Abstract
Sexual isolating mechanisms that act before fertilization are often considered the most important genetic barriers leading to speciation in animals. While progress has been made toward understanding the genetic basis of the postzygotic isolating mechanisms of hybrid sterility and inviability, little is known about the genetic basis of prezygotic sexual isolation. Here, we map quantitative trait loci (QTL) contributing to prezygotic reproductive isolation between the sibling species Drosophila santomea and D. yakuba. We mapped at least three QTL affecting discrimination of D. santomea females against D. yakuba males: one X-linked and one autosomal QTL affected the likelihood of copulation, and a second X chromosome QTL affected copulation latency. Three autosomal QTL also affected mating success of D. yakuba males with D. santomea. No epistasis was detected between QTL affecting sexual isolation. The QTL do not overlap between males and females and are not disproportionately concentrated on the X chromosome. There was some overlap in map locations of QTL affecting sexual isolation between D. santomea and D. yakuba with QTL affecting sexual isolation between D. simulans and D. mauritiana and with QTL affecting differences in pigmentation between D. santomea and D. yakuba. Future high-resolution mapping and, ultimately, positional cloning, will reveal whether these traits do indeed have a common genetic basis.
DESPITE the probable importance of sexual isolation as a primary reproductive barrier during the process of speciation (Coyne and Orr 2004), we know relatively little about the genetic basis of interspecific mate discrimination. Yet such genetic studies can answer important questions about speciation. Is sexual isolation based on few genes or many? If many, do a few genes contribute to most of the sexual isolation? Do “mate discrimination” genes tend to occur in similar regions of chromosomes among different species in the same group, implying that sexual isolation may involve identical genes in different speciation events? Do the same chromosome regions (and possibly the same genes) contribute to mate preference in males and females? Finally, what is the normal function of genes involved in sexual isolation? This last question can be answered only by identifying those genes, an endeavor that must begin by their fine-structure localization.
Previous studies of prezygotic isolation in Drosophila have mapped genes affecting sexual isolation to whole chromosomes, chromosome arms, or large sections of chromosomes (Zouros 1981; Coyne 1989, 1993, 1996a,b; Wu et al. 1995; Noor 1997; Ting et al. 2001; Williams et al. 2001; Gleason and Ritchie 2004; Takahashi and Ting 2004), but so far there have been only a few studies localizing quantitative trait loci (QTL) affecting sexual isolation between species with high resolution by linkage to molecular markers (Civetta and Cantor 2003; Moehring et al. 2004)—the first step toward positional cloning of candidate loci. Here, we report the results of mapping QTL causing sexual isolation between two sister species, Drosophila yakuba and D. santomea, using 32 species-specific molecular markers to localize chromosome regions involved in mate discrimination. D. yakuba is widespread across sub-Saharan Africa and on islands near the continent, inhabiting open areas such as savannas, montane grassland, and, in human-colonized areas, disturbed habitats such as plantations and cut-over fields. D. santomea, discovered in 1998, is endemic to São Tomé, an 860-km2 volcanic island 255 km off the coast of Gabon (Lachaise et al. 2000), where it inhabits only montane rain and mist forests. D. yakuba also inhabits São Tomé. On the mountain of Pico de São Tomé, D. yakuba lives at elevations below 1450 m, while D. santomea lives at elevations above 1150 m. Between these elevations, the species' ranges overlap at an ecotone between plantations and virgin rain forest, forming a hybrid zone in which one finds a low frequency (∼1%) of hybrids (Lachaise et al. 2000; Llopart et al. 2005). Molecular evidence puts the divergence between D. yakuba and D. santomea at ∼400,000 years ago (Cariou et al. 2001; Llopart et al. 2002).
The species show substantial sexual isolation in the laboratory (Coyne et al. 2002, 2005; Llopart et al. 2002): the two interspecific matings occur less frequently than intraspecific matings, with the mating between D. santomea females and D. yakuba males occurring very rarely. In this latter pairing, D. yakuba males court D. santomea females persistently, but are usually rejected. In the reciprocal pairing, weaker sexual isolation is evinced by D. santomea males courting D. yakuba females less ardently than conspecific females (Coyne et al. 2005). Thus most sexual isolation is due to discrimination against some traits of D. yakuba by D. santomea females. There is no enhanced sexual isolation (“reinforcement”) between strains of these species taken from the area of sympatry (Coyne et al. 2002).
