Abstract
A 20-month-old German shepherd with primary pancreatic acinar atrophy and exocrine pancreatic insufficiency that was treated with pancreatic enzyme supplementation, vitamin B12, and cimetidine developed oral bleeding. Following discontinuation of the cimetidine, increased preincubation of the enzymes with the food, and symptomatic therapy for the ulceration, the dog’s condition improved.
Résumé
Ulcération buccale et saignement associés à l’apport complémentaire d’enzymes pancréatiques chez un berger allemand atteint d’atrophie des acini pancréatiques. Un Berger allemand âgé de 20 mois atteint d’atrophie primaire des acini pancréatiques et d’insuffisance pancréatique exocrine a développé un saignement buccal après avoir été traité par l’apport complémentaire d’enzymes pancréatiques, de vitamine B-12 et de cimétidine. Suite à l’arrêt de la cimétidine, de l’augmentation de la préincubation des enzymes dans la nourriture et d’une thérapie symptomatique des ulcères, l’état de l’animal s’est amélioré.
(Traduit par Docteur André Blouin)
A 20-month-old, 27.8 kg, intact male German shepherd was referred to the Small Animal Clinic at the Western College of Veterinary Medicine with a 2-month history of chronic diarrhea with occasional fecal accidents in the house; marked flatulence; audible borborygmi; and weight loss, despite having a good appetite. The dog was first seen by his referring veterinarian (RDVM), 6 wk prior to referral, and was started on a bland potato diet trial for 10 d. No response was seen, so the owners were instructed to switch him to an easily digested dog food (Sensitive Stomach Adult Dry, Hill’s Science Diet; Hills Pet Nutrition, Topeka, Kansas, USA); a 2-week course of metronidazole, 16 mg/kg BW, PO, q12h, was also prescribed. No response was noted. A blood analysis, 2 wk prior to referral, showed a mild neutropenia (2.7 × 109/L; reference range 3.0 to 10.0 × 109/L), mild hyper-kalemia (6.3 mmol/L; reference range 3.8 to 5.6 mmol/L) with a sodium to potassium ratio of 23:1, mild hyperphosphatemia (2.67 mmol/L; reference range 0.63 to 2.41 mmol/L), mild hypocholesterolemia (2.44 mmol/L; reference range 2.7 to 5.94 mmol/L), and mild elevations in multiple liver enzymes, including alkaline phosphatase (ALP) (95 U/L; reference range 9 to 90 U/L), alanine aminotransferase (ALT) (228 U/L; reference range 19 to 59 U/L), and sorbital dehydrogenase (SDH) (35 U/L; reference range 0 to 4 U/L). Pre- and post-prandial bile acids were within normal limits. A 2-week trial of prednisone, 1.5 mg/kg BW, PO, q12h, for a suspicion of inflammatory bowel disease was prescribed and lack of improvement prompted referral. Primary differential diagnoses for the dog’s clinical signs included exocrine pancreatic insufficiency (EPI) due to pancreatic acinar atrophy (PAA), inflammatory bowel disease, gastrointestinal neoplasia, parasitic or infectious causes of chronic diarrhea, and hypoadrenocorticism.
