Abstract
Yeast prions are non-Mendelian genetic elements that are conferred by altered and self-propagating protein conformations. Such a protein conformation-based transmission is similar to that of PrPSc, the infectious protein responsible for prion diseases. Despite recent progress in understanding the molecular nature and epigenetic transmission of prions, the underlying mechanisms governing prion conformational switch and determining prion “strains” are not understood. We report here that the evolutionarily conserved heat-shock transcription factor (HSF) strongly influences yeast prion formation and strain determination. An hsf1 mutant lacking the amino-terminal activation domain inhibits the yeast prion [PSI+] formation whereas a mutant lacking the carboxyl-terminal activation domain promotes [PSI+] formation. Moreover, specific [PSI+] strains are preferentially formed in these mutants, demonstrating the importance of genetic makeup in determining de novo appearance of prion strains. Although these hsf1 mutants preferentially support the formation of certain [PSI+] strains, they are capable of receiving and faithfully propagating nonpreferable strains, suggesting that prion initiation and propagation are distinct processes requiring different cellular components. Our findings establish the importance of HSF in prion initiation and strain determination and imply a similar regulatory role of mammalian HSFs in the complex etiology of prion disease.
PRIONS or proteinaceous infectious particles are generally considered to be responsible for the class of fatal mammalian neurodegenerative diseases known as transmissible spongiform encephalopathies, including scrapie in sheep, bovine spongiform encephalopathy in cattle, and Creutzfeldt–Jakob disease in humans (Prusiner 2004). In each case, it is thought that a normal host protein, PrPC, changes conformation to adopt a pathogenic or prion isoform, PrPSc. Remarkably, PrPSc can self-propagate by converting additional PrPC to its prion conformation and is thus infectious (Prusiner 1998). There are at least four epigenetic elements in yeast, [PSI+], [URE3], [RNQ+], and [NU+], which are transmitted from mother to daughter cell as particular self-propagating protein conformations. They are known as yeast prions since their transmission mechanism is similar to that of PrPSc (for reviews see Liebman and Derkatch 1999; Uptain and Lindquist 2002; Chien et al. 2004; Wickner et al. 2004; Jones and Tuite 2005). Yeast prions share many features with their mammalian brethren, despite the absence of any structural or functional homologies among the implicated proteins. Similar to the mammalian prion protein, yeast prion proteins are capable of perpetuating particular conformational changes, forming amyloid (ordered protein aggregates) fibers under physiological conditions (Glover et al. 1997; King et al. 1997) and existing as distinct prion “strains” (Derkatch et al. 1996; Schlumpberger et al. 2001; Bradley and Liebman 2003). In mammals, several distinct strains of prion disease have been described, which differ in symptoms, incubation times, and brain pathologies (Prusiner 1998). Yeast prion strains are generally referred to as variants to distinguish them from the traditional classification of yeast strains (Derkatch et al. 1996).
Yeast Sup35, the protein determinant of prion [PSI+], is a homolog of the highly conserved eukaryotic release factor 3 (eRF3). When Sup35 is in its native conformation, it binds to Sup45 to form a functional translational termination factor to direct ribosomes to stop faithfully at stop codons (Stansfield et al. 1995). When Sup35 enters an altered conformation, [PSI+], it is sequestered from the translation termination machinery to result in translational read-through. As a consequence, [PSI+] cells that contain ade1-14, a nonsense mutation in ADE1, are able to grow in medium lacking adenine because of sufficient read-through of ade1-14 (Cox 1965; Firoozan et al. 1991). In contrast, isogenic [psi−] cells with the native Sup35 conformation cannot grow in adenine-deficient media since translation faithfully terminates at the nonsense codon. Thus, isogenic [PSI+] and [psi−] cells carrying ade1-14 exhibit strikingly different appearances on YPD (rich media): [psi−] cells are red due to the accumulation of a pigment byproduct whereas [PSI+] cells are white due to the translational read-through. Such differences in growth and appearance provide a sensitive and convenient assay for [PSI+] (Cox 1965).
Although there has been significant progress recently in understanding the “protein only” transmission of prions (King and Diaz-Avalos 2004; Legname et al. 2004; Tanaka et al. 2004; Castilla et al. 2005; Liebman 2005; Liebman and Mastrianni 2005), the molecular mechanisms underlying the prion conformational switch and strain determination remain unknown. To unveil the cellular machinery governing prion formation and transmission, we have investigated the role of yeast heat-shock transcription factor (HSF) in prion formation and propagation. HSFs are evolutionarily conserved transcription factors that play an essential role in protecting eukaryotic organisms against heat shock and other environmental stresses (Morano and Thiele 1999). For example, upon heat shock, HSF is activated to increase the production of heat-shock proteins (HSPs), most of which are molecular chaperones, a group of proteins that exercise protective functions in the cell by refolding or disaggregating denatured proteins produced during the stress (Pirkkala et al. 2001). Several molecular chaperones, Hsp104, Ssa and Ssb, and Sis1, are implicated in playing important roles in prion propagation (Chernoff et al. 1995b; Jung et al. 2000; Kushnirov et al. 2000; Jung and Masison 2001; Sondheimer et al. 2001; Allen et al. 2005). Hsp90 cochaperones Sti1 and Cpr7 were also shown to influence [PSI+] stability (Jones et al. 2004). Although studying the effect of individual factors and their simple combinations on yeast prion formation has proven to be fruitful, such an approach would likely fail to uncover factors with a functionally redundant homolog(s) or protein networks that are required for prion formation. Since HSFs are the master regulators in controlling the expression of molecular chaperones, elucidating the link between prion formation and HSF would possibly allow us to identify such cellular components.
HSFs are structurally and functionally conserved from yeast to humans, containing a winged helix-turn-helix DNA-binding domain, a hydrophobic stretch necessary for homotrimerization, followed by a transcription activation domain that is also conserved (Wu 1995; Littlefield and Nelson 2001). HSF binds to cis-acting DNA promoter elements known as heat-shock elements (HSEs), which are highly conserved as well (Amin et al. 1988). Unlike higher eukaryotes possessing multiple distinct HSF isomers, yeast has a single essential gene, HSF1, containing both an amino-terminal activation domain (NTA, residues 1–147) and a carboxyl-terminal activation domain (CTA, residues 584–833), which are thought to differentially regulate the expression of HSF target genes (Sorger 1990; Chen and Parker 2002). Recently, a genomewide analysis using chromatin immunoprecipation (CHIP)-based DNA microarray technology identified ∼165 direct HSF-target genes, which function in diverse processes including protein folding and degradation, transcription, energy generation, protein trafficking, and cell signaling (Hahn et al. 2004). In this study, we examined yeast prion formation and transmission in two HSF truncation mutants, ΔNTA-HSF (147–833) and ΔCTA-HSF (1–584). We report here that both HSF truncation mutants have profound and distinct effects on de novo formation as well as strain determination of the yeast prion [PSI+]. We have also shown that cellular factors essential for maintaining the yeast prion [PSI+] can be differentially expressed in different hsf1 mutants. Our results demonstrate that elucidating the link between HSF and yeast prions will provide valuable information on the mechanisms of prion initiation and propagation.
MATERIALS AND METHODS
Plasmids:
Plasmids described in this study are listed in Table 1. To construct pRS416GPD-HSF (URA3), the HSF1 ORF was obtained by a polymerase chain reaction (PCR) using pRS314 HSF (TRP1) as the template, 5′ TTGTTCCCGGGATGAATAATGCTGCA 3′ as the forward primer, and 5′ TTGCCCTGAATTCTATTTCTTAGC 3′ as the reverse primer. After digestion with SmaI and EcoRI, the PCR fragment was ligated to pRS416GPD (URA3) that was predigested with SmaI/EcoRI. To create the hsf1 disruption construct, we applied a PCR-based method using pFA6a-kanMX6 (KanR) as the template and two primers, HSF1 F1 5′ GAAACAAAAAAGACAAAAAGACAGCTGTATTGTTGGCGCCCGGATCCCCGGGTTAATTAA 3′ and HSF1 R1 5′ AAATGATTATATACGCTATTTAATGACCTTGCCCTGTGTAGAATTCGAGCTCGTTTAAAC 3′, to obtain a PCR product containing the KanR marker flanked with HSF1 sequence. To create pRS305-HSP104 (LEU2), pYS104 (URA3) was digested with XhoI and the resulting XhoI–XhoI fragment containing the HSP104 coding region as well as its flanking regions was ligated to pRS305 (LEU2) that was predigested with XhoI. To create pRS415-HSP104, pYS104 was digested with ClaI and blunt ended by DNA polymerase I treatment followed by XhoI digestion. The resulting ClaI (blunt-ended)–XhoI fragment containing HSP104 coding sequence and its flanking regions was ligated to p415GPD that was predigested with SacI (blunt ended by DNA polymerase I treatment) and XhoI (the GPD promoter of p415GPD was thus removed). To create p425GPD-HSP104 (LEU2), p2HG-104 (HIS3) (Li and Lindquist 2000) was digested with BamHI and the resulting BamHI–BamHI fragment containing the HSP104 coding region was ligated to p425GPD (LEU2) that was predigested with BamHI.