MATERIALS AND METHODS
Drosophila strains:
All flies were maintained in 8-dram vials containing standard cornmeal-agar-Karo media on a 12:12 hr light:dark cycle at 24°. We used a strain of D. yakuba named Taï18, an isofemale line collected by D. Lachaise in 1983 in the Taï rainforest on the border between Liberia and the Ivory Coast. D. yakuba Taï18 contains a polymorphic inversion on the second chromosome (2Rn) that distinguishes the species from D. santomea. We therefore eliminated this inversion from the stock to make it homosequential to D. santomea, enhancing our mapping capabilities. We inbred 30 lines of Taï18 by full-sib mating for seven generations. We tested these inbred lines for the presence of the inversion by crossing the inbred D. yakuba Taï18 males to D. santomea females and observing whether or not the polytene chromosomes of F1 larvae contained the loops diagnostic of inversion heterozygosity. We saved stocks in which all F1's lacked the inversion and thus were homosequential to D. santomea. Four of these inbred lines were intercrossed in equal numbers to create the D. yakuba ST strain used in this study. D. santomea STO.4 is an isofemale line collected in March of 1998 in the Obo Natural Reserve on São Tomé at 1300 m altitude (Lachaise et al. 2000). Further description can be found in Llopart et al. (2002).
Cytology:
The two species strains are homosequential, but their exact cytology in relation to D. melanogaster is not known, hindering our ability to accurately define QTL boundaries and candidate loci within QTL regions. Therefore, we determined the cytology of D. yakuba in relation to D. melanogaster by BLASTing (http://www.ncbi.nlm.nih.gov/BLAST/) 6-kb pieces of the D. yakuba genome (Release 1.0, April 2004; http://genome.ucsc.edu/cgi-bin/hgGateway) to D. melanogaster every 100 kb, for example, base pairs 100,000–106,000 of the right arm of chromosome 2. Occasionally it was necessary to use larger stretches of sequence or sequence closely adjacent to the 100-kb interval due to transposon insertions, microsatellites, etc. We also visually inspected the projected base pair alignment (http://genome.ucsc.edu/cgi-bin/hgGateway) for any genomic segments ≥5 kb that were out of order.
Crosses:
The sexual isolation between these species has been described extensively (Coyne et al. 2002, 2005). Heterospecific matings occur less often than conspecific matings, with a particular dearth of matings between D. santomea females and D. yakuba males (this lack of mating occurs despite ardent courtship by the D. yakuba males and thus is due largely to discrimination by D. santomea females). Table 1 shows the extent of discrimination between pure lines, demonstrating the typical pattern of sexual isolation described above. For matings that do occur, the copulation latency (time from introduction of flies into observation vials until copulation takes place) is particularly long in the mating between D. yakuba females and D. santomea males.
TABLE 1.
Sexual isolation between pure D. yakuba (ST strain) and D. santomea (STO.4 strain)
| Pairings
|
|||
|---|---|---|---|
| Female | Male | % mated | Mean CL (SE) |
| D. yakuba | D. yakuba | 85.6 | 15.28 (1.07) |
| D. yakuba | D. santomea | 47.8 | 22.23 (1.53) |
| D. santomea | D. yakuba | 14.4 | 14.38 (2.26) |
| D. santomea | D. santomea | 71.1 | 10.32 (0.93) |
| BC santomea | D. yakuba | NA | 14.97 (0.49) |
| D. santomea | BC yakuba | NA | 18.70 (0.50) |
Ninety pairs of flies were watched for 45 min for each of the four pairings of pure species. Females from the backcross to D. santomea were observed until 50% had mated and males from the backcross to D. yakuba were observed for 45 min, when ∼50% had mated. Each pair was observed individually. We recorded whether or not mating occurred during that period, and, if so, the mean copulation latency (CL) of the matings. Mean copulation latencies and their standard errors (SE) are given in minutes.