Case description
On physical examination, the dog was in poor body condition (BCS 1/5); he was a unilateral cryptorchid and had fecal staining evident around his perineum. A rectal examination revealed yellow, very soft stool, which the owner described as having a “cow-paddy” consistency. A complete blood (cell) count (CBC), serum biochemical analysis, urinalysis, fecal floatation, fecal culture, fecal fluorescent antibody (IFA) testing for Giardia sp. and Cryptosporidium spp. and abdominal radiographs and ultrasonographs were performed. Serum was submitted for trypsin-like immunoreactivity (TLI) determination (VitaTech International, Tustin, California, USA). Results from the CBC and urinalysis were unremarkable. Results from the serum biochemical analysis showed a low urea level (3.3 mmol/L; reference range 3.5 to 11.4 mmol/L), moderate hypocholesterolemia (1.35 mmol/L; reference range 2.7 to 5.94 mmol/L), and mild elevations in all liver enzymes, including ALP (156 U/L; reference range 9 to 90 U/L), ALT (246 U/L; reference range 19 to 59 U/L), gamma glutamyl transferase GGT (13 U/L; reference range 0 to 8 U/L), and SDH (23 U/L; reference range 0 to 4 U/L). Results from fecal floatation and IFA for Giardia sp. and Cryptosporidium spp. were negative. Results from the fecal culture were negative for Salmonella spp., Campylobacter jejuni, and Clostridium spp. but 4+ Streptococcus spp. and 3+ Escherichia coli were isolated. The latter were not considered pathogenic. Abdominal radiographs were nondiagnostic because of the dog’s lack of adipose tissue. Abdominal ultrasonographs revealed dilation of many of the hepatic venules, mild peritoneal effusion, and a prominent tortuous vessel near the left kidney, the significance of which remains unknown but which was less prominent on a follow-up ultrasonograph a day later. The layering and thickness of the gastric and intestinal walls were within normal limits. The dog was dewormed with fenbendazole (Intervet Canada, Whitby, Ontario), 50 mg/kg BW, PO, q24h for 5 d, and metronidazole (Apometronidazole; Apotex, Toronto, Ontario), 15 mg/kg PO BW q12h for 14 d, was prescribed for suspected secondary small intestinal bacterial overgrowth (SIBO). Ten days after initial referral, exocrine pancreatic insufficiency (TLI < 1 ng/mL; reference range 5 to 35 ng/mL) was confirmed by the TLI test. Given the dog’s signalment and the lack of supportive clinical or ultrasonographic signs of chronic pancreatitis, a primary diagnosis of pancreatic acinar atrophy (PAA) was made. At this time, the dog was started on pancreatic enzyme supplementation (Pancrease-V Powder; Bioniche, Belleville, Ontario) at 1.5 teaspoons to be mixed with each meal. The dog’s diet (Sensitive Stomach Adult Dry, Hill’s Science Diet) was not changed.
Six weeks after initial referral, the dog returned for a recheck. He had gained 3 kg in body weight but continued to have occasional fecal accidents in the house and loose stools. Due to financial concerns, the owners opted to switch from the pancreatic enzyme powder to the less expensive, but also less effective, pancreatic enzyme tablets (1 tablet with each meal) (Pancrease-V; Bioniche, Belleville, Ontario). In addition, the dog was administered weekly parenteral cobalamin (Cyanocobalamin; Vetoquinol, Lavaltrie, Quebec) injections, 500 μg, SC, q7d for 6 wk, followed by 500 μg, SC, q3wk, and prescribed a pet multivitamin (Visorbits; Pfizer, Kirkland, Quebec) and metronidazole (Apometronidazole; Apotex), 15 mg/kg BW, PO, q12h for 21 d, therapy for presumed secondary SIBO. Serum cobalamin levels were not measured due to financial constraints. An H2 blocker, cimetidine (Tagamet; Nu-Pharm, Richmond Hill, Ontario), 15 mg/kg BW, PO, q12h for 12 wk, to be given 30 min prior to feeding was also prescribed. To prevent the inappropriate defecation in the house, the owners were instructed to split his total caloric intake into 3 rather than 2 feedings, and to administer 1 tablet of pancreatic enzymes with each meal.
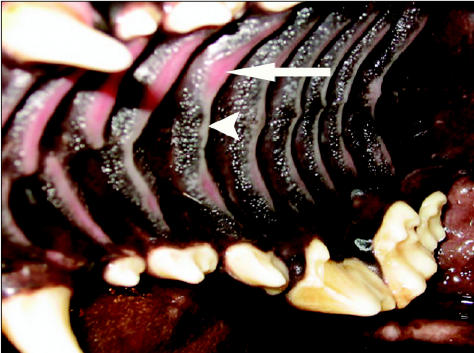
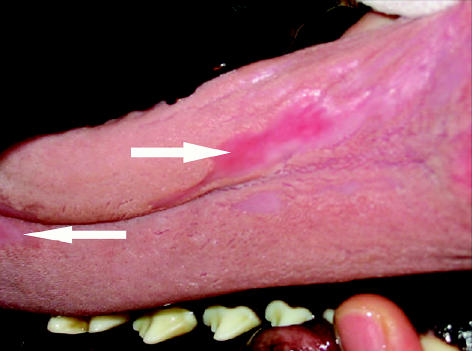
Ten weeks after initial referral, the dog had gained an additional 2 kg and had not had any more fecal accidents in the house; however, his stool consistency, although improved, was still soft. At this time, the dog was switched back to powdered pancreatic enzymes, as the manufacturer had discontinued production of the tablets. At his recheck in week 11, the owners reported that the dog had started dripping blood-tinged saliva from his mouth after eating. Under sedation, multiple superficial oral ulcers were visible on the roof of his mouth (ridges of the hard and soft palate) and dorsal surface of the tongue (Figures 1, 2). Differential diagnoses for the oral ulceration included ingestion of a caustic substance unknown to the owner, an adverse drug reaction, a manifestation of a systemic autoimmune disease, or a local reaction to the pancreatic enzyme supplementation. At this time, the only drug the dog was receiving was cimetidine, which was discontinued in case the ulcers were caused by an adverse drug reaction. Clotting times and oral biopsies were offered but declined. The owners were instructed to preincubate the enzymes with the food for 30 min, instead of the usual 20 min, prior to feeding. Symptomatic therapy for the oral ulcers consisting of sucralfate (Sulcrate; Axoan Pharma, Mont-Saint-Hilaire, Quebec), 5 mL, PO, q8h for 7 d, and oral chlorhexidine mouth rinse (Nolvadent; Ayerst, Fort Dodge, Iowa), 5 to 10 mL rinse q8h for 7 d, were initiated. One week later the oral bleeding had subsided and there were no visible oral ulcerations.