TABLE 1.
Plasmids used in this study
| Plasmid | Auxotrophic marker | Promoter | Copy number | Source |
|---|---|---|---|---|
| pRS416GPD-HSF1 | URA3 | GPD | CEN, low | This study |
| pRS314-HSF1 | TRP1 | HSF1 | CEN, low | Morano et al. (1999) |
| pRS314-HSF1ΔNTA(148–833) | TRP1 | HSF1 | CEN, low | This study |
| pRS314-HSF1ΔCTA(1–583) | TRP1 | HSF1 | CEN, low | Morano et al. (1999) |
| pCUP1-GFP | URA3 | CUP1 | CEN, low | Lindquist lab |
| pCUP1-Sup35GFP | URA3 | CUP1 | CEN, low | Lindquist lab |
| pCUP1-NMGFP | URA3 | CUP1 | CEN, low | Lindquist lab |
| pCUP1-Rnq1GFP | URA3 | CUP1 | CEN, low | Sondheimer and Lindquist (2000) |
| pCUP1-Ure2(1–65)-GFP | URA3 | CUP1 | CEN, low | Lindquist lab |
| pRS313CUP1-NMGFP | HIS3 | CUP1 | CEN, low | Derkatch et al. (2001) |
| pRS305-HSP104 | LEU2 | HSP104 | Integrating, single | This study |
| pRS415-HSP104 | LEU2 | HSP104 | CEN, low | This study |
| p425GPD-HSP104 | LEU2 | GPD | 2μ, high | This study |
| P426/PQ25 | URA3 | GPD | 2μ, high | Krobitsch and Lindquist (2000) |
| P426/PQ47 | URA3 | GPD | 2μ, high | Krobitsch and Lindquist (2000) |
| P426/PQ72 | URA3 | GPD | 2μ, high | Krobitsch and Lindquist (2000) |
| P426/PQ103 | URA3 | GPD | 2μ, high | Krobitsch and Lindquist (2000) |
Yeast strains:
Yeast strains are listed in Table 2. To obtain the hsf1 disruption strain we transformed 74D-694 (MATa: ade1-14, trp1-289, his3Δ-200, ura3-52, leu2-3, 112, [psi−], [RNQ+]) with pRS416GPD-HSF1 followed by chromosomal replacement of HSF1 with the PCR fragment containing the KanR marker flanked by HSF1 sequence as described above through “one-step” gene replacement (Sherman 1991). The resulting hsf1 disruption strain was named 74D-hsf1∷KanR.
TABLE 2.
S. cerevisiae strains used in this study
| Strain | Genotype description | Source |
|---|---|---|
| 74D-694 | MATa: ade1-14, trp1-289, his3Δ-200, ura3-52, leu2-3, 112, [psi−][PIN+] | Patino et al. (1996) |
| 74D-694-wt-HSF | MATa: ade1-14, trp1-289, his3Δ-200, ura3-52, leu2-3, 112, hsf1∷KANR, [psi−][PIN+], containing pRS314-HSF | This study |
| 74D-694-ΔNTA-HSF | MATa: ade1-14, trp1-289, his3Δ-200, ura3-52, leu2-3, 112, hsf1∷KANR, [psi−][PIN+], containing pRS314-HSF(148–833) | This study |
| 74D-694-ΔCTA-HSF | MATa: ade1-14, trp1-289, his3Δ-200, ura3-52, leu2-3, 112, hsf1∷KANR, [psi−][PIN+], containing pRS314-HSF(1–583) | This study |
| L1976 | MATα: SUQ5, ade2-1, lys1-1, his3-11, 15, leu1, kar1-1, cyhR, [PSI+]w [PIN+], | Liebman lab |
| L1977 | MATα: SUQ5, ade2-1, lys1-1, his3-11, 15, leu1, kar1-1, cyhR, [PSI+]S [PIN+], | Liebman lab |
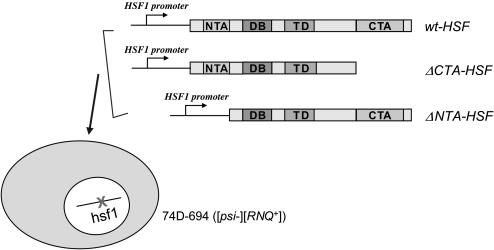
To create isogenic strains of wt-HSF, ΔNTA-HSF, and ΔCTA-HSF, 74D-hsf1∷KanR containing pRS416GPD-HSF1 was transformed with pRS413-wt-HSF, pRS413-ΔNTA-HSF, or pRS413-ΔCTA-HSF. The resulting transformants were grown on media containing 5-fluoroorotic acid to eliminate pRS416GPD-HSF1. The generated isogenic strains, 74D-wt-HSF, 74D-ΔNTA-HSF, and 74D-ΔCTA-HSF, express the full-length HSF, an NTA truncated HSF, and a CTA truncated HSF, respectively, under the control of the native HSF1 5′- and 3′-flanking regions (Figure 1). The growth of yeast cultures and other yeast genetic manipulations were performed according to established protocols (Sherman 1991).
Figure 1.
Creating isogenic ΔNTA-HSF, ΔCTA-HSF, and wt-HSF cells in strain 74D-694. A CEN plasmid, p416GPD-HSF, expressing wt HSF1 with a URA3 marker was transformed into a 74D-694 strain ([psi−][RNQ+]), whose chromosomal HSF1 was subsequently disrupted with a KanR gene through one-step gene replacement (see materials and methods). After transformation with a CEN/HIS3 plasmid, pRS314ΔNTA-HSF(148–833), pRS314ΔCTA-HSF(1–583), or pRS314wt-HSF, p416GPD-HSF was eliminated by growing the transformants in the presence of 5-FOA to obtain isogenic strains with ΔNTA-HSF, ΔCTA-HSF, or wt-HSF as the sole copy of HSF.
[RNQ+] sedimentation assay:
Single colonies of each strain were inoculated in 3 ml liquid YPD and grown overnight at 30° with shaking. Overnight yeast cultures were diluted into fresh media to a density of OD600 = 0.05 and grown to OD600 = 0.6 before harvesting. Spheroplasts were prepared, lysed, and centrifuged as described (Sondheimer and Lindquist 2000). The total lysate, soluble, and pellet fractions of each sample were analyzed by SDS–PAGE and immunoblot analysis using a polyclonal antibody, anti-Rnq1 [a kind gift from S. Lindquist's lab (Sondheimer and Lindquist 2000)].
[PSI+] induction and strain determination:
Exponential-phase cells of wt-HSF, ΔNTA-HSF, and ΔCTA-HSF containing CEN/URA3 plasmids pCUP1-GFP, pCUP1-NMGFP, or pCUP1-Rnq1GFP were induced by CuSO4 at a final concentration of 34 μm. After 4 hr or 24 hr induction, cells were examined by fluorescence microscopy for aggregation and/or were spotted onto YPD and SC-ade to score for [PSI+] formation using a fivefold serial dilution. Ade+ colonies on SC-ade plates were randomly selected and streaked onto YPD to view their colors. Potential [PSI+]S (white) and [PSI+]W (pink) were streaked onto YPD and replica plated onto YPD + 5mm guanidine hydrochloride (GdnHCl). Only ade+ colonies that were cured by GdnHCl were scored as [PSI+]. Sometimes, sequential streaking was carried out to obtain stable [PSI+] variants as newly induced [PSI+] are often unstable (Derkatch et al. 1996).
Cytoduction:
L1976 (c10B-H49 MATα, SUQ5, ade2-1, his3-11, lys1-1, leu1, kar1-1, cyhR, [PIN+][PSI+]W) and L1977 (c10B-H49 MATα, SUQ5, ade2-1, his3-11, lys1-1, leu1, kar1-1, cyhR, [PIN+][PSI+]S) containing a CEN plasmid (HIS3) were used as donors to cytoduce 74D-[psi−] wt-HSF, ΔNTA-HSF, and ΔCTA-HSF cells (MATa), respectively. Individual pairs of donors and recipients were mixed in YPD for 5 hr at 30° and spread onto SC (−lys, −his) plates to select for diploids and cytoductants. Colonies grown on SC (−lys, −his) plates were transferred onto a 5-FOA plate, which selects against the donor and diploid cells. Cytoductants were confirmed by a mating-type test and by the presence of the recipient's auxotrophic nuclear markers, for example, the LEU2.
RESULTS
The HSF truncation mutants, ΔNTA-HSF and ΔCTA-HSF, do not affect the propagation of preexisting [RNQ+] prions:
To examine the effect of HSF activity on yeast prions, we created a chromosomal hsf1 disruption mutant in yeast strain 74D-694 ([psi−][RNQ+]), which contains a premature stop codon in the ADE1 gene to allow sensitive and quantitative assays for [PSI+] formation (Cox 1965). In this particular strain, [PSI+] de novo appearance requires the presence of [RNQ+], which is also called [PSI+] inducibility ([PIN+]) (Derkatch et al. 2001). Since HSF is an essential gene, a CEN plasmid, pRS314-wt-HSF, pRS314-ΔNTA-HSF, or pRS314-ΔCTA-HSF was maintained in the hsf1 disruption strain to give isogenic strains expressing wt-HSF, ΔNTA-HSF, or ΔCTA-HSF, respectively (Figure 1).
