of Chlamydomonas is shown in (d). The values of νFe—O2 and νO—O are, respectively, 1136 (1068 with 18O2) and 554 (525) cm−1, for wild-type Chlamydomonas Hb; 1140 (1071) and 556 (527) cm−1 for the K(E10)A mutant of Chlamydomonas Hb; and 1133 (1065) and 554 (535) cm−1 for wild-type Synechocystis Hb.
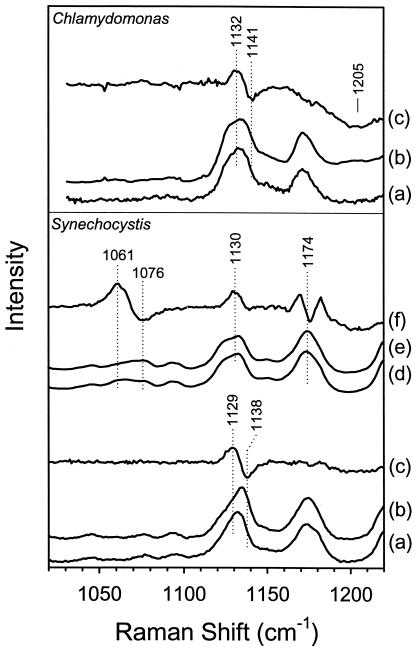
The H/D sensitivity of the νO—O region spectra of Chlamydomonas and Synechocystis oxyhemoglobins is shown in Fig. 2. The H2O − D2O difference spectra (spectra c) are obtained by subtracting spectra b (16O2 in D2O) from spectra a (16O2 in H2O). Difference features are observed in the νO—O region spectra of both hemoglobins (1132/1141 cm−1 in Chlamydomonas and 1129/1138 cm−1 in Synechocystis). Similarly, difference bands are observed at 1061/1076 cm−1 for the 18O2 derivative of Synechocystis Hb (spectra d–f). Thus, all of the observed νO—O mode isotope shifts are to higher frequency in D2O, as shown by the 8–15 cm−1 apparent upshift in the difference spectra. The absolute value of the shifts is substantially smaller than the maximum and minimum in the difference spectra for these H/D shifts and are estimated to be a few cm−1 by using mathematical methods described elsewhere (16). The νFe—O2 mode does not show any appreciable H/D sensitivity in either hemoglobin.
Figure 2.
The H/D sensitivity of the frequency of the O—O stretching mode in the resonance Raman spectra of Chlamydomonas and Synechocystis oxyhemoglobins. Spectra shown are (a) 16O2 in H2O, (b) 16O2 in D2O, and (c) the H2O − D2O difference spectrum for 16O2. The sensitivity of the νO—O mode to H2O/D2O is seen in the difference line at 1132/1141 cm−1 for Chlamydomonas HbO2, and at 1129/1138 cm−1 for Synechocystis HbO2. The broad line at ≈1205 cm−1 results from the D—O—D bending vibration of D2O. Also shown, for Synechocystis oxyHb, are (d) 18O2 in H2O, (e) 18O2 in D2O, and (f) the H2O − D2O difference spectrum for 18O2, which shows a difference feature at 1061/1076 cm−1.
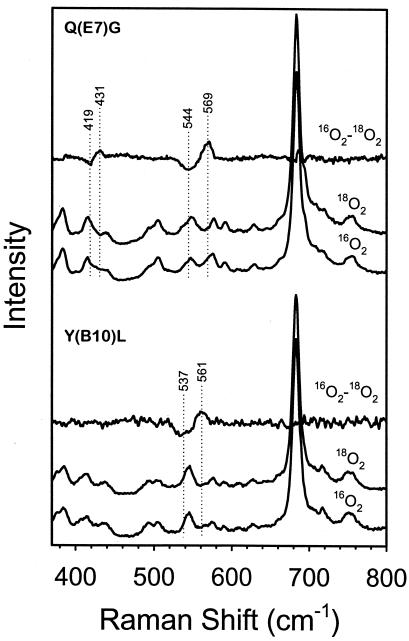
To identify the amino acid residues involved in hydrogen bonding to the iron-bound oxygen, three distal pocket mutants of Chlamydomonas Hb were studied. Single point mutations were made at the B10 (Y63), E7 (Q84), and E10 (K87) helical positions. The resonance Raman 16O2 − 18O2 difference spectra of the Y(B10)L and Q(E7)G oxyHb mutants of Chlamydomonas are presented in Fig. 3. The effects of the mutations are clearly seen in the frequency of the νFe—O2 mode, which increases from 554 cm−1 in the wild-type protein (Fig. 1) to 561 cm−1 in Y(B10)L and 569 cm−1 in Q(E7)G. However, in the oxy complex of K(E10)A (Fig. 1 Top, spectrum d), the νFe—O2 mode (556 cm−1) has a frequency similar to that found in the wild-type protein. The νO—O mode of K(E10)A is assigned at 1140 cm−1, close to that in the wild-type protein (1136 cm−1). In contrast, νO—O modes were not detected in the Y(B10)L and Q(E7)G mutants.