The strong sexual isolation between D. santomea females and D. yakuba males suggested that the genetics of sexual isolation in females should be studied by pairing pure D. yakuba males with D. santomea/D. yakuba backcross (BC) females (F1 hybrid males are sterile, so that individuals of mixed genotype must be generated in backcrosses) and the genetics of sexual isolation in males should be studied by pairing pure D. santomea females with D. santomea/D. yakuba BC males. Preliminary experiments and previous data (Coyne et al. 2002) showed that sexual isolation of mixed genotypes was strongest in males from the backcrosses of F1 hybrid females to D. yakuba males and strongest in females from the backcross of F1 hybrid females to D. santomea males.
To produce F1 females, 4-day-old virgin D. yakuba ST females were crossed to D. santomea STO.4 males. Virgin F1 females were then backcrossed in two ways: (A) to D. santomea STO.4 males, producing 535 BC females, or (B) to D. yakuba ST males, producing 539 BC males. BC females are either homozygous D. santomea or heterozygous D. yakuba/D. santomea and have mitochondrial DNA from D. yakuba. BC males are autosomally either homozygous D. yakuba or heterozygous D. yakuba/D. santomea, the X-linked loci are either pure D. yakuba or D. santomea, and the Y chromosome and mitochondrial DNA are from D. yakuba.
Mating behavior:
Two sets of no-choice mating assays were conducted in which single BC individuals were paired with single pure-species individuals: (A) BC females and D. yakuba ST males and (B) BC males and D. santomea STO.4 females. Experiment A reveals the QTL in BC females that lead to lack of mating with D. yakuba males. Since D. yakuba males mate readily with conspecific females in the assay time period, we presume that heterospecific alleles at QTL (i.e., those for D. santomea female mating behavior) are the likely cause of reduced mating success by D. yakuba males. Experiment B reveals QTL in BC males that lead to lack of mating with D. santomea females. Again, heterospecific alleles (i.e., those for D. yakuba male mating behavior) are the likely cause of this reduced mating success.
The BC flies were collected as virgins and sorted by sex under brief CO2 exposure and kept in uncrowded vials for 4 days before use in experiments. Four-day-old virgin BC and pure-species flies were transferred by aspiration to 8-dram vials containing standard cornmeal–agar–Karo media within 1.5 hr of “lights on.” Experiments were conducted at room temperature, which varied from 21° to 23°. Forty pairs of flies were watched during each observation session. For flies in experiment A, we watched flies for a variable period until about half of them had mated; this period varied from 20 to 57 min. We watched flies from experiment B for a constant period of 45 min, which also yielded an ∼50% frequency of copulation. In both experiments, flies that did not mate were given a mating score of “0,” while those that mated were given a score of “1.” For those flies that did copulate, copulation latency (time from introduction of flies into vials to copulation) was recorded in minutes; individuals that did not mate were not used in the analysis of copulation latency.
Molecular markers:
We used the same 32 strain-specific markers and conditions for genotyping described in Carbone et al. (2005), with two exceptions. We included Ngp between Sara and Kr and excluded janB between ymp and krz. The forward and reverse primers for Ngp were, respectively, 5′-AGAACAATTGGCCCAAAAGA-3′ and 5′-CCTCGGATCTAGCATCTTCG-3′. The primer annealing temperature for this marker was 55°, and the nucleotide difference was detected as a restriction length polymorphism following digestion with BamHI.
All BC flies from the mating behavior assays were stored at −80° in 0.5-ml Eppendorf tubes. Genomic DNA was extracted from each BC individual using the Puregene (Gentra Systems, Research Triangle Park, NC) single-fly DNA extraction protocol, with minor revisions involving increased centrifugation times and pipette transfer of supernatant rather than pouring. Genotyping was performed using restriction fragment length polymorphism analysis by PCR amplification from genomic DNA, using RedTaq DNA Polymerase (Sigma, St. Louis) followed by restriction enzyme digestion (see Carbone et al. 2005 for primers, restriction enzymes, and conditions). Digested products were run on a 3% agarose gel stained with ethidium bromide, imaged with the Bio-Rad ChemiDoc System PC RS-170 and Quantity One software (version 4.2.1), and manually genotyped. Individuals were scored as a 0 if homozygous and a 1 if heterozygous. The genotypes of the 1074 backcross hybrids were determined for all 32 markers (i.e., 34,368 genotypes). The marker map was constructed using Mapmaker.