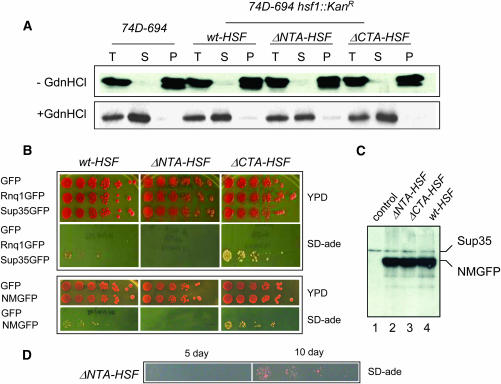
We first investigated if [RNQ+] was faithfully maintained in the hsf1 truncation mutants. It has been shown that Rnq1, the protein determinant of [RNQ+], exists in an aggregated form in [RNQ+] cells but remains soluble in isogenic [rnq−] cells (Sondheimer and Lindquist 2000). The difference in Rnq1 conformation in [RNQ+] and [rnq−] cells can be detected by sedimentation assays (Sondheimer and Lindquist 2000). By doing so, we found that Rnq1 was in the pellet fraction in all ΔNTA-HSF, ΔCTA-HSF, and wt-HSF cells (Figure 2A), suggesting that deletion of the HSF NTA or CTA had no effect on preexisting [RNQ+]. Yeast prions such as [RNQ+] can be eliminated by treatment of cells with a low concentration of GdnHCl (Tuite et al. 1981). After GdnHCl treatment, Rnq1 was found in the soluble fractions (Figure 2A), indicating [RNQ+] was eliminated. Thus, the hsf1 alleles have no effect on the propagation of preexisting [RNQ+] or GdnHCl curing of [RNQ+].
Figure 2.
The truncation mutants of hsf1, ΔNTA-HSF and ΔCTA-HSF, have no effect on preexisting [RNQ+] but dramatically influence the de novo formation of [PSI+]. (A) [RNQ+] sedimentation assay of isogenic strains of ΔNTA-HSF, ΔCTA-HSF, and wt-HSF, which are derived from a 74D-694 ([psi−][RNQ+]) strain (see materials and methods and Figure 1). Protein extract was prepared and centrifuged as described previously (Sondheimer and Lindquist 2000). T, total protein extract; S, soluble fraction; P, pellet fraction. Cells were treated either without (−GdnHCl) or with 5 mm GdnHCl (+GdnHCl). (B) De novo formation of [PSI+] in isogenic ΔNTA-HSF, ΔCTA-HSF, and wt-HSF cells. Yeast cells with specific genetic background indicated were transformed with pCUP1-GFP (vector control), pCUP1-Sup35GFP, pCUP1-NMGFP, or pCUP1-Rnq1GFP. Fresh transformants were grown in SC-ura media to early log phase before addition of CuSO4 (final concentration, 34 μm). After a 4-hr induction, cells were spotted onto either YPD or SC-ade in a fivefold dilution series. Pictures were taken after cells were grown for 3 days (YPD) or 5 days on SC-ade at 30°. (C) Immunoblot analysis of isogenic ΔNTA-HSF, ΔCTA-HSF, and wt-HSF cells after a 4-hr induction of Sup35NMGFP. Crude extracts prepared using the ethanol lysis method were analyzed by SDS–PAGE and immunoblot analysis using Sup35-1B, a polyclonal antibody against the Sup35M region. As shown: lanes 2, 3, and 4 are ΔNTA-HSF, ΔCTA-HSF, and wt-HSF, respectively. Lane 1 is a wt cell containing expression vector as a control. (D) ΔNTA-HSF cells were able to induce [PSI+] but with slower kinetics. The same ΔNTA-HSF transformant used for B was reassayed for its ability to induce [PSI+] after storage at 4° for 14 days. [PSI+] induction was carried out under identical conditions as described in B. The pictures were taken after 5 and 10 days of incubation at 30° on SC-ade.
Distinct HSF activation domains have profound effects on [PSI+] de novo formation:
We next examined if the hsf1 alleles influence de novo formation of [PSI+]. While [PSI+] and [psi−] cells interconvert at a low spontaneous rate of ∼10−6 (Tuite et al. 1981; Wickner 1994), overexpression of Sup35 (Chernoff et al. 1993) or its prion domain increases the rate of [PSI+] de novo formation up to 1000-fold (Ter-Avanesyan et al. 1994). To determine if ΔNTA-HSF and ΔCTA-HSF affect the de novo formation of [PSI+], isogenic ΔNTA-HSF, ΔCTA-HSF, and wt-HSF cells were transformed with pCUP1-Sup35GFP or pCUP1-NMGFP, plasmids expressing GFP fusions of full-length SUP35 or SUP35NM (the N-terminal and middle regions of Sup35), respectively. Freshly transformed cells were grown in SC-ura media and induced to form [PSI+] by CuSO4 addition. After 4 hr, cells were spotted onto SC-ade plates to select for translation termination suppressors, indicative of potential [PSI+] cells. As shown in Figure 2B and Table 3, upon Sup35GFP overexpression, the number of ade+ colonies formed by ΔCTA-HSF cells is approximately 7-fold more than that of isogenic wt-HSF cells, suggesting that the absence of the HSF-CTA promotes [PSI+] de novo formation. When fresh ΔNTA-HSF transformants containing pCUP1-SUP35GFP were induced under identical conditions, [PSI+] formation was inhibited (Figure 2B and Table 3). As expected, control cells containing pCUP1-Rnq1GFP or pCUP1-GFP did not give rise to ade+ colonies (Figure 2B and Table 3). Upon NMGFP overexpression, [PSI+] induction was also inhibited in ΔNTA-HSF cells but the [PSI+] promoting effect of ΔCTA-HSF was lessened to ∼2.5-fold when compared to [PSI+] induction in wt-HSF (Figure 2B and Table 3). Since CUP1 can be activated by HSF in response to a variety of growth and stress conditions, including heat shock, chemical stress, and glucose starvation (Tamai et al. 1994; Hahn et al. 2004), the observed differences in [PSI+] induction by hsf1 alleles could have resulted from different expression levels of NMGFP or Sup35GFP (Tamai et al. 1994; Hahn et al. 2004). To test this possibility, we carried out immunoblot analysis using a polyclonal antibody specific to Sup35M to estimate the expression levels of NMGFP. As shown in Figure 2C, similar amounts of NMGFP was expressed in all cell types after a 4-hr induction. Comparable intensities of the endogenous Sup35 band (the slower migrating band) in each lane confirmed the equal loading (Figure 2C). The expression levels of Sup35GFP are also similar in these hsf1 alleles (data not shown). These results demonstrate that the observed differences in [PSI+] de novo formation in wt-HSF, ΔNTA-HSF, and ΔCTA-HSF cells are not due to differences in NMGFP or Sup35GFP expression levels.
TABLE 3.
The effects of hsf1 alleles on [PSI+] de novo formation
| [PSI+] appearance %
|
|||||
|---|---|---|---|---|---|
| GFP | Sup35GFP | NMGFP | Rnq1GFP | ||
| 74D-694 | wt-HSF | <0.1 | 2.1 ± 0.9 | 2.5 ± 0.6 | <0.1 |
| (0/2242) | (48/2353) | (50/2020) | (0/2569) | ||
| ΔNTA-HSF | <0.1 | <0.1 | <0.1 | <0.1 | |
| (0/2387) | (0/2278) | (0/1905) | (0/2302) | ||
| ΔCTA-HSF | <0.1 | 15.4 ± 3.1 | 6.1 ± 2.0 | <0.1 | |
| (0/2206) | (330/2217) | (124/2155) | (0/2153) | ||
Although [PSI+] is nearly undetectable after 5 days at 30° for fresh ΔNTA-HSF transformants upon 4 hr Sup35 overexpression, [PSI+] formation can be detected upon a longer incubation period (data not shown). ΔNTA-HSF tranformants stored for an extended period (>10 days) also give rise to [PSI+] with faster kinetics upon induction of Sup35 overexpression (Figure 2D). Presumably, the leaky CUP1 promoter, which is also induced in response to glucose deprivation (Tamai et al. 1994; Hahn et al. 2004), allowed weak but constitutive expression of Sup35GFP and NMGFP. These observations suggest that [PSI+] de novo formation in ΔNTA-HSF cells is severely impaired but not completely abolished. Under our experimental conditions, we have not observed any detectable differences in growth among the isogenic strains of ΔNTA-HSF, ΔCTA-HSF, and wt-HSF cells. As shown in Figure 2B, the number and size of colonies on YPD are similar in all cell types, demonstrating that they have a similar growth rate. Thus, the inhibitory effect of ΔNTA-HSF or the stimulatory effect of ΔCTA-HSF on [PSI+] de novo formation was not caused by a difference in their growth rates.
