Abstract
Previous genetic analysis has shown that dos/soc-1/Gab1 functions positively in receptor tyrosine kinase (RTK)-stimulated Ras/Map kinase signaling through the recruitment of csw/ptp-2/Shp2. Using sensitized assays in Caenorhabditis elegans for let-23/Egfr and daf-2/InsR (insulin receptor-like) signaling, it is shown that soc-1/Gab1 inhibits phospholipase C-γ (PLCγ) and phosphatidylinositol 3′-kinase (PI3K)-mediated signaling. Furthermore, as well as stimulating Ras/Map kinase signaling, soc-1/Gab1 stimulates a poorly defined signaling pathway that represses class 2 daf-2 phenotypes. In addition, it is shown that SOC-1 binds the C-terminal SH3 domain of SEM-5. This binding is likely to be functional as the sem-5(n2195)G201R mutation, which disrupts SOC-1 binding, behaves in a qualitatively similar manner to a soc-1 null allele in all assays for let-23/Egfr and daf-2/InsR signaling that were examined. Further genetic analysis suggests that ptp-2/Shp2 mediates the negative function of soc-1/Gab1 in PI3K-mediated signaling, as well as the positive function in Ras/Map kinase signaling. Other effectors of soc-1/Gab1 are likely to inhibit PLCγ-mediated signaling and stimulate the poorly defined signaling pathway that represses class 2 daf-2 phenotypes. Thus, the recruitment of soc-1/Gab1, and its effectors, into the RTK-signaling complex modifies the cellular response by enhancing Ras/Map kinase signaling while inhibiting PI3K and PLCγ-mediated signaling.
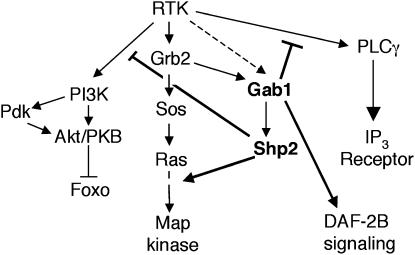
GROWTH factors commonly act through cell surface receptors with intrinsic tyrosine kinase activity to control a wide range of cellular activities including movement, differentiation, proliferation and survival. Receptor tyrosine kinases (RTKs) are activated by growth factor binding leading to the tyrosine phosphorylation of substrates. This may activate substrates directly or create specific binding sites for proteins containing Src homology-2 (SH2) domains. Recruitment of effector molecules leads to the activation of a small number of intracellular signaling cascades, including those mediated by Ras, phosphatidylinositol 3′-kinase (PI3K), and phospholipase C-γ (PLCγ) activation (Figure 1). How the specificity of the response is achieved is not completely understood, but it is believed that the context of the cell limits the possible responses to growth factor signaling and the magnitude and duration of RTK activation and, together with the relative degree of stimulation of particular intracellular signaling pathways, determines the specific response from this limited set (Marshall 1995; Schlessinger 2000). Distinct growth factors are differentiated by the relative abundance of their receptors upon the cell surface, coupled with the fact that distinct receptors activate intracellular signaling pathways differentially (Pawson and Scott 1997; Schlessinger 2000). The intracellular signaling pathways activated by a particular growth factor receptor are determined by the presence/abundance of effector binding sites on the receptor itself or on associated scaffolding/docking proteins (Pawson and Scott 1997). Scaffolding/docking proteins typically contain an N-terminal pleckstrin homology (PH) domain followed by a phosphotyrosine binding (PTB) domain and a C terminus containing multiple SH2 binding sites. The use of scaffolding/docking proteins broadly separates growth factor receptors into two classes. For example, the insulin-like growth factor-1 (IGF-1) receptor and the fibroblast growth factor (Fgf) receptor (Fgfr) primarily utilize scaffolding/docking adaptor proteins such as IRS1 (insulin receptor substrate 1) or FRS2 (Fgf receptor substrate 2) to mediate effector binding. By contrast, the epidermal growth factor receptor (Egfr) and platelet-derived growth factor receptor utilize tyrosines within C-terminal extensions as substrates onto which multi-protein signaling complexes are assembled (Pawson and Scott 1997; Schlessinger 2004). The Gab family of adaptor proteins is structurally similar to scaffolding/docking proteins but lacks a PTB domain and is utilized downstream from a broad range of growth factor receptors (reviewed in Liu and Rohrschneider 2002; Gu and Neel 2003; Nishida and Hirano 2003). Gab1 was initially identified as a Grb2-binding protein and contains SH2-binding sites for the regulatory subunits of PI3K, PLCγ, and Shp2 (Holgado-Madruga et al. 1996). Recruitment of Gab1 into the signaling complex usually occurs through its interaction with Grb2, although there is some evidence that Gab1 binding to the c-Met RTK may be more direct (Weidner et al. 1996). The interaction between Gab1 and Grb2 occurs between the C-terminal SH3 domain of Grb2 and an atypical proline motif within Gab1 (Lock et al. 2000; Feller et al. 2002). Recruitment of Gab1 is under control of a positive-feedback loop: Gab1 recruits PI3K, increasing phosphatidylinositol 3,4,5-trisphosphate [PtdIns(3,4,5)P3] production, which in turn promotes further binding of Gab1 through its PH domain (Isakoff et al. 1998; Rodrigues et al. 2000). Thus the recruitment of Gab1 may be regulated by the extent of RTK signaling.
Figure 1.
Schematic showing the effect of Gab1 on Ras/Map kinase-mediated signaling as revealed by genetic studies. Additional effects on PLCγ-, PI3K-, and DAF-2B-mediated signaling are derived from this study (thick lines). Arrows represent a positive/activating interaction. Lines ending in a perpendicular represent a negative/inhibitory interaction. IP3 receptor, inositol trisphosphate receptor. Dashed arrow shows that there is evidence that Gab1 can bind the Met RTK, but not other RTKs directly (Weidner et al. 1996).
Genetic systems have proven to be important in demonstrating the function of signaling components. In Drosophila, the Gab1 homolog, dos, was identified as a positively acting signaling component located downstream of the sev RTK (Herbst et al. 1996; Raabe et al. 1996). More recently, the Caenorhabditis elegans Gab1 homolog, soc-1, was identified as a positive signaling component downstream of the Fgfr (Schutzman et al. 2001). Previous genetic assays for Gab1 function, using these model organisms, utilized signaling mediated by the RTK/Ras/Map kinase pathway. In this context, the essential function of Gab1 is to recruit Shp2, a dual SH2 domain containing protein tyrosine phosphatase (Figure 1; Herbst et al. 1999; Bausenwein et al. 2000; Schutzman et al. 2001). The role of Gab1 in RTK signaling mediated by intracellular pathways other than the Ras/Map kinase-signaling pathway has not been previously addressed in a genetic model system. Here the role of Gab1 in RTK signaling utilizing intracellular signaling pathways mediated by PI3K and PLCγ is reported, using assays for Egfr and insulin receptor-like signaling in C. elegans.
In C. elegans, there is a single Egfr encoded by let-23, which functions during development to mediate induction of several specializations of the hypodermis through activation of the conserved Ras/Map kinase pathway (Sternberg and Han 1998). In addition, let-23 activity is required for hermaphrodite fertility. This adult function of let-23/Egfr is genetically separable from the developmental functions and is not mediated through Ras activation (Aroian et al. 1994; Jongeward et al. 1995; Lesa and Sternberg 1997). Instead, the let-23/Egfr fertility function is mediated through PLCγ signaling (Clandinin et al. 1998; Yin et al. 2004).
Similarly, there is a single insulin/IGF-1 receptor-like (InsR) molecule in C. elegans, encoded by the daf-2 gene (Kimura et al. 1997). Mutations in daf-2 were first identified in screens for animals that form dauers constitutively—the Daf-c phenotype (Riddle and Albert 1997). Dauers are stress resistant, diapausal, third stage larvae that normally develop in response to increased population density and starvation. There are two classes of daf-2 mutations. Class 1 daf-2 mutants form dauers constitutively, are intrinsically thermotolerant, and are long lived. Class 2 daf-2 mutants display these phenotypes plus one or more additional phenotypes, which include embryonic/early larval arrest, reduced pharyngeal pumping, and late progeny production (Gems et al. 1998). Class 1 and class 2 daf-2 alleles do not fall into a single allelic series, as class 1 alleles can be more severe for the Daf-c phenotype than many class 2 alleles. This suggests that the daf-2 gene may have distinct functional elements, hypothesized as daf-2A and daf-2B (Gems et al. 1998). However, unlike the let-23/Egfr gene, functional elements of the daf-2/InsR gene are not genetically separable. Thus, class 1 phenotypes may result from loss of daf-2A activity whereas class 2 phenotypes result from loss of both daf-2A and daf-2B activities (Gems et al. 1998).
daf-2/InsR signaling is primarily mediated through age-1/PI3K, which produces PtdIns(3,4,5)P3 from PtdIns(4,5)P2 (Morris et al. 1996). The production of PtdIns(3,4,5)P3 stimulates PDK-1 and the AKT-1 and AKT-2 protein kinase B proteins, which in turn inactivate the FOXO transcription factor DAF-16 (Figure 1; (Ogg et al. 1997; Paradis and Ruvkun 1998; Paradis et al. 1999). In addition, let-60/Ras modulates daf-2/InsR signaling (Nanji et al. 2005).
Here it is shown that soc-1/Gab1 has positive and negative regulatory functions in both Egfr and insulin receptor-like signaling in C. elegans. Downstream of let-23/Egfr, soc-1/Gab1 functions positively during vulval induction, but negatively upon let-23/Egfr-mediated hermaphrodite fertility. Downstream of daf-2/InsR, soc-1/Gab1 functions to negatively regulate dauer formation, but contributes positively to daf-2B signaling: mutation of soc-1 in a class 1 daf-2 background causes class 2 phenotypes to be displayed.