Figure 3.
Resonance Raman spectra of the oxy complex of Q(E7)G and the Y(B10)L mutants of Chlamydomonas oxyHb. The spectra with 16O2 and 18O2, as well as the difference spectra (16O2 − 18O2), are shown for both the mutants. The frequencies of the νFe—O2 mode are assigned at 569 (544 with 18O2) and 561 (537) cm−1 for Q(E7)G and Y(B10)L, respectively. The difference feature at 431/419 cm−1 in Q(E7)G may arise from the Fe—O—O bending mode (see refs. 23 and 35), which is not detected in Y(B10)L. The νO—O modes are not detected in these two mutants.
Discussion
The data reported here contain three major findings. (i) The frequency of the νO—O mode in oxyhemoglobin is identified in the resonance Raman spectra of iron-heme-containing globins. (ii) The frequencies of the νFe—O2 mode are lower than that reported in any other globin. (iii) Large effects of the distal E7 and B10 mutations on the νFe—O2 frequency suggest that these two residues are strong hydrogen bond donors to the bound oxygen in Chlamydomonas Hb.
The Frequency of the νO—O Mode.
The νO—O frequencies in heme proteins and model heme complexes all appear in the 1120–1200 cm−1 range, consistent with a ferric-iron-superoxide configuration (17) (νO—O ≈ 1100–1150 cm−1 in O2−, metal superoxides) and not a ferrous-iron-neutral oxygen complex (νO—O ≈ 1556 cm−1 in O2, molecular oxygen) (see Table 1). Location of the νO—O frequency at 1136 cm−1 in Chlamydomonas Hb and at 1133 cm−1 in Synechocystis Hb is consistent with this typical oxy-heme configuration. A low-spin ferric superoxide structure (Fe+3—O—O−) in these oxyHbs is also consistent with frequencies of the modes of the heme macrocycle oxidation state marker (ν4) and the spin-state marker (ν3) bands in Chlamydomonas oxyHb that appear at 1376 and 1502 cm−1, respectively (ν4 and ν3 for Synechocystis oxyHb are at 1374 and 1498 cm−1, respectively) (spectrum not shown), which are typical for low-spin ferric hemes. Similar frequencies of these two lines are observed in mammalian oxyHbs and oxymyoglobins. In ferrous 6-coordinate low-spin heme complexes (in which the axial ligand is not a π-acid, i.e., not O2, CO, or NO), ν4 and ν3 appear at ≈1360 and ≈1490 cm−1, respectively (13).
Table 1.