QTL mapping:
QTL for copulation latency and copulation duration were mapped in each backcross population using composite interval mapping (CIM) (Zeng 1994), implemented using QTL Cartographer software (Basten et al. 1999). CIM tests whether an interval between two markers contains a QTL affecting the trait while simultaneously controlling for the effect of QTL located outside the interval using multiple regression on marker cofactors. Marker cofactors were chosen by forward selection–backward elimination stepwise regression. The likelihood-ratio (LR) test statistic is −2 ln(L0/L1), where L0/L1 is the ratio of the likelihood under the null hypothesis (i.e., there is no QTL in the test interval) to the alternative hypothesis (there is a QTL in the test interval). LR test statistics were computed every 2 cM with marker cofactors ≥10 cM from the test location.
We used permutation analysis to determine appropriate significance thresholds that take into account the multiple tests performed and correlations among markers. We permuted trait and marker data 1000 times and recorded the maximum LR statistic across all intervals for each permutation. LR statistics calculated from the original data that exceed the 50th greatest LR statistic from the permuted data are significant at the experimentwise 5% level under the null hypothesis (Churchill and Doerge 1994; Doerge and Churchill 1996). We estimated the effects of each QTL as the difference between heterozygous yakuba/santomea genotypes and homozygous pure-species genotypes at the peak LR, scaled by the phenotypic standard deviation. The approximate boundaries of regions containing QTL were determined by taking 2-LOD intervals (9.22 LR) surrounding the point of greatest significance and interpolating the cytological location of the interval by dividing the cytology within the region according to the observed amount of recombination between flanking markers.
Although the assumption of normality when calculating CIM is violated by the analysis of the binary trait of copulation occurrence, a previous study (Moehring et al. 2004) has shown that using an extension of CIM based on logistic regression (Xu and Atchley 1996), which assumes that the binary trait is connected to its continuous underlying liability by a threshold model (Falconer and Mackay 1996), reveals the same QTL peaks as those found with CIM.
We evaluated pairwise epistatic interactions between all significant QTL within each experiment, using either the marker positioned at the highest LR of each QTL peak or the haplotype of the two markers flanking the QTL peak. Tests for epistasis were calculated for the binary trait of copulation occurrence with a log-linear model using PROC CATMOD and SAS 8.2 software (SAS Institute, Cary, NC). Significance thresholds were determined via a Bonferroni correction.
RESULTS AND DISCUSSION
Cytology:
The chromosome band order in D. yakuba differs from D. melanogaster by many inversions and translocations (Ashburner 1989). We used publicly available sequences of these species to define the cytological differences more precisely. The D. yakuba cytology relative to D. melanogaster is given at 100-kb intervals in supplemental Table 1 (http://www.genetics.org/supplemental/), the exact base pair breakpoints of each cytological segment are given in supplemental Table 2 (http://www.genetics.org/supplemental/), and an overall comparison between D. yakuba and D. melanogaster genomes is given in Figure 2.
Figure 2.
The significant regions from QTL mapping when compared to D. melanogaster cytology. Short vertical lines below the horizontal are every 250 kb; the short thick lines are every 1 Mb. Tall vertical lines above the horizontal represent the inversion/translocation breakpoints. Colored boxes represent QTL regions: BC female copulation occurrence (yellow) and latency (blue) when mated to D. yakuba males and BC male copulation occurrence (red) when mated to D. santomea females. “*” indicates the peak of the QTL. Centromeres are represented by gray circles. Open red triangles represent markers, with the markers in the same order as listed in materials and methods: y, per, sog, v, rux, f, bnb, Hex-A, AnnX, su(f), l(2)gl, Rad1, RpL27A, salr, Rep4, His3, barr, Sara, Ngp, Kr, Lsp1γ, dib, sfl, Est-6, Ssl1, ry, Rpn5, AP-50, Mlc1, ymp, and krz. Note that markers are spaced according to base pair distance.