The effect of N-terminal or C-terminal domain deletion of HSF on prion protein and poly(Q) aggregation:
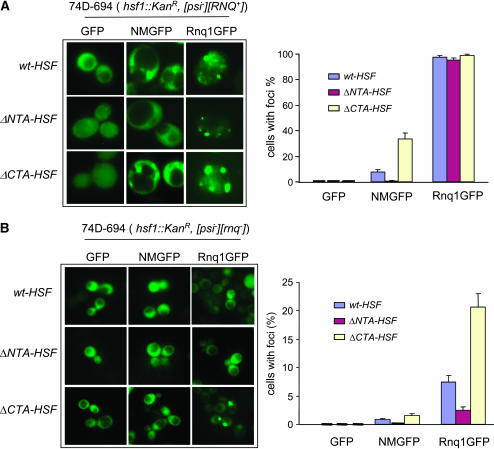
Consistent with our observation for [PSI+] induction, we observed that deletion of HSF NTA or CTA has a profound effect on Sup35 aggregation. We examined Sup35NMGFP fluorescent foci formation in isogenic hsf1 strains that are [psi−][RNQ+]. As shown in Figure 3A, fresh ΔNTA-HSF transformants containing pCUP1-NMGFP were unable to form detectable fluorescent foci after a 4-hr CuSO4 addition. Approximately 7% of wt-HSF cells exhibited fluorescent foci after a 4-hr induction, similar to previously reported results (Patino et al. 1996). However, ΔCTA-HSF cells formed approximately three times more foci than did wt-HSF cells (Figure 3A). For isogenic hsf1 alleles in [psi−][rnq−] strains (after GdnHCl treatment), Sup35NMGFP exhibited minimal fluorescent foci in all cell types (Figure 3B), suggesting that deletion of HSF-NTA or -CTA does not evade the requirement of [RNQ+] for NMGFP aggregation. Indeed, [PSI+] induction in all hsf1 alleles requires the presence of [RNQ+] (data not shown).
Figure 3.
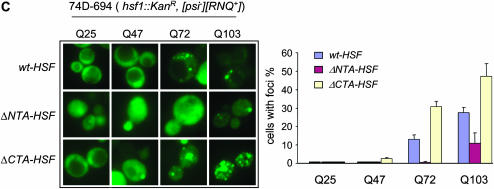
Fluorescent microscopic assay of NMGFP, Rnq1-GFP, and poly(Q)-GFP aggregation. Isogenic ΔNTA-HSF, ΔCTA-HSF, and wt-HSF cells were transformed, respectively, with pCUP1-GFP (vector control), pCUP1-NMGFP, pCUP1-Rnq1GFP, or p426-GPD-based poly(Q)-GFP expression plasmids varying in Q-lengths (Krobitsch and Lindquist 2000). (A) Visualization of NMGFP and Rnq1GFP aggregations in yeast cells ([psi−][RNQ+]) with specific hsf1 alleles indicated (left) and a quantitative summary of three independent experiments of NMGFP and Rnq1GFP aggregation (right). (B) Fluorescent microscopic assay of prion protein aggregation in [psi−][rnq−] cells with different hsf1 alleles. Cells from A were treated with 5 mm GdnHCl to eliminate [RNQ+]. Cells containing the respective plasmids were grown in SC-Ura to early log phase and induced to express GFP fusions by addition of CuSO4 (final concentration, 34 μm). Data shown were acquired after a 4-hr induction. (C) Visualization of poly(Q)-GFP aggregation in isogenic yeast cells of wt-HSF, ΔNTA-HSF, and ΔCTA-HSF (top) and a quantitative summary of three independent experiments of poly(Q)-GFP aggregation (bottom).
We next asked if the hsf1 alleles influence Rnq1 aggregation. In agreement with the solubility assay, Rnq1GFP (a GFP fusion containing the Rnq1 prion domain) formed fluorescent foci with a similar pattern of multiple dots (m.d.) (Bradley et al. 2002) in isogenic ΔNTA-HSF, ΔCTA-HSF, and wt-HSF cells that were derived from [psi−][RNQ+], confirming their [RNQ+] status (Figure 3A). To examine de novo aggregation of Rnq1, we subjected cells ([psi−][RNQ+]) containing pCUP1-Rnq1GFP as described in Figure 3A to GdnHCl treatment to eliminate [RNQ+]. After GdnHCl treatment, only a diffuse Rnq1GFP fluorescent pattern was seen for all hsf1 alleles upon a 0.5-hr induction, confirming that [RNQ+] was cured (data not shown). After a 4-hr induction, Rnq1GFP fluorescent patterns were strikingly different. ΔCTA-HSF cells formed about three times more fluorescent foci (21% of 378 cells) than did wt-HSF cells (7.5% of 349 cells). Rnq1GFP foci in ΔNTA-HSF cells were almost undetectable (Figure 3B). Our results demonstrate that the hsf1 alleles have similar effects on the aggregation of both Sup35 and Rnq1.
To test if the hsf1 alleles specifically affect prion proteins, we examined the aggregation of Huntingtin protein, which contains a poly(Q) tract with variable lengths among different individuals. Poly(Q) length is tightly associated with the etiology of Huntington's disease: the longer the tract is, the worse the symptoms are, and the earlier the onset of the disease is (Andrew et al. 1993; Duyao et al. 1993; Snell et al. 1993). There are at least nine poly(Q)-associated neurodegenerative diseases, including Huntington's disease (Walsh et al. 2005). Yeast has been a useful model for studying the aggregation and toxicity of poly(Q) proteins (Krobitsch and Lindquist 2000; Osherovich and Weissman 2001; Meriin et al. 2002; Derkatch et al. 2004; Gokhale et al. 2005). Huntingtin exon-1, with a long poly(Q) tract, forms detectable aggregates in yeast cells and the aggregation strength and the associated cell toxicity are proportional to the length of the Q-tract (Krobitsch and Lindquist 2000; Meriin et al. 2002). We transformed the isogenic ΔNTA-HSF, ΔCTA-HSF, and wt-HSF cells with a set of high-copy GPD plasmids containing Huntingtin exon-1-GFP with variable poly(Q) lengths: Q25, Q47, Q72, and Q103, respectively (Krobitsch and Lindquist 2000). As shown in Figure 3C, Q47-GFP formed no detectable aggregates in wt-HSF cells, in agreement with a previous report (Krobitsch and Lindquist 2000). However, the formation of Q47-GFP aggregates was obvious in ΔCTA-HSF cells (Figure 3C). For Huntingtin-Q72, ∼30% of ΔCTA-HSF cells (92/296) formed fluorescent foci compared to 15% of wt-HSF cells (56/428) and none in ΔNTA-HSF cells (0/392). For Q103, fluorescent foci were seen in 27% of wt-HSF cells (81/ 286), in ∼53% of ΔCTA-HSF cells (175/325), but only in ∼10% of ΔNTA-HSF cells (33/325) (Figure 3C). Thus, the effects of the distinct hsf1 alleles on protein aggregation are not specific to prion proteins.
The deletion of the N-terminal or C-terminal domain of HSF differentially affects [PSI+] variant formation:
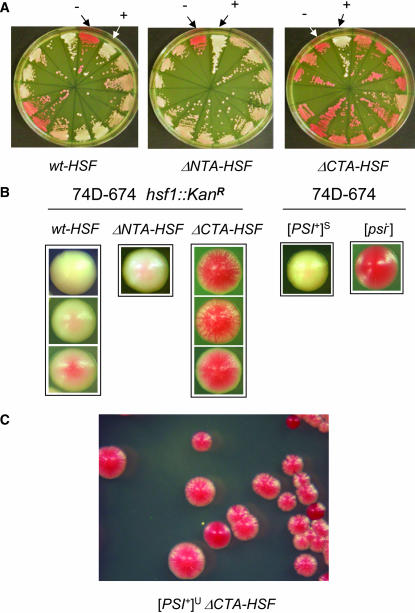
The dramatic effect of hsf1 alleles on [PSI+] de novo formation encouraged us to examine whether they also influence [PSI+] variant establishment. Overexpression of the Sup35 prion domain in wild-type (wt) cells typically gives rise to [PSI+] variants ranging from very weak to very strong (Derkatch et al. 1996). This is also the case when we examined the induced [PSI+] in wt-HSF cells upon NMGFP overexpression (Figure 4, A and B). Although ΔCTA-HSF significantly increased the de novo appearance of [PSI+], we were surprised to find that almost all ade+ colonies of ΔCTA-HSF cells appeared as very weak [PSI+] upon streaking on YPD (Figure 4A, right). Detailed examination revealed unique mosaic colony morphology associated with [PSI+] induced in ΔCTA-HSF cells (Figure 4, B and C). Upon restreaking, these mosaic colonies gave rise to a low frequency of [psi−] cells (∼15%) and [PSI+]S cells (∼1%). The majority of them retain their mosaic feature (Figure 4, B and C). Intriguingly, [PSI+]W was not observed. Once they become [psi−] or [PSI+]S, they can be stably transmitted as [psi−] or [PSI+]S. We named those mosaic [PSI+] induced in ΔCTA-HSF cells [PSI+]U because of their “unstable” and “undifferentiated” features. In contrast to the preference of forming [PSI+]U in ΔCTA-HSF cells, almost all ade+ colonies formed in ΔNTA-HSF cells exhibited a uniform [PSI+] strength. They are apparently pinker than the control [PSI+]S [Figure 4, A (middle) and B]. Table 4 summarizes [PSI+] induction results from three consecutive experiments. Although wt-HSF gave rise to mixed [PSI+] variants, 91% of [PSI+] derived from ΔNTA-HSF cells were [PSI+]S and 97% of [PSI+] induced from ΔCTA-HSF cells are [PSI+]U. Thus, specific [PSI+] variants are preferably formed in different hsf1 mutant strains.