The Fe—O2 (νFe—O2)a and O—O (νO—O) stretching mode assignments of the oxy complexes of globins, other hemeproteins, and some selective model complexes
| Type | Protein/porphyrin | νFe—O2 | νO—O | Ref |
|---|---|---|---|---|
| Globin | Synechocystis Hbb | 554 | 1133 | This work |
| Chlamydomonas WT Hbc | 554 | 1136 | This work | |
| Chlamydomonas K(E10)A Hbc | 556 | 1140 | This work | |
| Chlamydomonas Y(B10)L Hbc | 561 | — | This work | |
| Chlamydomonas Q(E7)G Hbc | 569 | — | This work | |
| Human adult HbA | 568 | 1155, 1125 | 35, 11 | |
| Human adult HbA | ≈1135 | 12 | ||
| Paramecium Hbd | 563 | — | 26 | |
| Mycobacterium HbNe | 564 | — | 27 | |
| Ascaris Hbf | 570 | — | 31 | |
| Scapharca HbIg | 570 | — | 36 | |
| Horse Mb | 571 | — | 35 | |
| Elephant Mb | 572 | — | 37 | |
| Soybean legHbh | 576 | — | 38 | |
| Cobalt Hb (CoO2)i | — | 1122, 1153 | 8 | |
| Cobalt Hb (CoO2)i | — | 1134 | 9 | |
| Cobalt Hb (CoO2)i | — | ≈1139 | 10 | |
| Peroxidase | HRPj | ≈562 | — | 22 |
| Oxygenase | Heme oxygenase | 565 | — | 15 |
| Heme-Cu oxidase | Bovine aa3k | 568–572 | — | 35,39,40 |
| E. coli bo3l | 568 | 41 | ||
| Model heme-6C | TPP(Pip)m | 575 | 1157 | 18 |
| TpivP(1,2-Me2Im)n | 564 | 1159 | 17 | |
| TpivP(1-MeIm)n | 568 | 1159 | 17,19,20 | |
| [(Piv)2C12](1-MeIm)o | 563 | — | 21 | |
| [(Piv)2C9](1-MeIm)o | 560 | — | 21 | |
| [(Piv)2C8](1-MeIm)o | 563 | — | 21 | |
| Model heme-5C | TPPm | 508 | 1195 | 42 |
| TMPp | 522 | 1171 | 34 | |
| Model heme-6C (—S−)q | TpivP(C6HF4S−)q | 527 | 1140 | 24 |
| TpivP(C6F5S−)q | 536 | 1147 | 24 | |
| P450 (—Cys−)r | Cytochrome P450 | 540–541 | 1139–1140 | 23,24 |
| NOSs | — | 1135 | 43 | |
| O2 gas | 1556 | 44 | ||
| O2− (metal-O2−) | 1100–1150 | 44 |
The νFe—O2 frequency is from the 16O2—18O2 difference spectrum where available; Ref, reference; Hb, hemoglobin; Mb, myoglobin.
Synechocystis PCC6803 Hb.
Chlamydomonas eugametos Hb; WT, wild-type protein.
Paramecium caudatum Hb.
Mycobacterium tuberculosis HbN.
Ascaris suum Hb.
HbI of Scapharca inaequivalvis.
Soybean leghemoglobin.
Cobalt-porphyrin substituted hemoglobin; νCo—O2 appears at 537 cm−1.
Horseradish peroxidase.
Bovine aa3 cytochrome c oxidase.
Eschericia coli bo3-type quinol oxidase.
TPP, tetraphenylporphyrin; Pip, piperidine.
TpivP, meso-tetra(α,α,α,α-o-pivalamidophenyl)porphyrin (picket fence porphyrin); MeIm, methylimidazole.
(Piv)2Cn, α,α-5,15-[2,2′-(X)diphenyl]-α,α-10,20-bis(o-pivalamidophenyl)porphyrin (n = 8, X = octanediamido; n = 9, X = nonanediamido; n = 12; X = dodecanediamido).
TMP, 5,10,15,20-tetramesitylporphyrin.
S−-ligation in the proximal side.
P450 type heme proteins that contain proximal Cys− ligand.
Oxygenase domain of rat brain neuronal nitric oxide synthase. 5C, five-coordinate; 6C, six-coordinate.
Unusually Low Frequency for the νFe—O2 Mode.
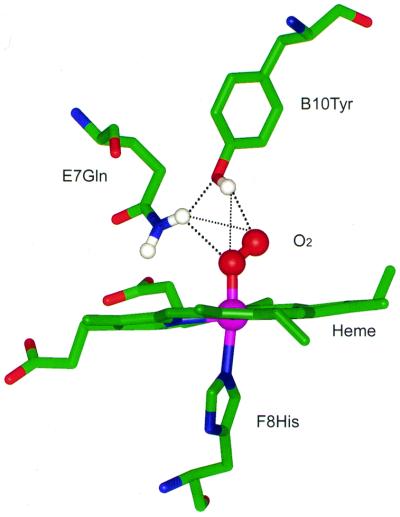
The νFe—O2 mode in Chlamydomonas and Synechocystis oxyHbs appears at an unusually low frequency (554 cm−1) compared with those in other hemeproteins containing an axial histidine ligand (see Table 1). This frequency is also the lowest among the axial imidazole-containing heme model compounds in solution (in the range of 560–576 cm−1) (17–21) and the peroxide complex of horseradish peroxidase (≈562 cm−1) (ref. 22; Table 1). However, for thiolate axial ligand-containing hemeproteins, such as cytochrome P450 in which the strength of the trans axial ligand (thiolate) is substantially higher in comparison with that in the histidine-ligated hemeproteins, the νFe—O2 band appears in a different frequency domain (≈540 cm−1) (refs. 23 and 24; Table 1). We attribute the significant lowering (≈14 cm−1) of the νFe—O2 frequency in both Chlamydomonas and Synechocystis oxyHbs relative to mammalian oxyHb (≈568 cm−1) to arise predominantly from relatively strong hydrogen bonding of the bound O—O moiety on the distal side of the heme. Both Chlamydomonas and Synechocystis hemoglobins contain a tyrosine at helical position B10 and a glutamine at E7, in the distal heme pocket (13, 14, 25). We postulate that these two residues serve as hydrogen bond donors (13, 14) to both the oxygen atoms in oxyHb (Fig. 4), which is also evident from mutagenesis data discussed below.