The cytological order in D. yakuba in relation to D. melanogaster is as follows, where “*” denotes the centromere: X chromosome (also see Figure 2), |1A1–2B14|11A9–11A1|5D3–6D7|5C5–2B14|11A9–15A1|10C10–9F5|19B3–18B1|8D9–6D7|5C5–5D3|11A1–10C10|15A1–18B1|8D9–9F5|19B3–20E|*; chromosome 2, |21A2–25B1|28D2–26B8|31E2–34E2|25C10–25B2|28D3–31E2|26B8–25C10|34E2–35B8|42B2–47A9|35F11–35B8|42A15–41F7|*|40F1–39E1|36A2–36D3|36A2–35F12|47A9–47F3|38F2–39D4|36D2–38D2|50D4–47F3|38F1–38D2|50D3–51F7|58C1–54C1|58E1–59B2|53E1–51F8|58C1–58E1|54C1–53E1|59B2–60F5|; and chromosome 3, |61A5–63D4|67C5–66B11|63B8–66B11|63B8–62D4|67C5–71B5|75E2–72F1|71B5–72D7|78F4–75E2|72F1–72D7|78F4–80C4|*|82A1–83B1|90A6–93F2|84E9–84A1|84E10–86E13|89F1–89D2|87A2–88A3|89D2–88A3|87A2–86E13|89F1–90A6|83B1–84A1|93F2–95C13|99D1–95C13|99D1–100D1|. Note that the molecular cytology differs substantially from that inferred from observations of banding patterns of salivary chromosomes (Ashburner 1989).
Molecular marker map:
We genotyped 1074 D. yakuba/D. santomea backcross hybrids for 32 molecular markers. The cytological locations of the markers (relative to D. melanogaster), recombination rates, and map distances are given in Table 2. Note that the map is expanded relative to D. melanogaster—by 21, 37, and 60%, respectively, for the X, second, and third chromosomes. Increased recombination rates relative to D. melanogaster have also been previously observed for the sibling species D. simulans and D. mauritiana (Ashburner 1989; Liu et al. 1996; Zeng et al. 2000; Moehring et al. 2004) and D. simulans and D. sechellia (Liu et al. 1996; Macdonald and Goldstein 1999; Civetta and Cantor 2003; Gleason and Ritchie 2004).
TABLE 2.
Molecular markers and map positions
| Chromosome | Marker | Cytological location | r | d | Prop. het. |
|---|---|---|---|---|---|
| X | y | 1A5 | 0.0000 | 0.00 | 0.4802 |
| per | 3B1–2 | 0.0255 | 2.62 | 0.4877 | |
| sog | 13E1 | 0.1316 | 17.89 | 0.4790 | |
| v | 9F11 | 0.0683 | 25.23 | 0.4742 | |
| rux | 5D2 | 0.2131 | 53.01 | 0.4788 | |
| f | 15F4–7 | 0.0643 | 59.89 | 0.4993 | |
| bnb | 17D6 | 0.0800 | 68.61 | 0.4998 | |
| Hex-A | 8E10 | 0.0366 | 72.41 | 0.4965 | |
| AnnX | 19C1 | 0.0453 | 77.16 | 0.4956 | |
| su(f) | 20E | 0.0255 | 79.77 | 0.4986 | |
| 2 | l(2)gl | 21A5 | 0.0000 | 0.00 | 0.5228 |
| Rad1 | 23A1 | 0.0623 | 6.65 | 0.5275 | |
| RpL27A | 24F3 | 0.0637 | 13.47 | 0.5285 | |
| salr | 32E4–F1 | 0.1371 | 29.49 | 0.5376 | |
| Rep4 | 34B4 | 0.0722 | 37.29 | 0.5527 | |
| His3 | 39D3–E1 | 0.1679 | 57.75 | 0.5558 | |
| barr | 38B1–2 | 0.0464 | 62.62 | 0.5546 | |
| Sara | 57E6 | 0.0994 | 73.70 | 0.5543 | |
| Ngp | 54C8 | 0.1409 | 90.25 | 0.5432 | |
| Kr | 60F5 | 0.3506 | 150.65 | 0.5134 | |
| 3 | Lsp1γ | 61A6 | 0.0000 | 0.00 | 0.5129 |
| dib | 64A5 | 0.1631 | 19.74 | 0.5199 | |
| sfl | 65B3–4 | 0.1168 | 33.04 | 0.5281 | |
| Est-6 | 69A1 | 0.2273 | 63.36 | 0.5108 | |
| Ssl1 | 80B2 | 0.2086 | 90.35 | 0.5346 | |
| ry | 87D9 | 0.1036 | 101.96 | 0.5277 | |
| Rpn5 | 83C4 | 0.1612 | 121.42 | 0.5417 | |
| AP-50 | 94A15–16 | 0.0803 | 130.17 | 0.5311 | |
| Mlc1 | 98A14–15 | 0.1697 | 150.90 | 0.5288 | |
| ymp | 96E | 0.0858 | 160.32 | 0.5261 | |
| krz | 100E3 | 0.1418 | 176.99 | 0.5019 | |
| 4 | ci | 102A1–3 | 0.0000 | 0.00 | 0.4984 |
