Abstract
Monitoring the passive transfer of immunoglobulins from colostrums particularly in noncentrifuged samples can be useful for calf health management programs. The results of total solids refractometry from centrifuge and noncentrifuge harvested sources of serum were highly correlated (R2 = 0.95). Results from a digital and a hand-held refractometer were highly correlated (R2 = 0.96).
Résumé
Comparaison des méthodes de récolte de sérum et des types de réfractomètres pour la détermination des solides totaux afin d’estimer l’échec du transfert passif chez les veaux. La surveillance du transfert passif des immunoglobulines du colostrum, particulièrement dans les échantillons non centrifugés, peut être utile dans la gestion de programmes de santé des veaux. Les résultats de la réfractométrie des solides totaux provenant de sérums centrifugés et non centrifugés montraient une forte corrélation (R2 = 0.95). Les résultats provenant d’un réfractomètre digital et d’un réfractomètre manuel étaient en forte corrélation (R2 = 0.96).
(Traduit par Docteur André Blouin)
As an important source of nutrients, vitamins, minerals, energy, and protein, colostrum is essential to health and survival of neonatal calves (1). Calves rely on the ingestion and absorption of maternal immunoglobulins in colostrum for immunity against specific pathogens during the neonatal period (1). Success of the passive transfer of immunoglobulins is determined by the amount, quality, and absorption of colostrum ingested by calves within 24 h after birth (2,3).
Many techniques are available to measure failure of passive transfer (FPT). Radial immunodiffusion and enzyme-linked immunosorbant assay (ELISA) directly measure serum immunoglobulin (Ig)G concentration (3). In newborn calves, serum total solids (TS) refractometry, sodium sulfite turbidity test, zinc sulfate turbidity test, serum gamma-glutamyl transferase activity, whole blood glutaraldehyde gelation can all be used to estimate serum IgG concentration indirectly (3). Measuring passive transfer can be a challenging, and time consuming onfarm endeavor (2).
Refractometry is a technique for measuring FPT that can be adapted for on-farm use. In brief, a beam of light is shone through a serum sample. The refractometer measures how much of that light is refracted from the total proteins in the sample. In calves, from 1 to 7 d of age, the greatest constituents of total proteins are immunoglobulins (4). Thus, the total proteins measured by refractometry can be used to estimate the passive transfer of immunoglobulins (4). Although refractometry for serum TS is an easy test to perform, it requires harvesting serum from blood samples. The necessity of having a centrifuge on-farm to harvest serum has kept this method from widespread adoption. In the current study, serum TS refractometry results were compared between duplicate samples that were centrifuged and noncentrifuged prior to harvesting the serum. In addition, since a digital refractometry device is now available, it was compared to the standard hand-held device.
Commercial dairy herds from across southern Ontario that were involved in a large project on the risk factors for and prevention of Cryptosporidia parvum in dairy calves were recruited to participate in the current study. Based upon herd size and calving frequency, each herd was visited on either a weekly or biweekly basis for the study period (June 1, 2004 to July 31, 2004). Duplicate blood samples were collected by jugular venipuncture on all calves between 1 and 7 d of age into tubes without anticoagulant and allowed to clot. One blood sample, from each calf, was centrifuged and the serum subsequently harvested and refrigerated. The duplicate sample was allowed to clot and then refrigerated. The sample color was recorded as an indication of sample hemolysis. The centrifuged serum and the noncentrifuged serum were analyzed concurrently by digital refractometry (Digital Refractometer # 300027; Sper Scientific, Scottsdale, Arizona, USA) 1 to 6 d following sample collection (the noncentrifuged serum was aspirated from around the clot).
A subset of centrifuged serum samples were also analyzed by hand-held refractometer (SPR-Ne; Atago Company, Kirkland, Washington, USA). A 2-tailed Fisher’s exact test was used to determine the statistical association between refractometry TS results on serum extracted from centrifuged versus noncentrifuged samples. In addition, the refractometry TS results for the 2 serum extraction methods were plotted and a Spearman rank coefficient of correlation determined. Finally, the TS results from centrifuged samples, as measured by digital refractometry, were plotted against the TS results, as measured by a hand-held refractometry instrument.
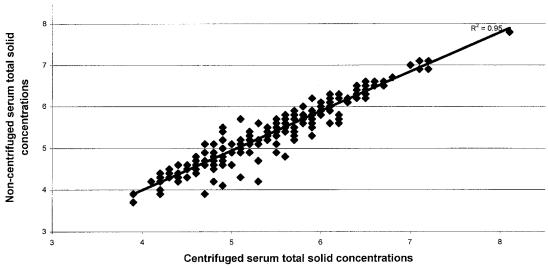
A total of 234 calves from 61 different dairy farms were enrolled in this study. The mean serum TS concentration, as measured by refractometry, was 5.5 g/dL (range from 3.9 to 8.1 g/dL) for the centrifuged serum and 5.4 g/dL (range from 3.7 to 7.8 g/dL) for the noncentrifuged serum. The serum TS results as measured by digital refractometry of serum from centrifuged samples versus serum collected from noncentrifuged samples are plotted in Figure 1. The Spearman rank correlation coefficient was 0.95. The frequency of hemolysis in both the centrifuged and noncentrifuged samples was 6%.