Furthermore, it is shown that a C-terminal SH3 domain mutation in sem-5/Grb2 has a qualitatively similar effect as a null mutation in soc-1/Gab1 in all assays performed for Egfr and InsR signaling. This same mutation in sem-5 blocks SOC-1 binding to SEM-5, consistent with a model where SOC-1/Gab1 recruitment into the signaling complex downstream of the Egfr and insulin receptor is dependent upon the C-terminal SH3 domain of SEM-5/Grb2. However, genetic analysis of ptp-2/Shp2 suggests that recruitment of PTP-2/Shp2 may not be the sole function of SOC-1/Gab1.
Together the data suggest that the recruitment of soc-1/Gab1 into the RTK-signaling complex regulates signaling output by potentiating Ras/Map kinase-mediated signaling while inhibiting PI3K- and PLCγ-mediated signaling. As Gab1 recruitment is itself regulated by the extent of signaling (Rodrigues et al. 2000), this provides a mechanism that could link the magnitude and duration of RTK signaling to distinct cellular responses.
MATERIALS AND METHODS
Strains and genetics:
The N2 strain was used as the wild-type C. elegans strain. The following mutant strains were used in this study: DR1567 daf-2(m577), CB1370 daf-2(e1370), MT5547 clr-1(e1745); soc-1(n1789), MT5998 sem-5(n2195), WS841 ptp-2(op194) unc-4(e120)/mln1[dpy-10(e128)]; him-5(e1490), HP36 let-23(sy10) unc-4(e120)/mnC1[dpy-10 unc-52]; let-60(n1046gf), MT2124 let-60(n1046gf) (Beitel et al. 1990; Clark et al. 1992; Gems et al. 1998; Gutch et al. 1998; Hopper et al. 2000; Schutzman et al. 2001). soc-1(n1789) results in a G87STOP within the N-terminal PH domain and is defined as a null mutation of soc-1 (Schutzman et al. 2001). The ptp-2(op194) null mutation deletes the entire phosphotyrosine phosphatase domain of ptp-2 (Gutch et al. 1998). The following strains were generated for this study using standard techniques: HP25 soc-1(n1789), HP26 daf-2(m577); sem-5(n2195), HP27 daf-2(e1370); sem-5(n2195), HP28 daf-2(m577); soc-1(n1789), HP29 daf-2(e1370); soc-1(n1789), HP30 let-23(sy10) unc-4(e120)/mnC1[dpy-10 unc-52]; let-60(n1046gf); sem-5(n2195), HP31 let-23(sy10) unc-4(e120)/mnC1[dpy-10 unc-52]; let-60(n1046gf); soc-1(n1789), HP32 let-23(sy10) unc-4(e120)/mnC1[dpy-10 unc-52]; let-60(n1046gf); sos-1(n1031) unc-46(e177), HP33 let-60(n1046gf); sem-5(n2195), HP34 let-60(n1046gf); soc-1(n1789), HP35 ptp-2(op194) unc-4(e120); let-60(n1046gf).
RNA-mediated interference experiments:
C. elegans animals were reared on HT115 bacteria expressing ptp-2 dsRNA to reduce ptp-2 gene activity. ptp-2 genomic DNA (nucleotides 67–2233 relative to start codon) was generated by PCR and inserted between the T7 promoters of the L4440 vector. HT115 bacteria were transformed with this ptp-2-containing vector and separately with L4440, which was used as a control. Expression of T7 RNA polymerase was induced in HT115 as described in Kamath et al. (2001). Animals were maintained on HT115 expressing dsRNA for at least two generations before assaying. Experimental (ptp-2) and control (L4440) worms were treated in exactly the same way except ptp-2(RNAi) worms were maintained on HT115 transformed with ptp-2 inserted into L4440 (as above) whereas L4440(RNAi) worms were maintained on HT115 transformed with L4440 alone.
Vulval induction and fertility assays:
Both assays were performed as previously described (Hopper et al. 2000). Briefly, animals were maintained at 20° and vulval induction scored at the early to mid-L4 stage. To control for possible temperature effects and the effect of starvation upon the let-60(n1046gf) phenotype (Battu et al. 2003), vulval induction assays were performed in parallel upon animals from well-fed plates incubated in the same location within the 20° incubator. For fertility assays, L4 animals were transferred to individual plates and fertility was defined as having more than two offspring.
Terminal arrest and dauer assays:
Adult animals were placed at the test temperature on fresh plates for 4–6 hr to lay eggs. The eggs were then transferred to fresh plates, counted, and incubated at the test temperature. For the dauer assays, animals were scored after 66–72 hr. L3d is defined as having a dauer-like appearance but actively pumping pharynx. For the terminal arrest experiments, animals were scored at 72 hr. The stage of arrest was defined by direct observation by DIC of gonad development. L1 arrest was between the 4- and 10-cell gonad stage. L2 arrest was beyond this but before a large proliferation of gonad.
Late offspring and longevity assays:
Animals were grown at 20° until the L4 stage of development. They were then picked to a fresh plate and placed at 25°. On each of the subsequent 8 days, they were moved to a fresh plate, as required. Control worms stopped producing offspring on days 4–5. On day 9, worms were placed individually on a plate to allow individual animals to be assayed for late progeny. These worms were moved to fresh plates again 10–14 days later. Animals dying as a result of bagging were censored from the longevity experiment. All worms dying before day 9 were censored from the late offspring experiment. Age at death was recorded to an accuracy of within 3 days. Death was defined as no longer being responsive to being poked repeatedly with a platinum wire. Survival analyses were performed using the Kaplan–Meier method upon censored data and P-values calculated for differences between survival curves using the log-rank test.
Yeast two-hybrid experiments:
Fragments of soc-1 cDNA encoding amino acids 179–268 and 286–357 were generated by PCR using primers to incorporate EcoRI and SmaI restriction sites in frame at each end, respectively. These fragments were cloned into pGBKT7 (CLONTECH, Palo Alto, CA) to create the bait vectors. Fragments of sem-5 cDNA encoding amino acids 1–212 were also generated by PCR using primers to incorporate SmaI and PstI restriction sites in frame at each end, respectively. The G201R mutation was introduced into the SEM-5 fragment using an extended reverse primer for the PCR. Fragments were cloned into pGAD424 (CLONTECH) to create the prey vectors. Yeast manipulations and two-hybrid experiments were performed as previously described (Hopper et al. 2000).
RESULTS
SOC-1 binds the C-terminal SH3 domain of SEM-5:
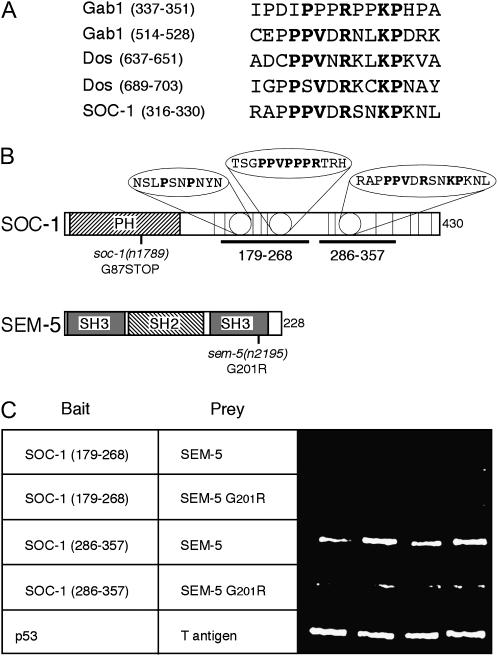
Studies of Drosophila and of mammalian cell culture have demonstrated that the C-terminal SH3 domain of Grb2 binds to an atypical proline-rich motif within Gab1 and that this interaction is required for recruitment of Gab1 downstream of the Egfr (Lock et al. 2000; Feller et al. 2002). This atypical proline-rich motif is conserved in soc-1, the C. elegans Gab1 homolog (Figure 2A). The C. elegans gene, sem-5, encodes Grb2. To test whether the binding of the C-terminal SH3 of Grb2 to Gab1 is also conserved in the C. elegans proteins, the yeast two-hybrid assay was employed. In these assays, SEM-5 bound the portion of SOC-1 containing the conserved atypical proline-rich motif but not to a region containing the two PxxP motifs also found in SOC-1 (Figure 2C). This interaction failed to occur when the conserved glycine at position 201 in SEM-5 was substituted to arginine (Figure 2C). This substitution is within the C-terminal SH3 domain of SEM-5/Grb2 and corresponds to the sem-5(n2195) mutation (Clark et al. 1992). Thus the interaction of the C-terminal SH3 of sem-5/Grb2 with soc-1/Gab1 is conserved in C. elegans. If this interaction is functionally important, then the sem-5(n2195) mutation should behave similarly to a null mutation in soc-1, with the caveat that the C-terminal SH3 domain of sem-5/Grb2 may have additional functions.
Figure 2.