Figure 4.
Proposed structural model of the active site in Chlamydomonas and Synechocystis HbO2. Only selective residues in the vicinity of the heme are shown. The model was built by using the structural coordinates of HbO2 of Ascaris suum (1ash.pdb, Brookhaven Protein Data Bank). The dotted lines represent hydrogen bonds (thick line, strong hydrogen bond; thin line, weak hydrogen bond). The E10 helical positions (not shown here) are occupied by a Lys and a His, respectively, in Chlamydomonas and Synechocystis Hb.
Factors That Modulate the Frequency of the νFe—O2 Mode.
The strength of the Fe—O2 bond in hemeproteins may be modulated by (i) direct distal interactions, (ii) proximal effects, and (iii) heme effects. We show below that the distal interactions play the major role in lowering the frequency of the νFe—O2 mode in Chlamydomonas and Synechocystis oxyHbs as compared with mammalian hemoglobins.
In the distal pocket, direct hydrogen bonding interactions between the bound oxygen and neighboring residues play a dominant role in influencing the frequency of the νFe—O2 mode. Lowering of the νFe—O2 frequency (to ≈560 cm−1) indeed has been observed in oxy complexes of some amide-basket-handle hemes and attributed to intramolecular hydrogen bonding of the secondary amide group with the oxygen (21). In the oxy complexes of Paramecium (26) and Mycobacterium (HbN) (27) Hbs (Table 1), observations of relatively lower frequencies have been attributed to distal hydrogen bonding with the bound oxygen. We postulate that the greater reduction in the frequency of the νFe—O2 mode in Chlamydomonas oxyHb (≈14 cm−1 in comparison with mammalian oxyHb) is caused by relatively stronger distal side hydrogen bonding. The involvement of the distal groups in providing hydrogen bonds is confirmed by the mutagenesis data. The νFe—O2 frequency increases from 554 cm−1 in the wild-type protein to 561 cm−1 in Y(B10)L. In Q(E7)G, the νFe—O2 frequency goes all the way to 569 cm−1, similar to that of HbA (Table 1). Thus, relative to the wild-type protein, the hydrogen bonding to the oxygen is reduced in both mutant proteins. This conclusion based on the spectroscopic results is consistent with the oxygen kinetics data, which show a large increase in oxygen dissociation rate in these mutants (0.948 and 0.444 s−1, respectively, in Y(B10)L and Q(E7)G) in comparison to a very low rate (0.014 s−1) in the wild-type protein (13). Interestingly, mutation of the E7 glutamine causes the νFe—O2 frequency to move to the 569 cm−1 region that is typical for νFe—O2 in mammalian oxyhemoglobin (and oxymyoglobin), whereas mutation of the B10 tyrosine shifts the νFe—O2 frequency only halfway. Thus, it is very likely that E7Gln interacts primarily with the proximal oxygen atom and that B10Tyr interacts primarily with the terminal oxygen. With such a hydrogen-bonding pattern, the distal hydrogen bonding structure of Chlamydomonas and Synechocystis oxyHbs (Fig. 4) resembles that of Ascaris oxyHb, which also has an E7 glutamine and a B10 tyrosine (28); and from the crystal structure of Ascaris oxyHb (28), it has been shown that the glutamine forms a hydrogen bond to the proximal oxygen atom. It may be noted that, in the Hbs with this type of distal pocket, E7Gln also is in hydrogen bonding contact with B10Tyr (25, 28), thus providing extra stability to the hydrogen bond network.