Figure 1.
Ulceration (arrow) and hypopigmentation (arrow-head) of the mucosa covering rugae of the hard palate in a 20-month-old German shepherd receiving pancreatic enzyme supplementation for exocrine pancreatic insufficiency secondary to pancreatic acinar atrophy.
Figure 2.
Ulceration of the tongue in a 20-month-old German shepherd receiving pancreatic enzyme supplementation for exocrine pancreatic insufficiency secondary to pancreatic acinar atrophy.
Discussion
At the time of writing, 8 mo after initial presentation, the dog continues to do well. He has gained back 10 kg in body weight and there has been no return of the oral bleeding or ulceration. Such an adverse drug reaction to cimetidine has not previously been reported in dogs or humans; however, pancreatic enzyme supplementation has been reported to cause oral bleeding in dogs, which supports this as the likely cause of the oral ulceration and subsequent oral bleeding in this dog.
Exocrine pancreatic insufficiency (EPI) refers to an inability of the pancreas to produce enough digestive enzymes to break down food in the small intestine (1–2). The resulting maldigestion leads to malabsorption and malnutrition. Causes of EPI in small animals include PAA, chronic pancreatitis, and, rarely, pancreatic neoplasia (1–2). In humans, cystic fibrosis and pancreatitis are the most common causes of EPI (2).
Pancreatic acinar atrophy is the most common cause of EPI in dogs, and the breeds with the highest prevalence of this disorder are the German shepherd and rough-coated collie (4). Multigenerational studies of affected German shepherds in the United States suggest that it is a familial disorder with an autosomal recessive mode of inheritance (5). Dogs usually develop clinical signs between 1 to 4 y of age (1–3). An immune-mediated pathogenesis has been proposed for PAA, because in the subclinical stage, lymphocytic pancreatic inflammation, which is presumed to lead to degeneration of the exocrine pancreas, is seen (1–3). However, by the time that clinical signs manifest, which occur only after greater than 90% of the pancreatic acinar cell reserve has been destroyed, there is typically very little evidence of pancreatic inflammation (1–3). The endocrine portion of the pancreas, the islets of Langerhans, although disorganized in dogs with PAA, is unaffected, so concurrent diabetes mellitus is not expected (2).
Clinical signs vary depending on the duration of disease, but voluminous soft stools and severe weight loss with polyphagia are most common. The feces of affected dogs are often yellow to grey, foul smelling, and fatty (1–3). Some dogs also exhibit vomiting, diarrhea, coprophagia, pica, marked borborygmi, and, in rare cases, bouts of anorexia. A poor hair coat may be seen, secondary to chronic malnutrition (1–3).
Definitive diagnosis of PAA requires pancreatic biopsy, but this is rarely required. Instead, less invasive indirect tests are relied on. Of these tests, the serum canine trypsin-like immunoreactivity (cTLI) is the most sensitive and specific (1–3). This assay quantifies the amounts of circulating trypsinogen and trypsin in the serum and a fasted cTLI concentration ≤ 2.5 μg/L is diagnostic of EPI (1–3). Because the assay is species specific, samples can be collected even when treatment with beef or pork pancreatic enzyme supplementation has already been initiated (1–3).