Figure 4.
[PSI+] induced in isogenic ΔNTA-HSF, ΔCTA-HSF, and wt-HSF cells exhibits distinct phenotypes. (A) Individual ade+ colonies of ΔNTA-HSF, ΔCTA-HSF, and wt-HSF formed by overexpression of Sup35NMGFP (see Figure 2) were randomly picked and streaked onto YPD plates to show their color. Also shown are [PSI+] (+) and [psi−] (−) control variants derived from wt 74D-694 cells. (B) Representative single colonies from A were enlarged to show their colony morphology. (C) A representative streak of [PSI+]U induced in a ΔCTA-HSF background.
TABLE 4.
The effects of hsf1 alleles on [PSI+] variant formation
| wt-HSF (%) | ΔNTA-HSF (%) | ΔCTA-HSF (%) | |
|---|---|---|---|
| [PSI+]S | 34 (55 ± 10) | 29 (91 ± 8) | 2 (3 ± 3) |
| [PSI+]W | 28 (45 ± 10) | 3 (9 ± 8) | 0 |
| [PSI+]U | 0 | 0 | 64 (97 ± 3) |
| Total no. examined | 62 | 32 | 66 |
The effect of N-terminal or C-terminal domain deletion of HSF on [PSI+] propagation:
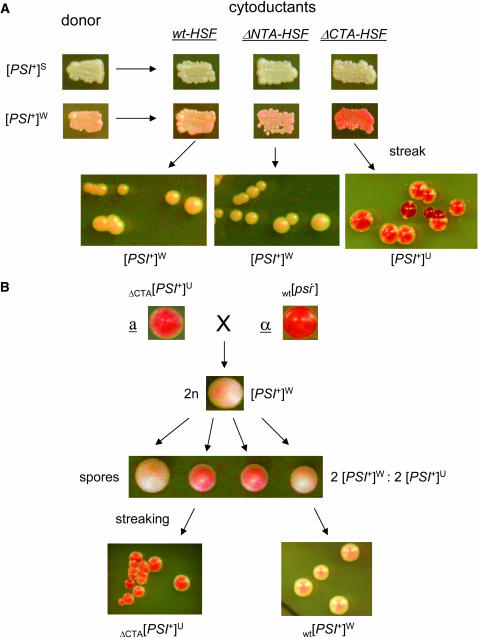
We next investigated if the hsf1 alleles are able to receive and propagate nonpreferred [PSI+] variants. We carried out cytoduction experiments using [PSI+]S and [PSI+]W as donors and isogenic wt-HSF, ΔNTA-HSF, and ΔCTA-HSF [psi−] cells as recipients. Cytoduction allows mating partners to share their cytoplasm but not their nuclear components (Conde and Fink 1976). If a particular genetic trait is mediated by a prion, it should be transmissible to a mating partner without contributing its nuclear materials since it is “infectious.” This is indeed the case for all yeast prions identified to date (Uptain and Lindquist 2002). When [PSI+]S was used as the donor and isogenic [psi−] cells of ΔNTA-HSF, ΔCTA-HSF, and wt-HSF were used as recipients, cytoductants in all cell types were [PSI+]S (Figure 5), indicating that deletion of the HSF NTA or CTA does not affect the ability of cells to faithfully receive and propagate [PSI+]S. When [PSI+]W was the donor, wt-HSF and ΔNTA-HSF cells were able to receive and faithfully maintain [PSI+]W whereas ΔCTA-HSF cytoductants of [PSI+]W exhibited similar colony morphology to that of [PSI+]U formed de novo in ΔCTA-HSF cells. They are mosaic and sectored (compare the enlarged cell streak in Figures 4B and 5). Their mosaic feature can be stably maintained upon multiple sequential streaking and even through plasmid transformation procedures. Table 5 summarizes results from three independent cytoduction experiments. All cytoductants of [PSI+]S were [PSI+]S, confirming that all hsf1 alleles are capable of receiving and propagating [PSI+]S. Approximately 45% ΔNTA-HSF cytoductants of [PSI+]W were [psi−], indicating a reduced ability of ΔNTA-HSF cells to receive and propagate [PSI+]W. The fact that ΔCTA-HSF cytoductants of [PSI+]W displayed the same colony morphology as that of [PSI+]U de novo formed in ΔCTA-HSF cells suggests that [PSI+]U is the same as [PSI+]W but with a unique phenotype in ΔCTA-HSF cells. To test this possibility, we crossed [PSI+]U of ΔCTA-HSF with wt [psi−] cells of the opposite mating type. The resulting diploid cells were stable [PSI+]W, suggesting that the [PSI+]U phenotype is not dominant (Figure 5B). Upon sporulation, a 2:2 ratio of stable [PSI+]W (wt) to [PSI+]U (ΔCTA-HSF) was obtained (Figure 5B). Upon streaking, the ΔCTA-HSF spores have the same colony morphology as that of [PSI+]U (Figure 5B). Thus, our results demonstrate that the ΔCTA-HSF genetic background cannot support a stable maintenance of [PSI+]W and confirm that [PSI+]U is essentially [PSI+]W but with a different readout in ΔCTA-HSF cells.
Figure 5.
The transmissibility of preexisting [PSI+] to hsf1 mutants. (A) Cytoduction: α-cells (c10B-H49) of [PSI+]S and [PSI+]W containing a kar-1 mutation (gifts of Susan Liebman) were used as donors to cytoduce isogenic a-cells of ΔNTA-HSF, ΔCTA-HSF, and wt-HSF (74D-694) that are [psi−]. Cytoduction was carried out as described in materials and methods. Cytoductants were verified by the presence of the recipient's nuclear marker (LEU2) and mating type, a. The [PSI+] status of each cytoductant was confirmed by its curability by GdnHCl. Also shown are patches of individual cytoductants on YPD for color visualization. Note that ΔCTA-HSF cytoductants of [PSI+]W exhibit [PSI+]U phenotype (see the enlarged cell streak), identical to the de novo formed [PSI+] in ΔCTA-HSF cells upon overexpression of Sup35NMGFP. (B) Mating and sporulation: wild-type α-cells (74D-694 [psi−][rnq−]) were crossed with ΔCTA-HSF a-cells that are [PSI+]U [pRS305-HSF(1–583) was integrated at leu2 locus]. The diploids were picked and incubated at 30° for 4 days on YPD plates before being subjected to sporulation (30° for 7 days on a sporulation plate). The tetrads were dissected and incubated at 30° for 4 days before streaking. The genome typing of spores was done by testing their ability to grow at 37° since wt-HSF cells are viable whereas ΔCTA-HSF cells are not at 37° (Morano et al. 1999).
TABLE 5.