The order of the markers in the first column reflects their relative positions in D. yakuba and D. santomea, inferred from the D. yakuba genome project (Release 1.0, April 2004; http://www.genome.wustl.edu/projects/yakuba/). Cytological locations are given on the basis of D. melanogaster cytology (Lemeunier and Ashburner 1976). r, the recombination rate between two adjacent markers; d, the genetic distance inferred from r using the Haldane map function [d = −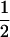 ln(1 − 2r)]; Prop. het, the proportion of genotypes that were heterozygous for that marker.
ln(1 − 2r)]; Prop. het, the proportion of genotypes that were heterozygous for that marker.
We assessed whether the markers exhibited segregation distortion, as would be expected if they were associated with differences in hybrid viability (Table 2). Although the proportion of heterozygotes was significantly skewed, averaged over all loci (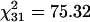 , P ≤ 0.001), no particular marker was especially deviant. It is interesting to note that there was a tendency toward an increased number of homozygotes for the X chromosome (Table 2), while there was a tendency toward an increased number of heterozygotes for autosomes. The X chromosome data are consistent with multiple polygenic X-linked loci, each contributing to small reductions in hybrid viability. The excess of heterozygotes on the autosomes may be due to inbreeding depression for viability that occurred in the parental species strains during long-term laboratory maintenance, so that the observed heterosis in the interspecific crosses is not related to speciation. The same explanation was previously proposed to account for the genomewide excess of heterozygotes in D. simulans/D. mauritiana backcross hybrids (Moehring et al. 2004).
, P ≤ 0.001), no particular marker was especially deviant. It is interesting to note that there was a tendency toward an increased number of homozygotes for the X chromosome (Table 2), while there was a tendency toward an increased number of heterozygotes for autosomes. The X chromosome data are consistent with multiple polygenic X-linked loci, each contributing to small reductions in hybrid viability. The excess of heterozygotes on the autosomes may be due to inbreeding depression for viability that occurred in the parental species strains during long-term laboratory maintenance, so that the observed heterosis in the interspecific crosses is not related to speciation. The same explanation was previously proposed to account for the genomewide excess of heterozygotes in D. simulans/D. mauritiana backcross hybrids (Moehring et al. 2004).
QTL for mating behavior:
We mapped QTL affecting the discrimination of BC to D. santomea females against pure-species D. yakuba males. We detected two additive QTL affecting copulation occurrence, one on the X chromosome and one with large effect on chromosome 3 (Table 3, Figures 1A and 2). One QTL for copulation latency mapped to the X chromosome in this hybridization (Table 3, Figures 1A and 2), but it should be noted that our power to detect QTL for copulation latency is lower than that for copulation occurrence since only half of the individuals mated and had latency scores. These results are consistent with those of Coyne et al. (2002), in which hybrid females actually have lower copulation latencies in tests to males of both species than do conspecific females.
TABLE 3.