The C-terminal SH3 domain of SEM-5/Grb2 binds an atypical proline-rich motif within SOC-1/Gab1. (A) Alignment of atypical proline-rich motifs of Gab1 and Dos with a similar sequence found in SOC-1. Gab1 (337–351) constitutes a weak Grb2-binding site, and Gab1(514–528) binds strongly (Lock et al. 2000). Both Dos sites shown contribute to Drk/Grb2 binding equally (Feller et al. 2002). Residues conserved in three of the four Gab1/Dos sites are in boldface type, as are the corresponding residues in SOC-1. (B) Domain structures of SOC-1 and SEM-5 showing location of mutations used in this study. Thick horizontal line shows regions of SOC-1 used in the yeast two-hybrid assay. Bubbles highlight proline-rich motifs. Vertical lines indicate positions of tyrosines. (C) Yeast two-hybrid analysis of the interaction between SEM-5 and SOC-1. Bait two-hybrid constructs were cloned into pGBKT7 and prey proteins were cloned into pGAD424 (CLONTECH) and two-hybrid analysis was performed as described previously (Hopper et al. 2000). SOC-1 (179–268) contains two PxxP motifs, most notably PPVPPPR at residues 236–242. The SEM-5 G201R construct bears the same mutation as found in the sem-5(n2195) allele.
soc-1 acts positively during let-23/Egfr-mediated vulval induction:
soc-1 was identified as a suppressor of the clear phenotype, resulting from hyperactivity of the Fgfr/Ras/Map kinase pathway in C. elegans (Schutzman et al. 2001). The sem-5(n2195) mutation was also recovered in the same screen (Clark et al. 1992). The function of soc-1 in this Fgfr assay is to recruit ptp-2 (Schutzman et al. 2001). The function of soc-1 in Egfr signaling in C. elegans has not been previously addressed. The vulva in C. elegans hermaphrodites is induced by an Egf signal produced from the gonadal anchor cell (Kornfeld 1997). A field of up to six cells, termed the vulva precursor cells (VPCs), can respond to this signal through the activation of an Egfr/Ras/Map kinase pathway. Normally three cells do so, but in animals carrying a weak gain-of-function Ras mutation, let-60(n1046gf), additional cells also respond, producing pseudovulvae. Null mutations in soc-1 and ptp-2 and the sem-5(n2195) mutation cause no observable defect in vulval differentiation in an otherwise wild-type background. However, previous studies have shown that both a null mutation in ptp-2 and sem-5(n2195)G201R weakly suppress the multi-vulva phenotype of let-60(n1046gf) mutant animals (Gutch et al. 1998; Hopper et al. 2000). Thus, if soc-1 functions to recruit ptp-2 during Ras/Map kinase signaling downstream of the Egfr, as it does downstream of the Fgfr, it is expected that soc-1 will also weakly suppress the multi-vulva phenotype of let-60(n1046gf) animals.
To determine whether the C-terminal SH3 domain of sem-5/Grb2, soc-1/Gab1, and ptp-2/Shp2 function together in Egfr/Ras/Map kinase signaling, double-mutant animals carrying let-60(n1046gf) with sem-5(n2195)G201R or a null mutation in either soc-1 or ptp-2 were generated and subjected to the vulval induction assay in parallel. As expected, all three mutations weakly suppressed let-60(n1046gf) (Table 1). The suppression seen by the soc-1(n1789) null mutation is identical to the suppression seen by the ptp-2(op194) null mutation in this and a previous study (Gutch et al. 1998). This is consistent with findings from other genetic assays for Ras/Map signaling, which suggest that the function of soc-1/Gab1 is to recruit ptp-2/Shp2 (Herbst et al. 1999; Bausenwein et al. 2000; Schutzman et al. 2001). Suppression of the let-60gf multi-vulva phenotype was significantly stronger by sem-5(n2195)G201R than by soc-1(n1789) or ptp-2(op194), suggesting that the C-terminal SH3 domain of sem-5/Grb2 has an additional function(s) other than recruiting soc-1/Gab1 (Fisher's exact test P < 0.05; Table 1). It has been reported that the C-terminal SH3 domain of Grb2 is also required for endocytosis of the Egfr in response to Egf (Wang and Moran 1996), which in turn is required for sustained Map kinase signaling (Vieira et al. 1996). In addition, the C-terminal SH3 domain of sem-5/Grb2 contributes to the binding of Sos, which in turn activates Ras (Figure 1; Sastry et al. 1995).
TABLE 1.
soc-1/Gab1 suppresses the multi-vulva phenotype arising from a gain-of-function Ras mutation
| Genotype | Average inductiona | % Muvb | n |
|---|---|---|---|
| +/+ | 3.0 | 0 | Many |
| let-60(n1046gf) | 3.9 | 80 | 45 |
| let-60(n1046gf); sem-5(n2195)G201R | 3.2 | 18 | 40 |
| let-60(n1046gf); soc-1(n1789) | 3.5 | 53 | 36 |
| ptp-2(op194); let-60(n1046gf) | 3.4 | 56 | 41 |
soc-1(n1789) and ptp-2(op194) are null mutations. ptp-2 was linked to unc-4(e120). Suppression of let-60(gf) by sem-5(n2195), soc-1(0), and ptp-2(0) was significant (Fisher's exact test P < 0.05). A significantly greater number of animals with more than three cells was induced in let-60ras(gf); soc-1(0) and ptp-2(0); let-60ras(gf) relative to let-60ras(gf); sem-5(n2195) animals (Fisher's exact test P < 0.05). There was no difference between let-60ras(gf); soc-1(0) and ptp-2(0); let-60ras(gf) (Fisher's exact test P = 0.82).
Total number of VPCs adopting a vulval cell fate divided by the number of animals scored.
Proportion of animals having more than three VPCs induced.
This, and previously published data, is consistent with a model in which the C-terminal SH3 domain of sem-5/Grb2, soc-1/Gab1, and ptp-2/Shp2 act in a genetic pathway to promote/sustain Ras/Map kinase signaling (Herbst et al. 1999; Bausenwein et al. 2000; Schutzman et al. 2001; Feller et al. 2002). As mutations in these genes in an otherwise wild-type background are mostly silent in Egfr and Fgfr assays, this pathway is not essential for Ras/Map kinase signaling per se, but rather acts to enhance its efficacy. To address whether this pathway also operates in RTK signaling not mediated by Ras/Map kinase, the function of these genes was assessed in assays for RTK signaling mediated by PLCγ and PI3K.
soc-1 functions negatively in let-23/Egfr fertility:
In addition to the function of let-23/Egfr in mediating inductive signaling during development in C. elegans, let-23 activity is required for hermaphrodite fertility. The focus of let-23/Egfr signaling for the fertility function is in the contractile tissue of the spermatheca and/or gonadal sheath cells (Clandinin et al. 1998; Yin et al. 2004). Unlike inductive signaling that utilizes the Ras/Map kinase pathway, the fertility function of let-23 is mediated through PLCγ and IP3 production (Clandinin et al. 1998; Yin et al. 2004). Thus, a Ras gain-of-function mutation, let-60(n1046gf), suppresses the defect in inductive signaling but not the sterility of animals homozygous for a non-null Egfr mutation, let-23(sy10).
To determine the function of soc-1/Gab1 in PLCγ-mediated signaling, an assay for let-23/Egfr hermaphrodite fertility was used. In the following experiments, it was necessary to have the let-60(n1046gf) mutation present to recover viable animals. Although reducing or increasing Ras activity has no effect upon the fertility function of let-23/Egfr, a null mutation in sem-5/Grb2 has previously been shown to suppress the sterile Egfr mutation, let-23(sy10) (Hopper et al. 2000). To test whether the inhibitory effect of sem-5/Grb2 is dependent upon the C-terminal SH3 domain, let-23(sy10); let-60(n1046gf); sem-5(n2195)G201R triple mutants were generated and assayed for fertility. It was found that sem-5(n2195)G201R suppressed let-23(sy10) sterility almost as well as the sem-5 null mutation (Table 2). Thus, the inhibitory function of sem-5/Grb2 upon PLCγ signaling is largely mediated through its C-terminal SH3 domain. To test whether this is, in turn, mediated through soc-1/Gab1 recruitment, let-23(sy10); let-60(n1046gf); soc-1(n1789) animals were generated. Again, it was found that soc-1/Gab1 suppressed let-23/Egfr sterility (Table 2). However, the suppression of let-23(sy10) sterility by soc-1(n1789) was not as strong as by sem-5(n2195)G201R (Fisher's exact test P < 0.05; Table 2). This indicates that the inhibitory function of sem-5/Grb2 upon PLCγ signaling is only partly mediated through soc-1/Gab1. To test whether the recruitment of sos-1/Sos by the C-terminal SH3 domain of sem-5/Grb2 is also required for the inhibition of PLCγ signaling, let-23(sy10); let-60(n1046gf); sos-1(s1031) animals were generated. Despite sos-1(s1031) being a genetic null, no significant suppression of let-23/Egfr sterility was observed (Table 2). This suggests an additional activity of the C-terminal SH3 domain of sem-5/Grb2 in the inhibition of PLCγ-mediated signaling other than in recruiting soc-1/Gab1 or Sos. This may be in the stimulation of endocytosis (Wang and Moran 1996), which is also expected to inhibit PLCγ activity (Vieira et al. 1996; Haugh et al. 1999; Haugh and Meyer 2002).
TABLE 2.
soc-1/Gab1 suppresses the let-23/Egfr sterility
| Genotype | % of fertile animals (n) |
|---|---|
| let-23(sy10); let-60(n1046gf) | 0 (45) |
| let-23(sy10); let-60(n1046gf); sem-5(ay73) | 55 (31)a |
| let-23(sy10); let-60(n1046gf); sem-5(n2195)G201R | 33 (110) |
| let-23(sy10); let-60(n1046gf); soc-1(n1789) | 18 (74) |
| let-23(sy10); let-60(n1046gf); ptp-2(RNAi) | 0 (64) |
| let-23(sy10); let-60(n1046gf); sos-1(s1031) | 3 (36) |
unc-4(e120) was present in all strains and used for a marker of let-23(sy10), which was balanced in the parental strain by mnC1. Animals carrying sos-1(s1031) also carried the tightly linked unc-46(e177) mutation. In both let-23(sy10); let-60(gf); soc-1(n1789) and let-23(sy10); let-60(gf); ptp-2(RNAi) animals, ∼50% of vulvae ruptured. These ruptured animals were not censored from the assay, as one let-23(sy10); let-60(n1046gf); soc-1(n1789) animal whose vulva ruptured was scored as fertile.