The primary treatment of PAA involves supplementing each meal with commercial pancreatic enzymes in powder, capsule, or tablet formulation (1–3,6). The powder or raw formulations are reported to be more effective clinically than the enteric coated tablets (1–3). The dose of pancreatic enzymes needed to treat EPI can be highly variable and should be individualized; however, a starting dose of 2 tsp/20 kg per meal is initially recommended (1–3,6). Once clinical improvement is noted, the dose should be reduced to the lowest possible effective dose. Raw chopped porcine or bovine pancreas (0.2 oz/kg BW) obtained from an abattoir is a cheaper alternative to commercial preparations, but there a risk of transmitting infectious diseases and it may be difficult to find a reliable source (1,2).
Dogs with EPI are predisposed to develop secondary SIBO (recently renamed antibiotic-responsive diarrhea [ARD]) due to the increased amount of substrate available to bacteria in the small intestine, a lack of bacteriostatic factors present in normal pancreatic secretions, and changes in intestinal motility and immune functions (3,7). In dogs with EPI that fail to respond to pancreatic enzyme supplements alone, empirical treatment for ARD is indicated. In rare cases, if ARD has been long-lasting, permanent villous atrophy may occur. This may explain why some dogs fail to respond completely to treatment (2).
Cobalamin deficiency and, rarely, deficiencies of vitamins E, A, and K may also occur with EPI (2). Small intestinal bacterial overgrowth and deficiency in pancreatic intrinsic factor and pancreatic proteases may be involved in malabsorption of cobalamin (2–3). These vitamin deficiencies may need to be addressed in cases that do not completely respond to pancreatic enzyme supplementation alone (1–3). For cobalamin, the serum concentration should be measured to determine if supplementation is required. If serum cobalamin is found to be low, supplementation with parenteral cobalamin, 500 μg, SC or IM, weekly for 6 wk, followed by less frequent dosing every 2 to 3 wk, will be required. In this case, multivitamins and cobalamin were empirically supplemented due to the suboptimal response to enzyme supplementation alone and the financial constraints of the owner.
Since most dogs with PAA display signs of dietary intolerance (or sensitivity), owners must be consistent with respect to the diet fed, the amount fed, and the frequency of feedings (3). Table scraps and treats should be avoided. Most dogs with PAA will do well eating a commercial adult maintenance dog food, which ideally should contain sources of highly digestible protein and carbohydrate. Diets too high in carbohydrates should be avoided, as undigested carbohydrates exert an osmotic effect and may potentiate or exacerbate diarrhea (2). Diets high in fiber should also be avoided, as certain types of fiber may interfere with the action of pancreatic enzymes (1–2).
Antibiotic therapy for suspected secondary SIBO may be necessary, if signs persist despite adequate enzyme supplementation. Good empirical antibiotic choices to treat secondary SIBO include metronidazole, 10 to 20 mg/kg BW, PO, q12h for 7 d; tylosin, 10 to 15 mg/kg BW, PO, q12h as needed; and oxytetracyline, 10 to 20 mg/kg BW, PO q12h for 7 to 28 d (1–3). If secondary SIBO is chronic and severe, it may lead to permanent mucosal damage that is only partially reversible. This fact may help to explain why some dogs with PAA have a suboptimal clinical response (1–3).
If the therapeutic response is suboptimal despite all of these steps, some dogs may also require short term prednisone therapy, 1 to 2 mg/kg BW, PO, q12h for 7 to 14 d, for possible concurrent inflammatory bowel disease (2).
The prognosis for dogs with PAA is generally good, but the treatment is expensive and life-long (1–3,6). If treatment is prohibitively expensive, euthanasia is warranted. Not all dogs with PAA will have an optimal response to treatment. Indeed, as many as 20% of affected dogs fail to completely respond (1–3). Some dogs fail to attain their former body weight and high fecal volume and flatulence may persist, but diarrhea can generally be controlled. A high prevalence of mesenteric torsion has been reported in German shepherds with PAA in Finland, but this same trend has not been reported in North America (2).