Figure 1.
Scatterplot of total solid refractometry results by 2 methods of serum harvesting.
Even though it is generally felt that using less than 5.0 g/dL as the cut-off value for defining FPT results in high specificity and low sensitivity (5), 25% and 28% of the serum TS values from centrifuged and noncentrifuged samples, respectively, were identified as having FPT, using this cut-off. A 2-tailed Fisher’s exact test indicated that the TS results, in categories of success and failure of passive transfer, did not differ significantly between the serum harvesting methods (P = 0.53).
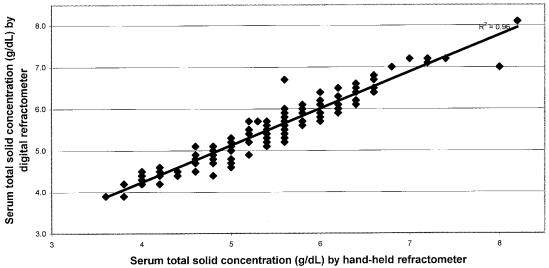
On a subset of 164 calves in this study, the serum from the centrifuged samples was measured for TS using both the digital and the hand-held refractometry instruments. The results for these 2 instruments on the same samples are plotted in Figure 2. The Spearman rank correlation coefficient applied to these data was 0.96.
Figure 2.
Scatterplot of centrifuged serum total solid concentration by 2 refractometers.
The Spearman rank coefficient assessing the linearity between TS results from centrifuged and noncentrifuged methods of serum harvesting was 0.95, indicating a high degree of correlation between the TS results from the 2 sources of serum. Furthermore, with a Spearman rank coefficient of 0.96, either the digital or hand-held refractometry instruments seem to be appropriate for measurement of serum TS to assess passive transfer.
Most dairy professionals consider a TS concentration of less than 5.0 g/dL (4) to indicate a failure of passive transfer. It is noteworthy that approximately 25% of calves on this large number of Ontario farms had FPT and, thus, increased risk of mortality (6).
The results of this study suggest that TS refractometry, measured in noncentrifuged serum, can be used successfully to identify calves at risk of FPT. Two refractometry instruments did not yield significantly different serum TS results. The importance of sample temperature at the time of refractometry reading and the potential impact of sample hemolysis are topics for future studies not addressed in the current research. Furthermore, studies to determine the impact of these various factors on the test characteristics of serum TS refractometry against a gold standard direct measure of immunoglobulin content, such as radial immunodiffusion, are warranted.
Acknowledgments
The contributions of Jen Wilstra, Beth Sockovie, Krista Belecky, and Erin Vernooy are gratefully acknowledged. CVJ
Footnotes
Reprints will not be available from the authors.
The financial contribution of the 2004 Ontario Veterinary College (OVC) Leadership Program is gratefully acknowledged.
References
- 1.McBeath DG, Penhale WJ, Logan EF. An examination of the influence of husbandry in the plasma immunoglobulin level of the newborn calf using a rapid refractometer test for assessing immunoglobulin content. Vet Rec. 1971;88:266–270. doi: 10.1136/vr.88.11.266. [DOI] [PubMed] [Google Scholar]
- 2.Quigley JD. 2000. Calf Note #62 — Calf Age, Total Protein, and FPT in Calves, [Online], Available at http://www.calfnotes.com Last accessed March 27, 2006.
- 3.Weaver DM, Tyler JW, VanMetre DC, Hostetler DE, Barrington GM. Passive transfer of colostral immunoglobulins in calves. J Vet Intern Med. 2000;14:569–577. doi: 10.1892/0891-6640(2000)014<0569:ptocii>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 4.Quigley JD. 2001. Calf Note #39 — Using a Refractometer, [Online], Available at http://www.calfnotes.com Last accessed March 27, 2006.
- 5.Tyler JW, Hancock DD, Parish SM, et al. Evaluation of three assays for failure of passive transfer in calves. J Vet Intern Med. 1996;10:304–307. doi: 10.1111/j.1939-1676.1996.tb02067.x. [DOI] [PubMed] [Google Scholar]
- 6.Tyler JW, Hancock DD, Thorne JG, Gay CC, Gay JM. Partitioning the mortality risk associated with inadequate passive transfer of colostral immunoglobulins in dairy calves. J Vet Intern Med. 1999;13:335–337. doi: 10.1892/0891-6640(1999)013<0335:ptmraw>2.3.co;2. [DOI] [PubMed] [Google Scholar]