The contribution to the distal hydrogen-bonding network from another residue in Chlamydomonas Hb, E10Lys, located close to the distal pocket, was also evaluated in this work. Mutation of this residue has a large effect on the heme structure in the absence of any exogenous ligand (29). However, in the oxy complex of K(E10)A, only minor perturbations on the FeO2 structure (increase in νFe—O2 and νO—O by 2 and 4 cm−1, respectively) relative to the wild-type proteins were observed. These small changes in the Fe—O—O modes are consistent with the observation of an oxygen dissociation rate (0.022 s−1) in K(E10)A similar to that in the wild-type protein (0.014 s−1) (13). In another exogenous ligand-bound form, the ferric cyanide derivative, mutation of E10Lys has no significant effect on the Fe—CN stretching frequency whereas the B10Tyr and E7Gln have significant effects on the Fe—CN mode (30). Thus, the binding of exogenous ligands (in both ferric and ferrous oxidation states) significantly modifies the structure of the heme pocket of Chlamydomonas (and also Synechocystis) Hb, demonstrating the adaptability of the heme pocket in these Hbs.
The H/D Sensitivity of the νO—O Mode.
The observation of a frequency increase of the νO—O mode in D2O relative to H2O (Fig. 2) in the present study may suggest that the upshift is caused by hydrogen bonding to the bound oxygen, assuming that there are no other latent changes in the spectra caused by the H/D exchange. An increase in the frequency of the νO—O mode in D2O in both the proteins could result from strong hydrogen bonding interactions that might cause an anomalous shift because of higher rigidity of the Fe—O—O⋅⋅⋅D— assembly compared with the Fe—O—O⋅⋅⋅H— assembly (31). Such an interpretation, however, could be flawed if some H/D-sensitive vibrational modes (either resonance enhanced or not) lie near the O—O stretching mode, which might couple with νO—O, causing anomalous shifts. Such frequency shifts may not reflect inherent sensitivity of νO—O to H/D. In a series of model cobalt-porphyrin oxy complexes, Kincaid and coworkers (10, 12, 32) have established that such vibrational couplings of the internal modes of trans axial ligands with νO—O are very abundant in the complexes and complicate the spectral pattern. They successfully extracted the inherent frequencies of the vibrational modes from the observed coupled frequencies, and provided an alternate interpretation of the H/D sensitivity of the νO—O mode in cobalt-porphyrin-substituted hemoglobins (9), concluding that the H/D-induced spectral changes arise solely from vibrational coupling and not from hydrogen bonding with the bound oxygen (10, 12). Thus, the H/D sensitivity observed here for the 16O2 complex, as well as for the 18O2 complex (Fig. 2), may be attributed either to vibrational coupling of νO—O with histidine internal modes or to hydrogen bonding of the distal residues with the bound oxygen. At this time, in the absence of internal mode assignments of proximal histidine in these hemoglobins, discrimination between these two possibilities is not straightforward. However, strong hydrogen bonds indeed exist between the distal residues and the heme-bound dioxygen in both of these hemoglobins, as indicated from the studies of the νFe—O2 mode discussed in earlier sections.
Correlation Between the Frequencies of the νO—O and νFe—O2 Modes.
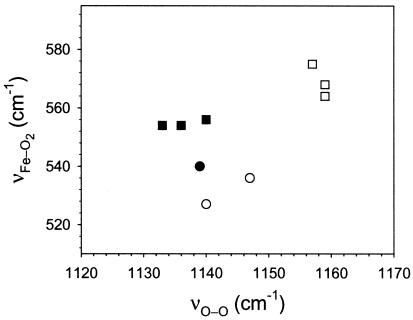
The correlation between the frequencies of Fe—XO and X—O (where X = C, N, O) in heme-ligand complexes has been a topic of considerable interest. Although there are ample data on the FeCO derivative available in the literature, studies on FeO2 and FeNO are limited. In the FeO2 complex of the hemeproteins containing an axial imidazole ligand, such a correlation could not be evaluated because of the non-availability of the νO—O frequency. However, for model heme complexes, some data are available, and it has been proposed that an inverse correlation between the νFe—O2 and νO—O frequencies exists just as in FeCO, albeit with a different slope (24, 33, 34). It is also argued that the 5-coordinate (no proximal ligand) and 6-coordinate FeO2 complexes lie in the same correlation line (34). The frequencies of νFe—O2 and νO—O for hemoglobins obtained from the present work are plotted along with the 6-coordinate model heme complexes (Fig. 5) to evaluate the correlation. From the data, no meaningful correlation between νFe—O2 and νO—O frequencies could be identified. In hemeproteins, at least in the present examples of Chlamydomonas and Synechocystis Hbs, distal interactions with the bound oxygen modulate the two frequencies. However, unlike CO, because of the small dπ—π* back-bonding in the FeO2 derivatives, modulation of the νFe—O2 frequency is not inversely correlated with the νO—O frequency.