The effects of hsf1 alleles on [PSI+] variant propagation
| Donor: | [PSI+]W
|
[PSI+]S
|
|||||
|---|---|---|---|---|---|---|---|
| Cytoductant phenotype | Recipient: | wt-HSF | ΔNTA-HSF | ΔCTA-HSF | wt-HSF | ΔNTA-HSF | ΔCTA-HSF |
| [PSI+]S | 0 | 0 | 0 | 18 | 14 | 13 | |
| [PSI+]W | 15 | 21 | 0 | 0 | 0 | 0 | |
| [PSI+]U | 0 | 0 | 23 | 0 | 0 | 0 | |
| [psi−] | 0 | 17 | 0 | 0 | 0 | 0 | |
| No. of cytoductants examined | 15 | 38 | 23 | 18 | 14 | 13 | |
The effect of N-terminal or C-terminal domain deletion of HSF on the expression of Hsp104:
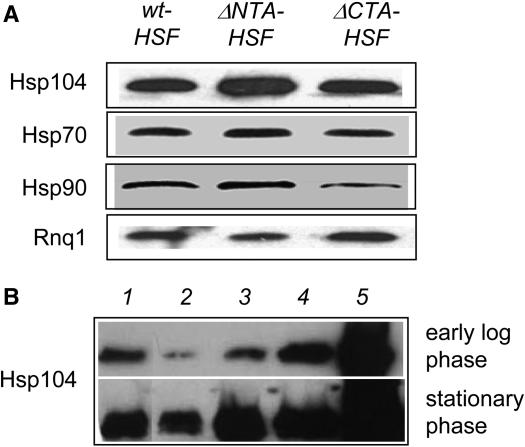
The molecular chaperone Hsp104 plays an essential role not only in prion propagation but also in poly(Q) aggregation in Saccharomyces cerevisiae (Chernoff et al. 1995b; Krobitsch and Lindquist 2000). It has been shown that HSP104 expression is under the regulation of HSF (Halladay and Craig 1995; Grably et al. 2002). To examine if the hsf1 alleles differentially regulate HSP104 expression, we conducted immunoblot analysis to assess the steady level of Hsp104 in isogenic wt-HSF, ΔNTA-HSF, and ΔCTA-HSF cells. As shown in Figure 6A, Hsp104 levels are similar in wt-HSF and ΔCTA-HSF cells. The level of Hsp104 in ΔNTA-HSF cells, however, is significantly elevated. To estimate the Hsp104 level in ΔNTA-HSF cells, we carried out a comparative immunoblot analysis using ΔNTA-HSF cells and isogenic wt cells containing various HSP104 expression plasmids. As shown in Figure 6B, the amount of Hsp104 in ΔNTA-HSF cells is slightly more than that in wt cells containing a single-copy integrated plasmid of HSP104 (lane 3) but less than that in wt cells containing a CEN or a multicopy HSP104 expression plasmid (lanes 4 and 5). This is true for cells harvested at both the early log phase and stationary phase (Figure 6B). We also examined the expression levels of Hsp70 and Hsp90, two additional HSF target genes (Hahn et al. 2004). The amount of Hsp70 is similar among all cell types (Figure 6A) as judged by immunoblot analysis using a monoclonal antibody against Drosophila Hsp70 (Velazquez and Lindquist 1984). The expression level of Hsp90 in ΔNTA-HSF cells is similar to that in wt-HSF cells. However, Hsp90 expression is significantly lower in ΔCTA-HSF cells, as reported previously (Morano et al. 1999) (Figure 6A).
Figure 6.
The effect of hsf1 alleles on the expression of molecular chaperones. (A) Isogenic ΔNTA-HSF, ΔCTA-HSF, and wt-HSF cells were grown in YPD to OD600 = 0.5. Whole cell lysates were prepared and analyzed by SDS–PAGE followed by immunoblot using a polyclonal antibody of yeast Hsp104. The same membrane was probed with a monoclonal antibody of Drosophila Hsp70, a polyclonal antibody of yeast Hsp90, or a polyclonal antibody of Rnq1 (as loading control) following sequential stripping. (B) ΔNTA-HSF cells (lane 1) and isogenic wt 74D-694 cells containing p415GPD (lane 2), integrated single-copy HSP104 (pRS305-HSP104) with HSP104 promoter and terminator (lane 3), p415-HSP104 with HSP104 promoter and terminator (lane 4), and p425GPD-HSP104 with GPD promoter and CYC1 terminator were grown under identical conditions and harvested at either early log phase or stationary phase (overnight). Whole cell lysates were prepared and analyzed by SDS–PAGE followed by immunoblot using a polyclonal antibody of yeast Hsp104. (All antibodies used were gifts from Susan Lindquist's lab).
DISCUSSION
We have observed a striking difference in [PSI+] formation between two truncation mutants of yeast HSF1, ΔNTA-HSF and ΔCTA-HSF: ΔCTA-HSF stimulates whereas ΔNTA-HSF inhibits the de novo appearance of [PSI+]. This result implies an important but complex role of HSF in prion formation. Early reports suggested that the NTA and CTA of HSF modulate distinct target genes upon different environmental stimuli (Sorger 1990). More recent studies suggest that the transcriptional activation domains of HSF are in a dynamic association with the DNA-binding and oligomerization domains (Bulman et al. 2001; Chen and Parker 2002). Maintaining such domain–domain interactions is important for preserving HSF in a repressive state under normal growth conditions (Hardy et al. 2000; Chen and Parker 2002). It is possible that the opposite effects of ΔNTA-HSF and ΔCTA-HSF on [PSI+] are due to their differences in perturbing such domain–domain interactions.
Our findings that ΔNTA-HSF and ΔCTA-HSF cells have a strikingly different ability with regard to [PSI+] de novo formation and variant determination suggest that important cellular factors required for prion formation are differently regulated in ΔNTA-HSF and ΔCTA-HSF cells. Indeed, we showed that HSP104 expression is significantly enhanced in ΔNTA-HSF, indicating that the N-terminal activation domain of HSF is inhibitory to HSP104 expression under nonstress conditions. The upregulation of HSP104 in ΔNTA-HSF cells demonstrates that particular mutations in HSF1 are able to differentially regulate cellular factors that are required for prion formation and propagation. It is possible that the elevated Hsp104 level is responsible for the observed inhibitory effect of ΔNTA-HSF on de novo [PSI+] formation since [PSI+] can be cured by overexpression of Hsp104 (Chernoff et al. 1995a). It has been shown and confirmed by us that the Hsp90 level is significantly reduced in ΔCTA-HSF (Morano et al. 1999) (Figure 6A). The expression levels of Sse1, a distant Hsp70 family member, and Sti1, an Hsp90 cochaperone, are also significantly lowered in ΔCTA-HSF cells (Morano et al. 1999). The involvement of Sti1 in [PSI+] propagation has been shown (Jones et al. 2004; Song and Masison 2005) but the role of Hsp90 and Sse1 in prion formation has not been reported. Comparative analysis of total gene expression profiles would allow us to identify additional genes that are differentially expressed in these hsf1 mutants and are important for prion formation.
An unsolved mystery in prion biology is the strain phenomenon, a single protein molecule existing in multiple inheritable conformations that are infectious (Derkatch et al. 1996; Prusiner 1998). Although mutations within a specific prion protein have been linked to the formation of particular strains of the corresponding prion (Chien et al. 2003; King and Diaz-Avalos 2004; Vanik et al. 2004), cellular factors required for de novo appearance of a particular strain remain to be identified. Yeast cells derived from one single colony are able to form prions with a wide range of variants, from very strong to very weak upon Sup35 overexpression (Derkatch et al. 1996) (Figure 4B). These observations suggest that prion strain determinants can be epigenetic modifiers. It has been shown that the cell cycle phase affects the number of [PSI+] seeding elements—“propagons” and their subsequent segregation (Cox et al. 2003). Environmental fluctuations, cell aging, and other unknown factors are also possible epigenetic modifiers influencing prion strains establishment. Our finding that the ΔNTA-HSF prefers [PSI+]S de novo formation whereas ΔCTA-HSF selectively gives rise to the [PSI+]U variant demonstrates for the first time that specific prion variants can be preferentially formed in defined genetic backgrounds. Thus, there is also a genetic basis for prion variant determination. Elucidating the relationship between HSF and [PSI+] variant formation might help us to reveal the identities of the variant determinants.
We demonstrated that >90% of [PSI+] formed in ΔNTA-HSF cells are [PSI+]S whereas >97% are [PSI+]U in ΔCTA-HSF cells (Figure 4 and Table 4). Both truncation mutants were, however, capable of receiving and faithfully propagating preformed [PSI+]S through cytoduction (Figure 5 and Table 5). Since [PSI+]U is a special readout of [PSI+]W in ΔCTA-HSF cells, we consider that both ΔNTA-HSF and ΔCTA-HSF cells are also capable of receiving and propagating [PSI+]W (Figure 5). These results strongly suggest that prion initiation and propagation are two separate processes that preferentially utilize distinct cellular machineries. The propagation process, including prion replication and subsequent segregation into daughter cells, has been extensively studied. For example, several cellular factors important for [PSI+] propagation, such as Hsp104, Ssa1 and Ssb1, Sis1, Sla1, Sti1, and Cpr7, have been identified (Chernoff et al. 1995b; Kushnirov et al. 2000; Sondheimer et al. 2001; Jones et al. 2004; Song and Masison 2005). In contrast, the initiation process is less understood. Our data suggest that the initiation process requires more stringent cellular environmental conditions than that of propagation, since both ΔNTA-HSF and ΔCTA-HSF cells are able to receive and propagate preformed [PSI+]S and [PSI+]W, but only a specific variant is preferably formed de novo in each mutant. Our finding demonstrates that a defined genetic background can give rise to a specific prion variant, offering a traceable system for identifying cellular factors that are important for prion initiation. Since both ΔNTA-HSF and ΔCTA-HSF cells we examined for [PSI+] de novo formation contained the same [RNQ+] variant, an m.d. form (Figure 2B) (Bradley and Liebman 2003), the differences of ΔNTA-HSF and ΔCTA-HSF cells in [PSI+] de novo formation are likely due to the influence of a non-[RNQ+] factor(s) that remains to be identified.