Data previously reported (Hopper et al. 2000).
In genetic assays for soc-1/Gab1 that utilize the Ras/Map kinase pathway, the function of soc-1/Gab1 is to recruit ptp-2/Shp2 (Herbst et al. 1999; Bausenwein et al. 2000; Schutzman et al. 2001; this study). ptp-2 maps very close to let-23 on chromosome II and ptp-2(op194) animals, like let-23(sy10) animals, have a fertility defect, making construction of a ptp-2(op194) let-23(sy10); let-60(n1046gf) strain exceedingly difficult. Therefore, to address whether ptp-2/Shp2 also suppresses let-23(sy10) sterility, let-23(sy10) unc-4(e120)/mnC1; let-60(n1046gf) animals were reared upon HT115 bacteria expressing ptp-2 dsRNA to reduce ptp-2/Shp2 gene function by RNA-mediated interference (RNAi) (Timmons and Fire 1998). After being reared for at least two generations upon these ptp-2 dsRNA-expressing bacteria, let-23(sy10); let-60(n1046gf) progeny remained sterile (Table 2). Although in these experiments there is no direct evidence that ptp-2 gene function is reduced in let-23(sy10); let-60(n1046gf) animals, daf-2 mutant animals reared on the same bacterial strain expressing ptp-2 dsRNA did show an effect (see below). In addition, previous work has revealed the tissue in which the let-23/Egfr fertility pathway acts to be sensitive to RNAi (Yin et al. 2004). Together, this suggests that ptp-2/Shp2 may not be the downstream effector of soc-1/Gab1 that mediates inhibition of PLCγ-mediated signaling.
soc-1 functions negatively in daf-2/InsR signaling during dauer formation:
Upon hatching, C. elegans larvae undergo four molts before becoming adults. Depending upon environmental conditions, L2 larvae either molt into the L3 stage or form dauers. The decision of which developmental pathway is used is made by L1 and L2 animals, depending upon temperature, nutritional status, and the concentration of dauer pheromone (Riddle and Albert 1997). daf-2 encodes an insulin receptor-like molecule that senses the nutritional status of the worm to represses dauer formation and promote reproductive development through the activation of PI3K-mediated signaling (Morris et al. 1996; Kimura et al. 1997). Mutations in daf-2 may result in a conditional or nonconditional constitive dauer formation (Daf-c) phenotype, depending upon the severity of the allele. To address the function of soc-1/Gab1 in insulin receptor-like-mediated signaling in C. elegans, two mutations in daf-2/InsR were used: daf-2(m577) and the stronger daf-2(e1370) allele, both of which are temperature sensitive for the Daf-c phenotype. Double-mutant animals combining either the daf-2 allele with sem-5(n2195)G201R or a null mutation in soc-1 were generated. A null mutation in ptp-2 is semisterile and this sterility is enhanced by either daf-2 mutation (not shown). Therefore the effects of ptp-2 upon DAF-2 signaling were assayed upon animals that had been fed ptp-2 dsRNA to reduce ptp-2 gene activity by RNAi (Timmons and Fire 1998).
sem-5(n2195)G201R weakly suppressed daf-2(m577) Daf-c under sensitive conditions (Table 3). However, sem-5(n2195)G201R did not suppress the more severe daf-2(e1370) allele under any conditions tested. soc-1(n1789) almost completely suppressed daf-2(m577) Daf-c under the sensitive conditions used (Table 3). Again, however, there was very little suppression of daf-2(e1370), with the non-dauers scored arresting as L3ds, having radial shrinkage typical of dauers, but exhibiting strong pharyngeal pumping. daf-2(m577) animals reared on bacteria expressing ptp-2 dsRNA were also suppressed for the Daf-c phenotype when compared to daf-2(m577) animals reared on control plates. Strikingly, however, daf-2(e1370) animals reared on bacteria expressing ptp-2 dsRNA were also strongly suppressed for dauer formation at 22.5° (Table 3). Almost all non-dauers on these plates, in contrast with the weak suppression seen by soc-1(n1789), were fully suppressed, forming L4's and adults. To test the strength of suppression by ptp-2 RNAi, daf-2(e1370) animals reared on bacteria expressing ptp-2 dsRNA were assayed for dauer formation at 25°. At this temperature, 231/233 animals formed dauers and the others arrested earlier (one each as an embryo and at the early L1 stage), indicating no suppression.
TABLE 3.
soc-1/Gab1 suppresses daf-2 Daf-c
| 22.5°
|
24°
|
|||
|---|---|---|---|---|
| Genotype | Dauers (%) | n | Dauers (%) | n |
| daf-2(m577) | 9 | 461 | 66 | 888 |
| daf-2(m577); sem-5(n2195)G201R | 5 | 356 | 48 | 524 |
| daf-2(m577); soc-1(n1789) | 0 | 254 | 1 | 253 |
| daf-2(m577); L4440[RNAi] | 20 | 327 | 77 | 202 |
| daf-2(m577); ptp-2[RNAi] | 2 | 327 | 8 | 146 |
| daf-2(e1370) | 100 | 309 | — | |
| daf-2(e1370); sem-5(n2195)G201R | 100 | 160 | — | |
| daf-2(e1370); soc-1(n1789) | 73a | 161 | — | |
| daf-2(e1370); L4440[RNAi] | 99a | 203 | — | |
| daf-2(e1370); ptp-2[RNAi] | 1b | 207 | — | |
Animals arresting development prior to L2 molt were excluded. —, not determined.
All non-dauers were scored as L3d (dauer-like appearance, but exhibiting strong pharyngeal pumping).
Non-dauers were scored as follows: L3d, 1%; L4s/adults, 98%.
It has recently been shown that the Daf-c phenotype of daf-2(m577) animals is also suppressed by a gain-of-function let-60/Ras mutation (Nanji et al. 2005), although this suppression is weaker than by soc-1(n1789) or by ptp-2 RNAi (not shown). Thus, in the daf-2 Daf-c assay, let-60/Ras functions positively and the C-terminal SH3 domain of sem-5/Grb2, soc-1/Gab1, and ptp-2/Shp2 function negatively. Therefore, the function of the C-terminal SH3 domain of sem-5/Grb2, soc-1/Gab1, and ptp-2/Shp2 downstream of DAF-2 in the dauer assay is likely to be distinct from the function in Ras/Map kinase signaling. As the repression of dauer formation by DAF-2 signaling is largely mediated through PtdIns(3,4,5)P3 production, this suggests that the C-terminal SH3 domain of sem-5/Grb2, soc-1/Gab1, and ptp-2/Shp2 have an inhibitory activity upon PI3K-mediated signaling (Figure 1).
That sem-5(n2195)G201R is a weaker suppressor of daf-2 Daf-c than a null mutation in soc-1/Gab1 may reflect that the C-terminal SH3 domain of sem-5/Grb2 has additional functions, which positively regulate DAF-2 signaling and act in opposition to the inhibitory effect of Gab1 recruitment. Since let-60/Ras is a weak positive modulator of DAF-2 signaling in the Daf-c assay (Nanji et al. 2005), these additional, positive functions are likely to be in the activation of Ras. Consistent with this, in the vulval induction assay for Egfr/Ras/Map kinase signaling, the C-terminal SH3 domain of sem-5/Grb2 has additional positive functions beyond recruiting Gab1 (Table 1). Thus, the degree of inhibition of PI3K-mediated signaling by sem-5(n2195)G201R is likely to be underestimated in the daf-2 Daf-c assay due to the additional and opposing effect upon Ras activation.
In the daf-2 Daf-c assay, ptp-2 RNAi is a stronger suppressor than soc-1. This presumably indicates that ptp-2/Shp2 recruitment may be only partially dependent upon soc-1/Gab1 in insulin receptor-like signaling. An IRS homolog, IST-1, which is predicted to contain an SH2-binding site for ptp-2/Shp2, is known to function in DAF-2 signaling (Wolkow et al. 2002).
soc-1 enhances daf-2 longevity:
In addition to being Daf-c, mutations in daf-2 also increase life span (Kenyon et al. 1993). To test the effects of soc-1/Gab1 on this daf-2 increased life-span (Age) phenotype, life-span trials were performed upon adult worms raised at 20° and shifted to 25° at the L4 stage. Introduction of the soc-1(n1789) mutation did not affect the mortality rate of daf-2(+) or daf-2(m577) animals (Table 4). However, it did significantly affect the mortality rate of daf-2(e1370) animals: the median life span of daf-2(e1370); soc-1(n1789) was enhanced by 40% compared to daf-2(e1370) animals in two of two trials (Table 4). Thus, although soc-1 suppresses daf-2 Daf-c, it enhances the daf-2 Age phenotype. In parallel experiments, sem-5(n2195)G201R reproducibly increased the life span of daf-2(+) animals, but not of daf-2(m577) or daf-2(e1370) animals (Table 4).
TABLE 4.