Figure 5.
A plot of the frequencies of the νFe—O2 vs. νO—O modes for the oxy complexes of hemeproteins and heme model compounds containing histidine as the proximal ligand. The frequencies are listed in Table 1. Chlamydomonas and Synechocystis Hb (filled square), six-coordinate heme model complexes (open square), Cytochrome P450 (filled circle), and 6-coordinate model heme complexes containing S−-ligation on the proximal side (open circle). No correlation between the frequencies of the νFe—O2 and νO—O modes is apparent from this plot.
Resonance Raman Enhancement of νO—O Mode.
The reason the simultaneous enhancement of the νFe—O2 and νO—O lines does not occur in the resonance Raman spectra of most oxyhemoglobins (and other histidine-ligated hemeproteins) but occurs in cobalt porphyrin-substituted oxyhemoglobins was suggested to be due to a charge-transfer transition from the occupied π* orbital to an empty antibonding σ* orbital (8). Following this suggestion, it was argued that, in the oxy complex of P450, strong electron donation from the axial thiolate ligand to the metal π* orbital makes the Fe(II) resemble Co(II), and thus both νFe—O2 and νO—O bands are enhanced (24). Obviously, this argument does not explain the simultaneous enhancement of both the modes in Chlamydomonas oxyHb because its Fe—His bond (232 cm−1) is much weaker than the Fe—Cys bond in P450 (351 cm−1) (7). Whereas it remains to be clarified as to what extent the proximal ligand influences the enhancement of the νFe—O2 and νO—O modes in oxyHb, we believe that distal interactions can contribute to the enhancement mechanism by altering the energy levels of the molecular orbitals of the FeO2 group. This conclusion is consistent with the silence of the νO—O mode in the resonance Raman spectra of oxy complexes of the two distal pocket mutants [Y(B10)L and Q(E7)G] of Chlamydomonas, in which the stereochemistry of the Fe—O—O moiety could be significantly different from that in the wild-type protein. It will be interesting to find out precisely what factors render the intensity of the O—O stretching mode weak in some hemeproteins, while it is moderately or strongly enhanced in others.
Conclusions
The present work reports the simultaneous detection of the O—O and Fe—O2 stretching modes in oxyhemoglobin, which until now had not been detected by resonance Raman spectroscopy in any iron hemeprotein containing a proximal histidine. The detection of νO—O at 1133−1140 cm−1 in Chlamydomonas and Synechocystis Hbs with simple 16O2 − 18O2 difference spectra, in contrast to those reported in cobalt-substituted globins, argues strongly for the assignment made by Kincaid and coworkers. Namely, the inherent O—O stretching mode is located in the 1133–1140 cm−1 region, and the extra lines seen in the infrared spectra and the resonance Raman spectra of the cobalt-substituted porphyrins are a consequence of mode coupling. It is also postulated that the bound oxygen is hydrogen-bonded not only to the terminal oxygen atom but also to the proximal atom, as has been shown by the crystal structure of Ascaris Hb. Deciphering the characteristics of the Fe—O and O—O bonds provides valuable insights not only for the mechanism of dioxygen stabilization and transport by oxyhemoglobin, but also for the dioxygen activation by histidine-ligated heme-enzymes such as cytochrome c oxidase and horseradish peroxidase. Dioxygen activation by cytochrome c oxidase is facilitated by the presence of redox-active groups such as CuB in close proximity to the heme-bound oxygen. In horseradish peroxidase, the O—O splitting after the peroxide reaction of the enzyme is aided by a trans effect from the proximal imidazolate as well as distal factors (hydrogen bonding of O—O with distal residues). The data reported here offer a clue for the understanding of the cellular function of these novel non-vertebrate hemoglobins. Strong hydrogen bonding of both oxygen atoms of FeO2 in Chlamydomonas as well as in Synechocystis Hb make it unlikely that they could serve as oxygen transport proteins because, with such a low oxygen off-rate, the O2 delivery would be very inefficient. Instead, the strong polar environment around the bound oxygen suggests that these proteins may activate dioxygen for an as yet undetermined enzymatic process. Experiments on the turnover rate of oxygen by the protein in a reducing environment will be a useful starting point to elucidate the mechanism of these novel proteins.