Deposition of protein aggregates is a hallmark of several devastating protein-folding diseases, including Huntington's disease, Alzheimer's disease, Parkinson's disease, and prion diseases (Soto 2003). Although the underlying mechanisms that confer each disease are poorly understood, the involvement of molecular chaperones in their etiologies has been implicated, including the prion disease (Muchowski and Wacker 2005). Our results that HSF greatly affects the aggregation of poly(Q) strongly suggest that it is possible that mutations in mammalian HSF can lead to anti-aggregation or aggregation-promoting phenotypes. Thus modulation of HSF activity can contribute to the etiology of protein-misfolding diseases. In this regard, the effect of HSF activity on the life span of Caenorhabditis elegans has been recently reported: overexpressing HSF prolonged the life span of C. elegans whereas loss-of-function mutations of hsf shortened it (Hsu et al. 2003; Morley and Morimoto 2004). Mammals have multiple members of HSFs, each of which responds differentially to distinct environmental stimuli (Morimoto 1998). Although the role of each member in protein aggregation is unclear, our finding that yeast hsf1 mutations profoundly affect the aggregation of both prion protein and poly(Q) suggests that deciphering the regulatory mechanism of HSF on protein aggregation might provide valuable information to facilitate the development of novel therapeutic drugs for protein-misfolding diseases.
Acknowledgments
We thank Susan Liebman for MATα strains of [PSI+]S and [PSI+]W for cytoduction and Susan Lindquist for Sup35, Rnq1, Hsp70, Hsp90, and Hsp104 antibodies. We also thank L. Mohanity for editing the manuscript. This work was supported by grants from the United States Army (0650-370-R744) and the Ellison Medical Foundation to L.L. and from the National Institutes of Health (GM59911) to D.T.
References
- Allen, K. D., R. D. Wegrzyn, T. A. Chernova, S. Muller, G. P. Newnam et al., 2005. Hsp70 chaperones as modulators of prion life cycle: novel effects of Ssa and Ssb on the Saccharomyces cerevisiae prion [PSI+]. Genetics 169: 1227–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin, J., J. Ananthan and R. Voellmy, 1988. Key features of heat shock regulatory elements. Mol. Cell. Biol. 8: 3761–3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew, S. E., Y. P. Goldberg, B. Kremer, H. Telenius, J. Theilmann et al., 1993. The relationship between trinucleotide (CAG) repeat length and clinical features of Huntington's disease. Nat. Genet. 4: 398–403. [DOI] [PubMed] [Google Scholar]
- Bradley, M. E., and S. W. Liebman, 2003. Destabilizing interactions among [PSI(+)] and [PIN(+)] yeast prion variants. Genetics 165: 1675–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley, M. E., H. K. Edskes, J. Y. Hong, R. B. Wickner and S. W. Liebman, 2002. Interactions among prions and prion “strains” in yeast. Proc. Natl. Acad. Sci. USA 99(Suppl. 4): 16392–16399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulman, A. L., S. T. Hubl and H. C. Nelson, 2001. The DNA-binding domain of yeast heat shock transcription factor independently regulates both the N- and C-terminal activation domains. J. Biol. Chem. 276: 40254–40262. [DOI] [PubMed] [Google Scholar]
- Castilla, J., P. Saa, C. Hetz and C. Soto, 2005. In vitro generation of infectious scrapie prions. Cell 121: 195–206. [DOI] [PubMed] [Google Scholar]
- Chen, T., and C. S. Parker, 2002. Dynamic association of transcriptional activation domains and regulatory regions in Saccharomyces cerevisiae heat shock factor. Proc. Natl. Acad. Sci. USA 99: 1200–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernoff, Y. O., I. L. Derkatch and S. G. Inge-Vechtomov, 1993. Multicopy SUP35 gene induces de-novo appearance of psi-like factors in the yeast Saccharomyces cerevisiae. Curr. Genet. 24: 268–270. [DOI] [PubMed] [Google Scholar]
- Chernoff, Y. O., S. W. Liebman, M. M. Patino and S. L. Lindquist, 1995. a Prions of yeast and heat-shock protein 104: coprion and cure: response from Chernoff et al. Trends Microbiol. 3: 369. [DOI] [PubMed] [Google Scholar]
- Chernoff, Y. O., S. L. Lindquist, B. Ono, S. G. Inge-Vechtomov and S. W. Liebman, 1995. b Role of the chaperone protein Hsp104 in propagation of the yeast prion-like factor [PSI+]. Science 268: 880–884. [DOI] [PubMed] [Google Scholar]
- Chien, P., A. H. DePace, S. R. Collins and J. S. Weissman, 2003. Generation of prion transmission barriers by mutational control of amyloid conformations. Nature 424: 948–951. [DOI] [PubMed] [Google Scholar]
- Chien, P., J. S. Weissman and A. H. DePace, 2004. Emerging principles of conformation-based prion inheritance. Annu. Rev. Biochem. 73: 617–656. [DOI] [PubMed] [Google Scholar]
- Conde, J., and G. R. Fink, 1976. A mutant of Saccharomyces cerevisiae defective for nuclear fusion. Proc. Natl. Acad. Sci. USA 73: 3651–3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, B., 1965. [PSI], a cytoplasmic suppressor of super-suppression in yeast. Heredity 20: 505–521. [Google Scholar]
- Cox, B., F. Ness and M. Tuite, 2003. Analysis of the generation and segregation of propagons: entities that propagate the [PSI+] prion in yeast. Genetics 165: 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkatch, I. L., Y. O. Chernoff, V. V. Kushnirov, S. G. Inge-Vechtomov and S. W. Liebman, 1996. Genesis and variability of [PSI] prion factors in Saccharomyces cerevisiae. Genetics 144: 1375–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkatch, I. L., M. E. Bradley, J. Y. Hong and S. W. Liebman, 2001. Prions affect the appearance of other prions: the story of [PIN+]. Cell 106: 171–182. [DOI] [PubMed] [Google Scholar]
- Derkatch, I. L., S. M. Uptain, T. F. Outeiro, R. Krishnan, S. L. Lindquist et al., 2004. Effects of Q/N-rich, polyQ, and non-polyQ amyloids on the de novo formation of the [PSI+] prion in yeast and aggregation of Sup35 in vitro. Proc. Natl. Acad. Sci. USA 101: 12934–12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duyao, M., C. Ambrose, R. Myers, A. Novelletto, F. Persichetti et al., 1993. Trinucleotide repeat length instability and age of onset in Huntington's disease. Nat. Genet. 4: 387–392. [DOI] [PubMed] [Google Scholar]
- Firoozan, M., C. M. Grant, J. A. Duarte and M. F. Tuite, 1991. Quantitation of readthrough of termination codons in yeast using a novel gene fusion assay. Yeast 7: 173–183. [DOI] [PubMed] [Google Scholar]
- Glover, J. R., A. S. Kowal, E. C. Schirmer, M. M. Patino, J. J. Liu et al., 1997. Self-seeded fibers formed by Sup35, the protein determinant of [PSI+], a heritable prion-like factor of S. cerevisiae. Cell 89: 811–819. [DOI] [PubMed] [Google Scholar]
- Gokhale, K. C., G. P. Newnam, M. Y. Sherman and Y. O. Chernoff, 2005. Modulation of prion-dependent polyglutamine aggregation and toxicity by chaperone proteins in the yeast model. J. Biol. Chem. 280: 22809–22818. [DOI] [PubMed] [Google Scholar]
- Grably, M. R., A. Stanhill, O. Tell and D. Engelberg, 2002. HSF and Msn2/4p can exclusively or cooperatively activate the yeast HSP104 gene. Mol. Microbiol. 44: 21–35. [DOI] [PubMed] [Google Scholar]
- Hahn, J. S., Z. Hu, D. J. Thiele and V. R. Iyer, 2004. Genome-wide analysis of the biology of stress responses through heat shock transcription factor. Mol. Cell. Biol. 24: 5249–5256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halladay, J. T., and E. A. Craig, 1995. A heat shock transcription factor with reduced activity suppresses a yeast HSP70 mutant. Mol. Cell. Biol. 15: 4890–4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy, J. A., S. T. Walsh and H. C. Nelson, 2000. Role of an alpha-helical bulge in the yeast heat shock transcription factor. J. Mol. Biol. 295: 393–409. [DOI] [PubMed] [Google Scholar]
- Hsu, A. L., C. T. Murphy and C. Kenyon, 2003. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science 300: 1142–1145. [DOI] [PubMed] [Google Scholar]
- Jones, G. W., and M. F. Tuite, 2005. Chaperoning prions: the cellular machinery for propagating an infectious protein? BioEssays 27: 823–832. [DOI] [PubMed] [Google Scholar]
- Jones, G., Y. Song, S. Chung and D. C. Masison, 2004. Propagation of Saccharomyces cerevisiae [PSI+] prion is impaired by factors that regulate Hsp70 substrate binding. Mol. Cell. Biol. 24: 3928–3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, G., and D. C. Masison, 2001. Guanidine hydrochloride inhibits Hsp104 activity in vivo: a possible explanation for its effect in curing yeast prions. Curr. Microbiol. 43: 7–10. [DOI] [PubMed] [Google Scholar]