The effect of sem-5(n2195)G201R, soc-1(n1789), and feeding ptp-2 dsRNA on aging
| Genotype | Trial | Median life span | 75% mortalitya | Maximum life span | nb | Pc |
|---|---|---|---|---|---|---|
| +/+ | 1 | 18 | 18 | 22 | 56 (6) | — |
| 2 | 17 | 19 | 25 | 71 (36) | — | |
| sem-5(n2195) | 1 | 19 | 21 | 25 | 83 (39) | 0.0005 |
| 2 | 18 | 22 | 31 | 77 (62) | 0.006 | |
| soc-1(n1789) | 1 | 15 | 18 | 25 | 44 (9) | 0.5 |
| 2 | 15 | 19 | 27 | 58 (56) | 0.2 | |
| daf-2(m577) | 1 | 32 | 38 | 44 | 36 (15) | — |
| 2 | 33 | 40 | 56 | 102 (59) | — | |
| daf-2(m577); sem-5(n2195) | 1 | 25 | 36 | 60 | 45 (42) | 0.2 |
| 2 | 40 | 49 | 66 | 80 (51) | 0.0002 | |
| daf-2(m577); soc-1(n1789) | 1 | 30 | 44 | 60 | 52 (41) | 0.7 |
| 2 | 32 | 40 | 57 | 84 (55) | 0.6 | |
| daf-2(e1370) | 1 | 38 | 44 | 56 | 24 (64) | — |
| 2 | 59 | 70 | 89 | 93 (98) | — | |
| daf-2(e1370); sem-5(n2195) | 1 | 38 | 44 | 74 | 54 (5) | 0.6 |
| 2 | 68 | 81 | 104 | 96 (16) | <0.0001 | |
| daf-2(e1370); soc-1(n1789) | 1 | 53 | 71 | 107 | 81 (6) | 0.0002 |
| 2 | 87 | 98 | 115 | 117 (41) | <0.0001 | |
| L4440(RNAi) | — | 21 | 25 | 28 | 37 (1) | — |
| ptp-2(RNAi) | — | 13 | 15 | 17 | 44 (16) | <0.0001 |
| daf-2(m577); L4440(RNAi) | — | 35 | 37 | 42 | 52 (11) | — |
| daf-2(m577); ptp-2(RNAi) | — | 23 | 35 | 44 | 62 (15) | 0.007 |
| daf-2(e1370); L4440 | — | 32 | 44 | 60 | 48 (58) | — |
| daf-2(e1370); ptp-2(RNAi) | — | 37 | 42 | 61 | 46 (41) | 0.6 |
Life span was measured at 25°.
The time (in days) at which 75% of animals in the trial had died.
Deaths scored (number of censored values).
P, probability of being identical to the isogenic control strain (log-rank test on censored data).
It has recently been shown that reducing Ras pathway signaling in C. elegans increases mortality of daf-2 animals and that the weak gain-of-function Ras allele, let-60(n1046gf), enhances maximum life span of daf-2 animals (Nanji et al. 2005). Thus, as in the daf-2 Daf-c assay, let-60/Ras and soc-1/Gab1 have opposing functions in a life-span assay. The failure of sem-5(n2195)G201R to enhance the daf-2(e1370) Age phenotype, which would be expected if sem-5/Grb2 acted upstream of soc-1/Gab1, may therefore be due to the opposing effect that reduced let-60/Ras activation would be expected to have upon daf-2 mortality.
Similar experiments were performed with daf-2 mutant animals fed on ptp-2 dsRNA. In these experiments, reduction of ptp-2 activity by RNAi increased daf-2(+) mortality. daf-2(m577) animals fed ptp-2 dsRNA displayed enhanced early mortality, but in this case maximum life span was unaffected (Table 4). Feeding daf-2(e1370) animals ptp-2 dsRNA had little effect on mortality (Table 4).
soc-1 enhances daf-2 early larval arrest:
As mutation of soc-1/Gab1 has opposing effects upon the constitutive dauer formation phenotype and the long-lived phenotype of daf-2 animals, the interaction between soc-1 and daf-2 was addressed using other daf-2 assays. daf-2 mutations form two classes. Class 1 mutants form dauer larvae constitutively, are thermotolerant, and are long lived. Class 2 mutants also exhibit these traits plus one or more additional phenotypes, including embryonic/early larval arrest, reduced pharyngeal pumping, and late progeny production (Gems et al. 1998). It has been hypothesized that daf-2 has two functional elements, daf-2A and daf-2B. Under this hypothesis, class 1 phenotypes would result from loss of daf-2A signaling and class 2 phenotypes from loss of daf-2A and daf-2B signaling (Gems et al. 1998). This does not preclude the possibility that daf-2B signaling affects class 1 phenotypes (Gems et al. 1998; see below). Thus, one possible explanation for the opposing effects of soc-1/Gab1 on daf-2 signaling is that soc-1/Gab1 modulates daf-2A and daf-2B signaling distinctly. Therefore, the effect of soc-1/Gab1 upon class 2 daf-2 phenotypes was addressed using the class 1 daf-2(m577) allele, which does not exhibit class 2 phenotypes, and the weak class 2 allele daf-2(e1370), which displays partially penetrant class 2 phenotypes.
Animals homozygous for the severe class 2 allele daf-2(e979), when raised at 25°, arrest development either prior to hatching or early in the L1 stage (Vowels and Thomas 1992; Gems et al. 1998). daf-2(e979) arrested worms are typically at the four-cell gonad stage (L1), are nonpumping, and are nonvacuolated (Nanji et al. 2005). Similarly, ∼3% of daf-2(e1370) animals raised at 25° arrest at this same stage (Table 5). This arrest is distinguishable from the rod-like L1 lethality phenotype produced by reduced Ras signaling, which is typified by a high degree of vacuolization. Moreover, the Ras lethality is irreversible, but the daf-2 L1 arrest is largely recoverable by placing daf-2 animals at 15°. The class 1 mutant, daf-2(m577), does not arrest (Table 5). Also, control worms, singly mutant for a null mutation in soc-1/Gab1 or with the C-terminal SH3 domain mutation in sem-5/Grb2, do not exhibit the daf-2 L1 arrest. However, a small proportion of sem-5(n2195)G201R animals suffered the rod-like L1 lethality phenotype typical of reduced Ras signaling at 25° and 2 of 143 soc-1(n1789) animals arrested development at the L2 stage (Table 5).
TABLE 5.
daf-2 L1 arrest is enhanced by sem-5(n2195)G201R and soc-1 but not by feeding ptp-2 dsRNA
| Genotype | Dead eggs (%) | L1 let (%) | L1 (%) | L2 (%) | n |
|---|---|---|---|---|---|
| +/+ | 6 | 0 | 0 | 0 | 102 |
| sem-5(n2195)G201R | 0.5 | 3 | 0 | 0 | 213 |
| soc-1(n1789) | 7 | 0 | 0 | 1 | 143 |
| L4440(RNAi) | 0 | 0 | 0 | 0 | 112 |
| ptp-2(RNAi) | 1 | 0 | 0 | 0 | 109 |
| daf-2(m577) | 3 | 0 | 0 | 0 | 316 |
| daf-2(m577); sem-5(n2195)G201R | 4 | 2 | 2 | 0.4 | 258 |
| daf-2(m577); soc-1(n1789) | 4 | 0 | 17 | 6 | 434 |
| daf-2(m577); L4440(RNAi) | 1 | 0 | 0 | 0 | 148 |
| daf-2(m577); ptp-2(RNAi) | 0 | 0 | 0 | 0 | 228 |
| daf-2(e1370) | 2 | 0 | 3 | 0 | 229 |
| daf-2(e1370); sem-5(n2195)G201R | 7 | 9 | 60 | 0 | 232 |
| daf-2(e1370); soc-1(n1789) | 9 | 0.3 | 55 | 2 | 351 |
| daf-2(e1370); L4440(RNAi) | 0 | 0 | 0 | 0 | 170 |
| daf-2(e1370); ptp-2(RNAi) | 0.4 | 0 | 0.4 | 0 | 232 |
Percentage of animals at each stage 72 hr post-egg laying (at 25°).
In addition to displaying a low frequency of rod-like dead L1's (Ras lethality), a small proportion of daf-2(m577); sem-5(n2195)G201R animals arrested development at the L1 stage at 25° in a manner typical of class 2 daf-2 alleles (Table 5; 0/316 vs. 4/258: Fisher's exact test P < 0.05). Although this effect is small, it is also seen in daf-2(m577); soc-1(n1789) animals (17% of daf-2(m577); soc-1(n1789) animals arrest as nonvacuolated L1's; Table 5). Thus the addition of sem-5(n2195)G201R or soc-1(n1789) to daf-2(m577) causes this class 1 allele to display this class 2 phenotype. Consistent with this finding, both sem-5(n2195)G201R and soc-1(n1789) strongly enhanced the L1 arrest phenotype of daf-2(e1370) animals (Table 5). In contrast, rearing daf-2(m577) or daf-2(e1370) animals on bacteria expressing ptp-2 dsRNA did not produce the L1 arrest phenotype (Table 5).
soc-1 acts positively in daf-2B signaling:
The L1 arrest phenotype of daf-2(e979) is suppressed by the weak gain-of-function Ras allele, let-60(n1046gf) (Nanji et al. 2005). At 22.5°, daf-2(m577) enhanced the L1 lethality of let-60(n2021) animals to 100%, all animals being vacuolated, precluding the test of whether reducing Ras signaling would enhance the daf-2 early larval arrest phenotype (Nanji et al. 2005). In addition, let-60(n1046gf) weakly suppresses the reduced pharyngeal pumping of daf-2(e1370) and reducing Ras signaling causes daf-2(m577) to feed less (Nanji et al. 2005). Thus, the finding that both sem-5(n2195)G201R and soc-1(n1789) enhance daf-2 L1 arrest could reflect their positive role in Ras signaling. An alternative, but not mutually exclusive, possibility is that soc-1/Gab1 contributes positively to daf-2B signaling. To test this, the effect of sem-5(n2195)G201R and soc-1(n1789) upon class 2 daf-2 signaling was determined using an assay for late progeny production. Increasing or decreasing Ras signaling in daf-2 mutants has little effect upon this class 2-specific phenotype (Nanji et al. 2005).