A rare complication of therapy, which was seen in this case, is oral ulceration and bleeding associated with feeding powdered pancreatic enzyme supplements (9). One other report in the literature describes 3 dogs receiving pancreatic enzyme supplementation for EPI that developed oral bleeding (9). In 2 of these dogs, coagulation profiles were normal and oral ulceration was not documented; oral biopsies were not performed in any of the dogs. In all 3 of these dogs, the oral bleeding resolved when the dose of pancreatic enzyme supplements added to the food was reduced (9). In the case described here, a longer preincubation period with the food prior to feeding has proved sufficient to prevent recurrence of the clinical signs. In humans, other complications that have been linked to pancreatic enzyme supplements include fibrosing colonopathy and colonic strictures in children with cystic fibrosis on high doses of pancreatic enzymes for secondary EPI, hyperuricosuria, and premature loss of teeth (9–12). To date, fibrosing colonopathy, colonic strictures, hyperuricosuria, and premature loss of teeth have not been reported in dogs receiving pancreatic enzyme supplements.
Despite the fact that all cases of oral bleeding in dogs with EPI are linked to supplementation with the powdered form of the pancreatic enzymes, this formulation is still recommended over the capsules or enteric coated tablets, due to the greater reported clinical efficacy. If this complication arises, the dose of the pancreatic enzymes can be decreased, or if higher doses are needed to control the clinical signs of EPI, longer pre-incubation periods with the food prior to feeding and other supportive treatments for oral ulcerations, such as sucralfate suspension and chlorhexidine mouth rinse, may be beneficial.
Acknowledgments
The author thanks the students that helped with this case, including Kristi Jacobson, Dana Marsden, and Hugh Millar. CVJ
References
- 1.Rutz GM, Steiner JM, Williams DA. Pancreatic acinar atrophy in German shepherds. Compend Contin Educ Pract Vet. 2001;23:347–356. [Google Scholar]
- 2.Williams DA. Exocrine pancreatic disease. In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine 5th ed. Philadelphia: WB Saunders, 2000:1345–1367.
- 3.Westermarck E, Wiberg M, Steiner JM, Williams DA. Exocrine pancreatic insufficiency in dogs and cats. In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine, 6th ed. Philadelphia: Elsevier Saunders, 2005:1492–1495.
- 4.Wiberg ME, Lautala HM, Westermarck E. Response to long-term enzyme replacement treatment in dogs with exocrine pancreatic insufficiency. J Am Vet Med Assoc. 1998;213:86–90. [PubMed] [Google Scholar]
- 5.Moeller EM, Steiner JM, Clark LA, et al. Inheritance of pancreatic acinar atrophy in German shepherd dogs. Am J Vet Res. 2002;63:1429–1434. doi: 10.2460/ajvr.2002.63.1429. [DOI] [PubMed] [Google Scholar]
- 6.Simpson JW, Maskell IE, Quigg J, Markwell PJ. Long term management of canine exocrine pancreatic insufficiency. J Small Anim Pract. 1994;35:133–138. [Google Scholar]
- 7.Williams DA, Batt RM, McLean L. Bacterial overgrowth in the duodenum of dogs with exocrine pancreatic insufficiency. J Am Vet Med Assoc. 1987;191:201–206. [PubMed] [Google Scholar]
- 8.Westermarck E, Rimaila-Parnanen E. Mesenteric torsion in dogs with exocrine pancreatic insufficiency: 21 cases (1978–1987) J Am Vet Med Assoc. 1989;195:1404–1406. [PubMed] [Google Scholar]
- 9.Rutz GM, Steiner JM, Williams DA. Oral bleeding associated with pancreatic enzyme supplementation in three dogs with exocrine pancreatic insufficiency. J Am Vet Med Assoc. 2002;221:1716–1718. doi: 10.2460/javma.2002.221.1716. [DOI] [PubMed] [Google Scholar]
- 10.FitzSimmons SC, Burkhart GA, Borowitz D, et al. High-dose pancreatic-enzyme supplements and fibrosing colonopathy in children with cystic fibrosis. N Eng J Med. 1997;336:1283–1289. doi: 10.1056/NEJM199705013361803. [DOI] [PubMed] [Google Scholar]
- 11.Taylor CJ, Dodge JA. High-strength pancreatic enzyme supplements and large-bowel stricture in cystic fibrosis. Lancet. 1994;343:110. [PubMed] [Google Scholar]
- 12.Stapleton FB, Kennedy J, Nousia-Arvanitakis S, Linshaw MA. Hyperuricosuria due to high-dose pancreatic extract therapy in cystic fibrosis. N Engl J Med. 1976;295:246–248. doi: 10.1056/NEJM197607292950503. [DOI] [PubMed] [Google Scholar]