Acknowledgments
This work was supported by National Institutes of Health Grants GM54806 and GM54812 (to D.L.R.) and Natural Sciences and Engineering Research Council of Canada Grant 06P0046306 (to M.G.).
Abbreviations
- Hb
hemoglobin
- H/D
H2O/D2O
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Liu L, Zeng M, Stamler J S. Proc Natl Acad Sci USA. 1999;96:6643–6647. doi: 10.1073/pnas.96.12.6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stamler J S, Jia L, Eu J P, McMahon T J, Demchenko I T, Bonaventura J, Gernert K, Piantadosi C A. Science. 1997;276:2034–2037. doi: 10.1126/science.276.5321.2034. [DOI] [PubMed] [Google Scholar]
- 3.Wittenberg J B, Wittenberg B A. Annu Rev Biophys Biophys Chem. 1990;19:217–241. doi: 10.1146/annurev.bb.19.060190.001245. [DOI] [PubMed] [Google Scholar]
- 4.Hardison R. J Exp Biol. 1998;201:1099–1117. doi: 10.1242/jeb.201.8.1099. [DOI] [PubMed] [Google Scholar]
- 5.Weber, R. E. & Vinogradov, S. N. (2001) Physiol. Rev., in press. [DOI] [PubMed]
- 6.Hu S, Smith K M, Spiro T G. J Am Chem Soc. 1996;118:12638–12646. [Google Scholar]
- 7.Wang J, Caughey W S, Rousseau D L. In: Methods in Nitric Oxide Research. Feelish M, Stamler J S, editors. New York: Wiley; 1996. pp. 427–454. [Google Scholar]
- 8.Tsubaki M, Yu N T. Proc Natl Acad Sci USA. 1981;78:3581–3585. doi: 10.1073/pnas.78.6.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitagawa T, Ondrias M R, Rousseau D L, Ikeda-Saito M, Yonetani T. Nature (London) 1982;298:869–871. doi: 10.1038/298869a0. [DOI] [PubMed] [Google Scholar]
- 10.Proniewicz L M, Kincaid J R. J Am Chem Soc. 1990;112:675–681. [Google Scholar]
- 11.Potter W T, Tucker M P, Houtchens R A, Caughey W S. Biochemistry. 1987;26:4699–4707. doi: 10.1021/bi00389a016. [DOI] [PubMed] [Google Scholar]
- 12.Bruha A, Kincaid J R. J Am Chem Soc. 1989;110:6006–6014. doi: 10.1021/ja00226a014. [DOI] [PubMed] [Google Scholar]
- 13.Couture M, Das T K, Lee H C, Peisach J, Rousseau D L, Wittenberg B A, Wittenberg J B, Guertin M. J Biol Chem. 1999;274:6898–6910. doi: 10.1074/jbc.274.11.6898. [DOI] [PubMed] [Google Scholar]
- 14.Couture M, Das T K, Savard P-Y, Ouellet Y, Wittenberg J B, Wittenberg B A, Rousseau D L, Guertin M. Eur J Biochem. 2000;267:4770–4780. doi: 10.1046/j.1432-1327.2000.01531.x. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi S, Ishikawa K, Takeuchi N, Ikeda-Saito M, Yoshida T, Rousseau D L. J Am Chem Soc. 1995;117:6002–6006. [Google Scholar]
- 16.Rousseau D L. J Raman Spec. 1981;10:94–99. [Google Scholar]
- 17.Collman J P, Brauman J I, Halbert T R, Suslick K S. Proc Acad Natl Sci USA. 1976;73:3333–3337. doi: 10.1073/pnas.73.10.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wagner W D, Paeng I R, Nakamoto K. J Am Chem Soc. 1988;110:5556–5557. [Google Scholar]
- 19.Burke J M, Kincaid J R, Peters S, Gagne R R, Collman J P, Spiro T G. J Am Chem Soc. 1978;100:6083–6088. [Google Scholar]
- 20.Walters M A, Spiro T G, Suslick K S, Collman J P. J Am Chem Soc. 1980;102:6857–6858. [Google Scholar]
- 21.Desbois A, Momenteau M, Lutz M. Inorg Chem. 1989;28:825–834. [Google Scholar]