Wild-type worms will cease producing offspring in the absence of mating within 5 days of adult life. However, class 2 daf-2 mutant animals can produce a small number of progeny relatively late in life (Gems et al. 1998). Late progeny production in this assay is defined as producing offspring after 9 days of adult life in the absence of mating at 25° (Nanji et al. 2005). Control worms singly mutant for sem-5(n2195)G201R or soc-1 did not produce late progeny. Neither did N2 worms reared on bacteria expressing ptp-2 dsRNA. As expected for a class 1 allele, animals mutant for daf-2(m577) also did not produce late offspring. However, 49% of daf-2(m577); sem-5(n2195)G201R animals and 17% of daf-2(m577); soc-1(n1789) animals produced late progeny (Table 6). Thus, in this second assay for a class 2-specific phenotype, the addition of sem-5(n2195)G201R or soc-1(n1789) to daf-2(m577) causes these class 1 mutant animals to display class 2 phenotypes.
TABLE 6.
sem-5(n2195)G201R and soc-1, but not feeding ptp-2 dsRNA, causes daf-2(m577) to display the class 2-specific late offspring phenotype
| Genotype | Animals producing late offspring (%) | n |
|---|---|---|
| +/+ | 0 | 87 |
| sem-5(n2195)G201R | 0 | 105 |
| soc-1(n1789) | 0 | 34 |
| L4440 [RNAi] | 0 | 34 |
| ptp-2[RNAi] | 0 | 29 |
| daf-2(m577) | 0 | 71 |
| daf-2(m577); sem-5(n2195)G201R | 49 | 76 |
| daf-2(m577); soc-1(n1789) | 17 | 88 |
| daf-2(m577); L4440(RNAi) | 0 | 64 |
| daf-2(m577); ptp-2(RNAi) | 0 | 63 |
| daf-2(e1370) | 65 | 60 |
| daf-2(e1370); sem-5(n2195)G201R | 64 | 67 |
| daf-2(e1370); soc-1(n1789) | 50 | 107 |
| daf-2(e1370); L4440(RNAi) | 72 | 54 |
| daf-2(e1370); ptp-2(RNAi) | 70 | 46 |
Late offspring are defined as appearing 9 days post-L4. Animals dying before day 9 were censored from assay.
About two-thirds of the class 2 daf-2(e1370) mutant hermaphrodites produce late progeny at 25°. This proportion is not significantly affected by introducing sem-5(n2195)G201R or soc-1(n1789) (Table 6). In addition, feeding daf-2(m577) or daf-2(e1370) animals ptp-2 dsRNA did not produce or enhance late progeny production, respectively.
Thus both sem-5(n2195)G201R and soc-1(n1789) cause daf-2(m577) to display class 2 phenotypes and so convert a class 1 daf-2 allele into a class 2 allele (Tables 5 and Table 6). This suggests that both the C-terminal SH3 domain of sem-5/Grb2 and soc-1/Gab1 contribute positively to class 2 daf-2 signaling. As feeding animals ptp-2 dsRNA did not cause class 2 phenotypes in daf-2(m577) or enhance them in daf-2(e1370), it is unlikely that ptp-2/Shp2 mediates this positive daf-2 signaling.
DISCUSSION
Previous genetic studies have suggested that the multi-substrate adaptor protein SOC-1/Dos/Gab1 contributes positively to RTK signaling (Herbst et al. 1996; Raabe et al. 1996; Schutzman et al. 2001). However, the assays used in these studies were for RTK signaling mediated by the Ras/Map kinase pathway. In this study, using sensitized assays for Egfr and InsR signaling in C. elegans, we show that soc-1/Gab1 has both positive and negative effects upon RTK signaling. The opposing effects of soc-1/Gab1 upon RTK signaling using distinct assays is likely to reflect the relative contribution of the various intracellular signaling pathways activated by the RTK to the tissue-specific function that forms the basis of the assay (Figure 1). Thus, when RTK signaling achieves a particular function through activation of the Ras/Map kinase pathway, soc-1/Gab1 acts positively (Table 1; Herbst et al. 1996; Raabe et al. 1996; Schutzman et al. 2001). When Ras activation downstream of the RTK signaling is not essential for the function of RTK in a particular cell(s), then additional functions for soc-1/Gab1 may be observed. For example, let-23/Egfr activates PLCγ, leading to IP3 production, which mediates its function in fertility (Clandinin et al. 1998; Yin et al. 2004). Ras activation downstream of the Egfr is not a requirement for fertility and soc-1/Gab1 acts negatively in this assay (Table 2). Likewise, although Ras signaling modulates daf-2/InsR signaling in C. elegans, it is not essential and soc-1/Gab1 has the opposite function to let-60/Ras in daf-2/InsR signaling of repressing dauer formation and promoting aging (this study and Nanji et al. 2005). Thus, soc-1/Gab1 has activities in addition to its function in Ras/Map kinase-mediated signaling. These activities are likely to be regulatory in nature as they are readily observed only in sensitized backgrounds.
The recruitment of soc-1/Gab1 into the RTK-signaling complex is likely to be mediated through its interaction with the C-terminal SH3 domain of sem-5/Grb2. This interaction maps to two atypical SH3-binding sites on Gab1 (Lock et al. 2000). Likewise, the Drosophila Gab1 homolog Dos has two atypical SH3-binding sites that bind Grb2 and these sites are necessary for Dos function in sev RTK and Egfr signaling (Feller et al. 2002). The soc-1 gene is predicted to produce a protein containing a single atypical SH3-binding site matching the consensus from Gab1 and Dos (Figure 2A). Furthermore, the interaction between the Gab1 and the C-terminal SH3 domain of Grb2 is also likely to be conserved in C. elegans as the atypical SH3-binding site in SOC-1 can bind SEM-5, the C. elegans Grb2 homolog, in yeast two-hybrid experiments (Figure 2C). In addition, the introduction of the sem-5(n2195) mutation, which substitutes glycine at position 201 (corresponding to 203 in Grb2) for arginine within the C-terminal SH3 domain, abolishes this interaction with SOC-1. In all the genetic assays performed here, sem-5(n2195)G201R behaves qualitatively similarly to a null mutation in soc-1, although there are quantitative differences (see below). Together, this provides strong evidence that recruitment of soc-1/Gab1 downstream of the Egfr and InsR in C. elegans is dependent upon its interaction with the C-terminal SH3 domain of sem-5/Grb2.
The function of soc-1/Gab1 in Ras/Map kinase signaling is to recruit Shp2 (Herbst et al. 1999; Bausenwein et al. 2000; Schutzman et al. 2001). Shp2 contains two SH2 domains preceding a tyrosine phosphatase domain. Shp2 is not required for initial Map kinase activation, but is required for sustained Map kinase signaling (Hadari et al. 1998; Maroun et al. 2000; Zhang et al. 2002). Evidence that sustained Map kinase signaling is focused in endosomes is accumulating (Vieira et al. 1996; Kranenburg et al. 1999; Wu et al. 2001; Teis et al. 2002). Thus it is possible that the function of Shp2 is to establish a sustained Map kinase signaling complex within an endosomal compartment. In this process, the C-terminal SH3 domain of sem-5/Grb2 is likely to have three distinct activities. First, it weakly contributes to Sos binding and subsequent Ras activation (Sastry et al. 1995). Second, it recruits Gab1, which in turn binds Shp2. And third, it promotes endocytosis of the activated RTK, presumably through interaction with dynamin (Wang and Moran 1996). Thus, although sem-5(n2195)G201R and null mutations in soc-1/Gab1 and ptp-2/Shp2 all suppress a gain-of-function let-60/Ras allele, sem-5(n2195)G201R has the stronger effect (Table 1).
sem-5(n2195)G201R is a stronger suppressor of let-23/Egfr sterility than soc-1/Gab1, suggesting that sem-5/Grb2 has additional, negative functions in fertility other than binding soc-1/Gab1 (Table 2). In this case, it is unlikely that the function of binding Sos is important, as sos-1 did not suppress let-23/Egfr sterility. Instead, the role of the C-terminal SH3 domain of sem-5/Grb2 in the binding of signaling attenuators such as ark-1, which inhibits the let-23/Egfr fertility function, may be important (Hopper et al. 2000). This may lead, in turn, to the stimulation of endocytosis of the activated RTK, a process known to involve the C-terminal SH3 domain of sem-5/Grb2 (Wang and Moran 1996). The fertility function of let-23/Egfr is mediated through PLCγ and IP3 production (Clandinin et al. 1998; Yin et al. 2004). Blocking endocytosis has been shown to increase phosphorylation of PLCγ whose activity may be confined to the cell surface (Vieira et al. 1996; Haugh et al. 1999; Haugh and Meyer 2002).