- Jung, G., G. Jones, R. D. Wegrzyn and D. C. Masison, 2000. A role for cytosolic Hsp70 in yeast [PSI+] prion propagation and [PSI+] as a cellular stress. Genetics 156: 559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, C. Y., and R. Diaz-Avalos, 2004. Protein-only transmission of three yeast prion strains. Nature 428: 319–323. [DOI] [PubMed] [Google Scholar]
- King, C. Y., P. Tittmann, H. Gross, R. Gebert, M. Aebi et al., 1997. Prion-inducing domain 2–114 of yeast Sup35 protein transforms in vitro into amyloid-like filaments. Proc. Natl. Acad. Sci. USA 94: 6618–6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krobitsch, S., and S. Lindquist, 2000. Aggregation of huntingtin in yeast varies with the length of the polyglutamine expansion and the expression of chaperone proteins. Proc. Natl. Acad. Sci. USA 97: 1589–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushnirov, V. V., D. S. Kryndushkin, M. Boguta, V. N. Smirnov and M. D. Ter-Avanesyan, 2000. Chaperones that cure yeast artificial [PSI+] and their prion-specific effects. Curr. Biol. 10: 1443–1446. [DOI] [PubMed] [Google Scholar]
- Legname, G., I. V. Baskakov, H. O. Nguyen, D. Riesner, F. E. Cohen et al., 2004. Synthetic mammalian prions. Science 305: 673–676. [DOI] [PubMed] [Google Scholar]
- Li, L., and S. Lindquist, 2000. Creating a protein-based element of inheritance. Science 287: 661–664. [DOI] [PubMed] [Google Scholar]
- Liebman, S. W., 2005. Structural clues to prion mysteries. Nat. Struct. Mol. Biol. 12: 567–568. [DOI] [PubMed] [Google Scholar]
- Liebman, S. W., and I. L. Derkatch, 1999. The yeast [PSI+] prion: making sense of nonsense. J. Biol. Chem. 274: 1181–1184. [DOI] [PubMed] [Google Scholar]
- Liebman, S. W., and J. A. Mastrianni, 2005. Tracking the elusive prion. Trends Mol. Med. 11: 439–441. [DOI] [PubMed] [Google Scholar]
- Littlefield, O., and H. C. Nelson, 2001. Crystal packing interaction that blocks crystallization of a site-specific DNA binding protein-DNA complex. Proteins 45: 219–228. [DOI] [PubMed] [Google Scholar]
- Meriin, A. B., X. Zhang, X. He, G. P. Newnam, Y. O. Chernoff et al., 2002. Huntington toxicity in yeast model depends on polyglutamine aggregation mediated by a prion-like protein Rnq1. J. Cell Biol. 157: 997–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morano, K. A., and D. J. Thiele, 1999. Heat shock factor function and regulation in response to cellular stress, growth, and differentiation signals. Gene Expr. 7: 271–282. [PMC free article] [PubMed] [Google Scholar]
- Morano, K. A., N. Santoro, K. A. Koch and D. J. Thiele, 1999. A trans-activation domain in yeast heat shock transcription factor is essential for cell cycle progression during stress. Mol. Cell. Biol. 19: 402–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto, R. I., 1998. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 12: 3788–3796. [DOI] [PubMed] [Google Scholar]
- Morley, J. F., and R. I. Morimoto, 2004. Regulation of longevity in Caenorhabditis elegans by heat shock factor and molecular chaperones. Mol. Biol. Cell 15: 657–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchowski, P. J., and J. L. Wacker, 2005. Modulation of neurodegeneration by molecular chaperones. Nat. Rev. Neurosci. 6: 11–22. [DOI] [PubMed] [Google Scholar]
- Osherovich, L. Z., and J. S. Weissman, 2001. Multiple Gln/Asn-rich prion domains confer susceptibility to induction of the yeast [PSI+] prion. Cell 106: 183–194. [DOI] [PubMed] [Google Scholar]
- Patino, M. M., J. J. Liu, J. R. Glover and S. Lindquist, 1996. Support for the prion hypothesis for inheritance of a phenotypic trait in yeast. Science 273: 622–626. [DOI] [PubMed] [Google Scholar]
- Pirkkala, L., P. Nykanen and L. Sistonen, 2001. Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. FASEB J. 15: 1118–1131. [DOI] [PubMed] [Google Scholar]
- Prusiner, S. B., 1998. Prions. Proc. Natl. Acad. Sci. USA 95: 13363–13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner, S. B., 2004. Prion Biology and Diseases. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Schlumpberger, M., S. B. Prusiner and I. Herskowitz, 2001. Induction of distinct [URE3] yeast prion strains. Mol. Cell. Biol. 21: 7035–7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman, F., 1991. Getting started with yeast, pp. 3–21 in Guide to Yeast Genetics and Molecular Biology, edited by C. Guthrie and G. R. Fink. Academic Press, San Diego.
- Snell, R. G., J. C. MacMillan, J. P. Cheadle, I. Fenton, L. P. Lazarou et al., 1993. Relationship between trinucleotide repeat expansion and phenotypic variation in Huntington's disease. Nat. Genet. 4: 393–397. [DOI] [PubMed] [Google Scholar]
- Sondheimer, N., and S. Lindquist, 2000. Rnq1: an epigenetic modifier of protein function in yeast. Mol. Cell 5: 163–172. [DOI] [PubMed] [Google Scholar]
- Sondheimer, N., N. Lopez, E. A. Craig and S. Lindquist, 2001. The role of Sis1 in the maintenance of the [RNQ+] prion. EMBO J. 20: 2435–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, Y., and D. C. Masison, 2005. Independent regulation of Hsp70 and Hsp90 chaperones by Hsp70/90 organizing protein Sti1 (Hop1). J. Biol. Chem. 280: 34178–34185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorger, P. K., 1990. Yeast heat shock factor contains separable transient and sustained response transcriptional activators. Cell 62: 793–805. [DOI] [PubMed] [Google Scholar]
- Soto, C., 2003. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat. Rev. Neurosci. 4: 49–60. [DOI] [PubMed] [Google Scholar]
- Stansfield, I., K. M. Jones, V. V. Kushnirov, A. R. Dagkesamanskaya, A. I. Poznyakovski et al., 1995. The products of the SUP45 (eRF1) and SUP35 genes interact to mediate translation termination in Saccharomyces cerevisiae. EMBO J. 14: 4365–4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamai, K. T., X. Liu, P. Silar, T. Sosinowski and D. J. Thiele, 1994. Heat shock transcription factor activates yeast metallothionein gene expression in response to heat and glucose starvation via distinct signalling pathways. Mol. Cell. Biol. 14: 8155–8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, M., P. Chien, N. Naber, R. Cooke and J. S. Weissman, 2004. Conformational variations in an infectious protein determine prion strain differences. Nature 428: 323–328. [DOI] [PubMed] [Google Scholar]
- Ter-Avanesyan, M. D., A. R. Dagkesamanskaya, V. V. Kushnirov and V. N. Smirnov, 1994. The SUP35 omnipotent suppressor gene is involved in the maintenance of the non-Mendelian determinant [PSI+] in the yeast Saccharomyces cerevisiae. Genetics 137: 671–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuite, M. F., C. R. Mundy and B. S. Cox, 1981. Agents that cause a high frequency of genetic change from [PSI+] to [psi−] in Saccharomyces cerevisiae. Genetics 98: 691–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uptain, S. M., and S. Lindquist, 2002. Prions as protein-based genetic elements. Annu. Rev. Microbiol. 56: 703–741. [DOI] [PubMed] [Google Scholar]
- Vanik, D. L., K. A. Surewicz and W. K. Surewicz, 2004. Molecular basis of barriers for interspecies transmissibility of mammalian prions. Mol. Cell 14: 139–145. [DOI] [PubMed] [Google Scholar]
- Velazquez, J. M., and S. Lindquist, 1984. hsp70: nuclear concentration during environmental stress and cytoplasmic storage during recovery. Cell 36: 655–662. [DOI] [PubMed] [Google Scholar]
- Walsh, R., E. Storey, D. Stefani, L. Kelly and V. Turnbull, 2005. The roles of proteolysis and nuclear localisation in the toxicity of the polyglutamine diseases. A review. Neurotox. Res. 7: 43–57. [DOI] [PubMed] [Google Scholar]
- Wickner, R. B., 1994. [URE3] as an altered Ure2 protein: evidence for a prion analog in Saccharomyces cerevisiae. Science 264: 566–569. [DOI] [PubMed] [Google Scholar]
- Wickner, R. B., H. K. Edskes, E. D. Ross, M. M. Pierce, U. Baxa et al., 2004. Prion genetics: new rules for a new kind of gene. Annu. Rev. Genet. 38: 681–707. [DOI] [PubMed] [Google Scholar]
- Wu, C., 1995. Heat shock transcription factors: structure and regulation. Annu. Rev. Cell Dev. Biol. 11: 441–469. [DOI] [PubMed] [Google Scholar]