- 22.Van Wart H E, Zimmer J. J Biol Chem. 1985;260:8372–8377. [PubMed] [Google Scholar]
- 23.Macdonald I D G, Sligar S G, Christian J F, Unno M, Champion P M. J Am Chem Soc. 1999;121:376–380. [Google Scholar]
- 24.Hu S, Schneider A, Kincaid J R. J Am Chem Soc. 1991;113:4815–4822. [Google Scholar]
- 25.Pesce A, Couture M, Dewilde S, Guertin M, Yamauchi K, Ascenzi P, Moens L, Bolognesi M. EMBO J. 2000;19:2424–2434. doi: 10.1093/emboj/19.11.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Das T K, Weber R E, Dewilde S, Wittenberg J B, Wittenberg B A, Yamauchi K, Van Hauwaert M-L, Moens L, Rousseau D L. Biochemistry. 2000;39:14330–14340. doi: 10.1021/bi001681d. [DOI] [PubMed] [Google Scholar]
- 27.Couture M, Yeh S R, Wittenberg B A, Wittenberg J B, Ouellet Y, Rousseau D L, Guertin M. Proc Natl Acad Sci USA. 1999;96:11223–11228. doi: 10.1073/pnas.96.20.11223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang J, Kloek A P, Goldberg D E, Mathews F S. Proc Natl Acad Sci USA. 1995;92:4224–4228. doi: 10.1073/pnas.92.10.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Das T K, Couture M, Lee H C, Peisach J, Rousseau D L, Wittenberg B A, Wittenberg J B, Guertin M. Biochemistry. 1999;38:15360–15368. doi: 10.1021/bi991237e. [DOI] [PubMed] [Google Scholar]
- 30.Das T K, Couture M, Guertin M, Rousseau D L. J Phys Chem B. 2000;104:10750–10756. [Google Scholar]
- 31.Das T K, Friedman J M, Kloek A P, Goldberg D E, Rousseau D L. Biochemistry. 2000;39:837–842. doi: 10.1021/bi9922087. [DOI] [PubMed] [Google Scholar]
- 32.Proniewicz L M, Bruha A, Nakamoto K, Kyuno E, Kincaid J R. J Am Chem Soc. 1989;111:7050–7056. [Google Scholar]
- 33.Mizutani Y, Hashimoto S, Tatsuno Y, Kitagawa T. J Am Chem Soc. 1990;112:6809–6814. [Google Scholar]
- 34.Vogel K M, Kozlowski P M, Zgierski M Z, Spiro T G. J Am Chem Soc. 1999;121:9915–9921. [Google Scholar]
- 35.Hirota S, Ogura T, Appleman E H, Shinzawa-Itoh K, Yoshikawa S, Kitagawa T. J Am Chem Soc. 1994;116:10564–10570. [Google Scholar]
- 36.Song S, Boffi A, Chiancone E, Rousseau D L. Biochemistry. 1993;32:6330–6336. doi: 10.1021/bi00076a005. [DOI] [PubMed] [Google Scholar]
- 37.Kerr E A, Yu N T, Bartnicki D E, Mizukami H. J Biol Chem. 1985;260:8360–8365. [PubMed] [Google Scholar]
- 38.Irwin M J, Armstrong R S, Wright P E. FEBS Lett. 1981;133:239–243. [Google Scholar]
- 39.Han S, Ching Y C, Rousseau D L. Nature (London) 1990;348:89–90. doi: 10.1038/348089a0. [DOI] [PubMed] [Google Scholar]
- 40.Varotsis C, Woodruff W H, Babcock G T. J Biol Chem. 1990;265:11131–11136. [PubMed] [Google Scholar]
- 41.Hirota S, Mogi T, Ogura T, Hirano T, Anraku Y, Kitagawa T. FEBS Lett. 1994;352:67–70. doi: 10.1016/0014-5793(94)00919-8. [DOI] [PubMed] [Google Scholar]
- 42.Proniewicz L M, Paeng I R, Nakamoto K. J Am Chem Soc. 1991;113:3294–3303. [Google Scholar]
- 43.Couture M, Stuehr D J, Rousseau D L. J Biol Chem. 2000;275:3201–3205. doi: 10.1074/jbc.275.5.3201. [DOI] [PubMed] [Google Scholar]
- 44.Herzberg G. Spectra of Diatomic Molecules, Molecular Spectra and Molecular Structure. Vol. 1. New York: Van Nostrand; 1950. [Google Scholar]