The C-terminal SH3 domain of sem-5/Grb2, soc-1/Gab1, and ptp-2/Shp2 also inhibit daf-2/InsR signaling during dauer formation (Table 3). In this case, ptp-2/Shp2 had the strongest effect. This is likely to be because downstream of the daf-2/InsR, in contrast with let-23/Egfr signaling, ptp-2/Shp2 is not completely dependent upon recruitment by soc-1/Gab1. The IRS homolog, IST-1, is predicted to contain an SH2-binding site for ptp-2/Shp2 (Wolkow et al. 2002). This activity of soc-1/Gab1 and ptp-2/Shp2 is distinct from that which promotes Ras/Map kinase signaling, as Ras signaling positively modulates daf-2/InsR signaling to repress dauer formation (Nanji et al. 2005), whereas soc-1/Gab1 and ptp-2/Shp2 activity negatively modulates daf-2/InsR signaling in the same assay. DAF-2 signaling is primarily mediated through AGE-1/PI3K, which produces PtdIns(3,4,5)P3 from PtdIns(4,5)P2 to inhibit dauer formation and promote reproductive development (Morris et al. 1996). This suggests that the C-terminal SH3 domain of sem-5/Grb2, soc-1/Gab1, and ptp-2/Shp2 inhibits PI3K-mediated signaling. It may be that ptp-2/Shp2 leads to the establishment of a sustained Ras/Map kinase signaling complex and that this indirectly inhibits PI3K-mediated signaling by removing the receptor-signaling complex from a source of PIP2, the precursor of PIP3 (Haugh and Meyer 2002). Alternatively, the inhibitory effect of ptp-2/Shp2 may be more direct, through the dephosphorylation of PI3K-binding sites (Myers et al. 1998; Zhang et al. 2002).
In contrast to their inhibitory effect upon daf-2/InsR signaling during dauer formation, the C-terminal SH3 domain of sem-5/Grb2 and soc-1/Gab1 contributes positively to class 2 daf-2/InsR signaling. The daf-2 gene has been proposed to contain distinct functional elements, hypothesized as daf-2A and daf-2B, with class 1 daf-2 phenotypes arising from loss of daf2A signaling and with class 2 daf-2 phenotypes arising from loss of both daf-2A and daf-2B signaling (Gems et al. 1998). The class 1 allele, daf-2(m577), displays class 2 phenotypes when the C-terminal SH3 domain of sem-5/Grb2 or soc-1/Gab1 is mutated (Tables 5 and Table 6). Under the above hypothesis, this positive function of the C-terminal SH3 domain of sem-5/Grb2 and soc-1/Gab1 in daf-2/InsR signaling may be specific to daf-2B signaling.
sem-5(n2195)G201R was more potent than soc-1/Gab1 in causing the class 1 daf-2(m577) allele to display the class 2-specific phenotype of late progeny production (Fisher's exact test P < 0.0001; Table 6). This may reflect a role for Ras in daf-2B signaling, although reducing Ras signaling in daf-2(m577) does not produce an appreciable late offspring phenotype (Nanji et al. 2005). An alternative possibility is that the C-terminal SH3 domain of sem-5/Grb2 promotes endocytosis of daf-2/InsR as it does for the Egfr and this additional effect upon endocytosis produces the stronger late progeny phenotype.
The suppression of daf-2 Daf-c and the enhancement of the daf-2 L1 arrest and late offspring phenotypes suggests that soc-1/Gab1 modulates daf-2A and daf-2B signaling distinctly. It is hypothesized that soc-1 negatively regulates daf-2A signaling and positively regulates daf-2B signaling. However, soc-1/Gab1 enhances the Age phenotype of daf-2(e1370) animals, a class 1 phenotype. If soc-1 negatively regulates daf-2A signaling, this enhancement of the Age phenotype could be explained only if daf-2B signaling also contributes to class 1 signaling. There is evidence that this is the case from the comparison of the Daf-c phenotypes of daf-2(e1369) and daf-2(e1370). daf-2(e1369) is a class 1 allele and daf-2(e1370) is a class 2 allele; therefore, daf-2(e1370) is more severely affected for daf-2B signaling than daf-2(e1369). As daf-2(e1369) is more severe in terms of Daf-c than daf-2(e1370), it follows that daf-2(e1369) is more severely affected for daf-2A signaling than daf-2(e1370) (Gems et al. 1998). daf-18(e1375) completely suppresses daf-2(e1369) Daf-c, but not daf-2(e1370) Daf-c (Nanji et al. 2005). This implication of this is twofold: daf-2A but not daf-2B signaling is strongly suppressed by daf-18(e1375) and a defect in daf-2B signaling in daf-2(e1370) contributes to its Daf-c phenotype (Nanji et al. 2005). The enhancement of the daf-2(e1370) Age phenotype by soc-1(n1789) might therefore be explained by the positive contribution that soc-1/Gab1 makes to daf-2B signaling.
Thus, the recruitment of soc-1/Gab1 by the C-terminal SH3 domain of sem-5/Grb2 into the RTK-signaling complex enhances Ras/Map kinase signaling and depresses PI3K- and PLCγ-mediated signaling. What is the function of this change in RTK signaling output? Recruitment of Gab1 into the RTK-signaling complex is regulated by the extent of signaling (Rodrigues et al. 2000). Subsequent Shp2 recruitment is required for sustained Map kinase signaling, which can result in distinct cellular responses to transient signaling (Marshall 1995). Different intracellular signaling pathways are regulated distinctly by soc-1/Gab1 recruitment. The regulated recruitment of soc-1/Gab1 therefore provides a mechanism whereby the magnitude and duration of RTK signaling can subtly alter the signaling output and produce distinct cellular responses.
Acknowledgments
I thank Anne Wooller for technical support and for performing the yeast two-hybrid experiments (presented in Figure 2C). Some strains in this study were gratefully received from the Caenorhabditis Genetics Center (funded by the National Institutes of Health) and David Gems (who provided DR1567). Thanks also to David Gems, Lindy Holden-Dye, Giovanni Lesa, and Vincent O'Connor for critical reading of the manuscript and discussions. This work was supported by the Wellcome Trust. The author was a Wellcome Trust Research Career Development Fellow (064988).
References
- Aroian, R. V., G. M. Lesa and P. W. Sternberg, 1994. Mutations in the Caenorhabditis elegans let-23 EGFR-like gene define elements important for cell-type specificity and function. EMBO J. 13: 360–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battu, G., E. F. Hoier and A. Hajnal, 2003. The C. elegans G-protein-coupled receptor SRA-13 inhibits RAS/MAPK signalling during olfaction and vulval development. Development 130: 2567–2577. [DOI] [PubMed] [Google Scholar]
- Bausenwein, B. S., M. Schmidt, B. Mielke and T. Raabe, 2000. In vivo functional analysis of the daughter of sevenless protein in receptor tyrosine kinase signaling. Mech. Dev. 90: 205–215. [DOI] [PubMed] [Google Scholar]
- Beitel, G. J., S. G. Clark and H. R. Horvitz, 1990. Caenorhabditis elegans ras gene let-60 acts as a switch in the pathway of vulval induction. Nature 348: 503–509. [DOI] [PubMed] [Google Scholar]
- Clandinin, T. R., J. A. DeModena and P. W. Sternberg, 1998. Inositol trisphosphate mediates a RAS-independent response to LET-23 receptor tyrosine kinase activation in C. elegans. Cell 92: 523–533. [DOI] [PubMed] [Google Scholar]
- Clark, S. G., M. J. Stern and H. R. Horvitz, 1992. C. elegans cell-signalling gene sem-5 encodes a protein with SH2 and SH3 domains. Nature 356: 340–344. [DOI] [PubMed] [Google Scholar]
- Feller, S. M., H. Wecklein, M. Lewitzky, E. Kibler and T. Raabe, 2002. SH3 domain-mediated binding of the Drk protein to Dos is an important step in signaling of Drosophila receptor tyrosine kinases. Mech. Dev. 116: 129–139. [DOI] [PubMed] [Google Scholar]
- Gems, D., A. J. Sutton, M. L. Sundermeyer, P. S. Albert, K. V. King et al., 1998. Two pleiotropic classes of daf-2 mutation affect larval arrest, adult behavior, reproduction and longevity in Caenorhabditis elegans. Genetics 150: 129–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, H., and B. G. Neel, 2003. The “Gab” in signal transduction. Trends Cell Biol. 13: 122–130. [DOI] [PubMed] [Google Scholar]
- Gutch, M. J., A. J. Flint, J. Keller, N. K. Tonks and M. O. Hengartner, 1998. The Caenorhabditis elegans SH2 domain-containing protein tyrosine phosphatase PTP-2 participates in signal transduction during oogenesis and vulval development. Genes Dev. 12: 571–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadari, Y. R., H. Kouhara, I. Lax and J. Schlessinger, 1998. Binding of Shp2 tyrosine phosphatase to FRS2 is essential for fibroblast growth factor-induced PC12 cell differentiation. Mol. Cell. Biol. 18: 3966–3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugh, J. M., and T. Meyer, 2002. Active EGF receptors have limited access to PtdIns(4,5)P(2) in endosomes: implications for phospholipase C and PI 3-kinase signaling. J. Cell Sci. 115: 303–310. [DOI] [PubMed] [Google Scholar]
- Haugh, J. M., K. Schooler, A. Wells, H. S. Wiley and D. A. Lauffenburger, 1999. Effect of epidermal growth factor receptor internalization on regulation of the phospholipase C-gamma1 signaling pathway. J. Biol. Chem. 274: 8958–8965. [DOI] [PubMed] [Google Scholar]
- Herbst, R., P. M. Carroll, J. D. Allard, J. Schilling, T. Raabe et al., 1996. Daughter of sevenless is a substrate of the phosphotyrosine phosphatase Corkscrew and functions during sevenless signaling. Cell 85: 899–909. [DOI] [PubMed] [Google Scholar]
- Herbst, R., X. Zhang, J. Qin and M. A. Simon, 1999. Recruitment of the protein tyrosine phosphatase CSW by DOS is an essential step during signaling by the sevenless receptor tyrosine kinase. EMBO J. 18: 6950–6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holgado-Madruga, M., D. R. Emlet, D. K. Moscatello, A. K. Godwin and A. J. Wong, 1996. A Grb2-associated docking protein in EGF- and insulin-receptor signalling. Nature 379: 560–564. [DOI] [PubMed] [Google Scholar]
- Hopper, N. A., J. Lee and P. W. Sternberg, 2000. ARK-1 inhibits EGFR signaling in C. elegans. Mol. Cell 6: 65–75. [PubMed] [Google Scholar]
- Isakoff, S. J., T. Cardozo, J. Andreev, Z. Li, K. M. Ferguson et al., 1998. Identification and analysis of PH domain-containing targets of phosphatidylinositol 3-kinase using a novel in vivo assay in yeast. EMBO J. 17: 5374–5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongeward, G. D., T. R. Clandinin and P. W. Sternberg, 1995. sli-1, a negative regulator of let-23-mediated signaling in C. elegans. Genetics 139: 1553–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath, R. S., M. Martinez-Campos, P. Zipperlen, A. G. Fraser and J. Ahringer, 2001. Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans. Genome Biol. 2: RESEARCH0002. [DOI] [PMC free article] [PubMed]
- Kenyon, C., J. Chang, E. Gensch, A. Rudner and R. Tabtiang, 1993. A C. elegans mutant that lives twice as long as wild type. Nature 366: 461–464. [DOI] [PubMed] [Google Scholar]
- Kimura, K. D., H. A. Tissenbaum, Y. Liu and G. Ruvkun, 1997. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 277: 942–946. [DOI] [PubMed] [Google Scholar]
- Kornfeld, K., 1997. Vulval development in Caenorhabditis elegans. Trends Genet. 13: 55–61. [DOI] [PubMed] [Google Scholar]
- Kranenburg, O., I. Verlaan and W. H. Moolenaar, 1999. Dynamin is required for the activation of mitogen-activated protein (MAP) kinase by MAP kinase kinase. J. Biol. Chem. 274: 35301–35304. [DOI] [PubMed] [Google Scholar]
- Lesa, G. M., and P. W. Sternberg, 1997. Positive and negative tissue-specific signaling by a nematode epidermal growth factor receptor. Mol. Biol. Cell 8: 779–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y., and L. R. Rohrschneider, 2002. The gift of Gab. FEBS Lett. 515: 1–7. [DOI] [PubMed] [Google Scholar]
- Lock, L. S., I. Royal, M. A. Naujokas and M. Park, 2000. Identification of an atypical Grb2 carboxyl-terminal SH3 domain binding site in Gab docking proteins reveals Grb2-dependent and -independent recruitment of Gab1 to receptor tyrosine kinases. J. Biol. Chem. 275: 31536–31545. [DOI] [PubMed] [Google Scholar]
- Maroun, C. R., M. A. Naujokas, M. Holgado-Madruga, A. J. Wong and M. Park, 2000. The tyrosine phosphatase SHP-2 is required for sustained activation of extracellular signal-regulated kinase and epithelial morphogenesis downstream from the met receptor tyrosine kinase. Mol. Cell. Biol. 20: 8513–8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall, C. J., 1995. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 80: 179–185. [DOI] [PubMed] [Google Scholar]
- Morris, J. Z., H. A. Tissenbaum and G. Ruvkun, 1996. A phosphatidylinositol-3-OH kinase family member regulating longevity and diapause in Caenorhabditis elegans. Nature 382: 536–539. [DOI] [PubMed] [Google Scholar]
- Myers, M. G., Jr., R. Mendez, P. Shi, J. H. Pierce, R. Rhoads et al., 1998. The COOH-terminal tyrosine phosphorylation sites on IRS-1 bind SHP-2 and negatively regulate insulin signaling. J. Biol. Chem. 273: 26908–26914. [DOI] [PubMed] [Google Scholar]
- Nanji, M., N. A. Hopper and D. Gems, 2005. LET-60 RAS modulates effects of insulin/IGF-1 signaling on development and aging in Caenorhabditis elegans. Aging Cell 4: 235–245. [DOI] [PubMed] [Google Scholar]
- Nishida, K., and T. Hirano, 2003. The role of Gab family scaffolding adapter proteins in the signal transduction of cytokine and growth factor receptors. Cancer Sci. 94: 1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogg, S., S. Paradis, S. Gottlieb, G. I. Patterson, L. Lee et al., 1997. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature 389: 994–999. [DOI] [PubMed] [Google Scholar]
- Paradis, S., and G. Ruvkun, 1998. Caenorhabditis elegans Akt/PKB transduces insulin receptor-like signals from AGE-1 PI3 kinase to the DAF-16 transcription factor. Genes Dev. 12: 2488–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis, S., M. Ailion, A. Toker, J. H. Thomas and G. Ruvkun, 1999. A PDK1 homolog is necessary and sufficient to transduce AGE-1 PI3 kinase signals that regulate diapause in Caenorhabditis elegans. Genes Dev. 13: 1438–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson, T., and J. D. Scott, 1997. Signaling through scaffold, anchoring, and adaptor proteins. Science 278: 2075–2080. [DOI] [PubMed] [Google Scholar]
- Raabe, T., J. Riesgo-Escovar, X. Liu, B. S. Bausenwein, P. Deak et al., 1996. DOS, a novel pleckstrin homology domain-containing protein required for signal transduction between sevenless and Ras1 in Drosophila. Cell 85: 911–920. [DOI] [PubMed] [Google Scholar]
- Riddle, D. L., and P. S. Albert, 1997. Genetic and environmental regulation of dauer larva development, pp. 739–768 in C. elegans II, edited by D. L. Riddle, T. Blumenthal, B. J. Meyer and J. L. Priess. Cold Spring Harbor Laboratory Press, Plainview, NY. [PubMed]
- Rodrigues, G. A., M. Falasca, Z. Zhang, S. H. Ong and J. Schlessinger, 2000. A novel positive feedback loop mediated by the docking protein Gab1 and phosphatidylinositol 3-kinase in epidermal growth factor receptor signaling. Mol. Cell. Biol. 20: 1448–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastry, L., W. Lin, W. T. Wong, P. P. Di Fiore, C. A. Scoppa et al., 1995. Quantitative analysis of Grb2-Sos1 interaction: the N-terminal SH3 domain of Grb2 mediates affinity. Oncogene 11: 1107–1112. [PubMed] [Google Scholar]
- Schlessinger, J., 2000. Cell signaling by receptor tyrosine kinases. Cell 103: 211–225. [DOI] [PubMed] [Google Scholar]
- Schlessinger, J., 2004. Common and distinct elements in cellular signaling via EGF and FGF receptors. Science 306: 1506–1507. [DOI] [PubMed] [Google Scholar]
- Schutzman, J. L., C. Z. Borland, J. C. Newman, M. K. Robinson, M. Kokel et al., 2001. The Caenorhabditis elegans EGL-15 signaling pathway implicates a DOS-like multisubstrate adaptor protein in fibroblast growth factor signal transduction. Mol. Cell. Biol. 21: 8104–8116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg, P. W., and M. Han, 1998. Genetics of RAS signaling in C. elegans. Trends Genet. 14: 466–472. [DOI] [PubMed] [Google Scholar]
- Teis, D., W. Wunderlich and L. A. Huber, 2002. Localization of the MP1-MAPK scaffold complex to endosomes is mediated by p14 and required for signal transduction. Dev. Cell 3: 803–814. [DOI] [PubMed] [Google Scholar]
- Timmons, L., and A. Fire, 1998. Specific interference by ingested dsRNA. Nature 395: 854. [DOI] [PubMed] [Google Scholar]
- Vieira, A. V., C. Lamaze and S. L. Schmid, 1996. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science 274: 2086–2089. [DOI] [PubMed] [Google Scholar]
- Vowels, J. J., and J. H. Thomas, 1992. Genetic analysis of chemosensory control of dauer formation in Caenorhabditis elegans. Genetics 130: 105–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z., and M. F. Moran, 1996. Requirement for the adapter protein GRB2 in EGF receptor endocytosis. Science 272: 1935–1939. [DOI] [PubMed] [Google Scholar]
- Weidner, K. M., S. Di Cesare, M. Sachs, V. Brinkmann, J. Behrens et al., 1996. Interaction between Gab1 and the c-Met receptor tyrosine kinase is responsible for epithelial morphogenesis. Nature 384: 173–176. [DOI] [PubMed] [Google Scholar]
- Wolkow, C. A., M. J. Munoz, D. L. Riddle and G. Ruvkun, 2002. Insulin receptor substrate and p55 orthologous adaptor proteins function in the Caenorhabditis elegans daf-2/insulin-like signaling pathway. J. Biol. Chem. 277: 49591–49597. [DOI] [PubMed] [Google Scholar]
- Wu, C., C. F. Lai and W. C. Mobley, 2001. Nerve growth factor activates persistent Rap1 signaling in endosomes. J. Neurosci. 21: 5406–5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, X., N. J. Gower, H. A. Baylis and K. Strange, 2004. Inositol 1,4,5-trisphosphate signaling regulates rhythmic contractile activity of myoepithelial sheath cells in Caenorhabditis elegans. Mol. Biol. Cell 15: 3938–3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S. Q., W. G. Tsiaras, T. Araki, G. Wen, L. Minichiello et al., 2002. Receptor-specific regulation of phosphatidylinositol 3′-kinase activation by the protein tyrosine phosphatase Shp2. Mol. Cell. Biol. 22: 4062–4072. [DOI] [PMC free article] [PubMed] [Google Scholar]