QTL affecting copulation occurrence and latency between D. yakuba and D. santomea
| BC cross | Trait | QTL | Peak | LR | Effect | Effect/σp | R2 |
|---|---|---|---|---|---|---|---|
| F1 females × D. santomea males | BC female mating to D. yakuba male | 1A–3B | 4B | 13.42 | −0.21 | −9.52 | 0.0225 |
| 82A–88B | 85E | 48.21 | −0.30 | −14.06 | 0.0908 | ||
| F1 females × D. santomea males | Latency to BC female mating D. yakuba male | 7D–16D | 10E | 11.81 | 4.91 | 0.42 | 0.0439 |
| F1 females × D. yakuba males | BC male mating to D. santomea female | 48D–50D | 48A | 10.22 | −0.16 | −7.44 | 0.0188 |
| 68A–73E | 69A | 12.73 | −0.16 | −7.60 | 0.0200 | ||
| 97D–95C | 96E | 14.67 | −0.16 | −7.22 | 0.0231 |
QTL regions are estimated from 2 LOD support intervals (P ≤ 0.05) and the cytological locations were extrapolated from recombination rate between markers in comparison to the D. yakuba cytological map. The peak is the cytological location with the highest likelihood ratio (LR). Effects were estimated from the least-squares means of the two genotype classes as [homozygous − heterozygous] and are also given scaled by the phenotypic standard deviation (σp). R2 is the proportion of the variance accounted for the QTL and is estimated by 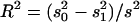 , where s2 is the variance of the trait,
, where s2 is the variance of the trait, 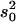 is the sample variance of the residuals, and
is the sample variance of the residuals, and 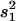 is the variance of the residuals (Basten et al. 1999).
is the variance of the residuals (Basten et al. 1999).
Figure 1.
QTL for the X, second, and third chromosomes affecting copulation latency (time to copulation) and occurrence (whether or not copulation occurred) in backcross hybrids between D. yakuba and D. santomea. There were no QTL for the small fourth chromosome. (A) F1 females backcrossed to D. santomea males. Resulting females are tested against D. yakuba males. (B) F1 females backcrossed to D. yakuba males. Resulting males are tested against D. santomea females. Plots are the likelihood-ratio (LR) test statistics for copulation latency (purple) and copulation occurrence (blue) as determined by composite interval mapping. The significance thresholds were determined by permutation testing, are represented by correspondingly colored dashed horizontal lines, and are all approximately LR = 10. Marker locations are represented by black triangles on the x-axis and are in the same order from left to right as the order listed in materials and methods: y, per, sog, v, rux, f, bnb, Hex-A, AnnX, su(f), l(2)gl, Rad1, RpL27A, salr, Rep4, His3, barr, Sara, Ngp, Kr, Lsp1γ, dib, sfl, Est-6, Ssl1, ry, Rpn5, AP-50, Mlc1, ymp, and krz. Note that markers are spaced according to recombination distance.
We mapped QTL affecting the discrimination of BC to D. yakuba males against pure-species D. santomea females. We detected three autosomal QTL, one on the second chromosome and two on chromosome 3 affecting copulation occurrence (Table 3, Figures 1B and 2). These QTL displayed additive gene action, as there was no evidence for pairwise epistasis between significant QTL. We did not detect any QTL affecting variation in copulation latency in this mapping population. Coyne et al. (2002) evaluated the mating success of reciprocal F1 D. santomea/D. yakuba male hybrids paired with both pure species. Hybrid males with a D. yakuba X chromosome mated as frequently to either D. santomea or D. yakuba females as did conspecific males, indicating a negligible effect of the X chromosome on mating occurrence, as observed here. However, Coyne et al. (2002) also observed that hybrid males with a D. santomea X chromosome had reduced mating success with both D. yakuba and D. santomea females. One interpretation of this unusual result is that QTL on the D. santomea X chromosome interact epistatically with QTL on the D. yakuba autosomes, causing behavioral sterility. While this does not appear to be the case—no significant epistatic interactions were detected between X chromosome markers with autosomal markers (after Bonferroni correction)—it is possible that increased sample size would increase the power to detect these interactions. Our observation that no QTL for copulation latency were detected in pairings of backcross males to D. santomea females is also consistent with the previous observations of Coyne et al. (2002).
Comparison with other studies:
Previously, we mapped QTL affecting sexual isolation in the D. simulans/D. mauritiana hybridization and observed at least seven QTL affecting traits in D. mauritiana females leading to reduced mating success with D. simulans males and at least three QTL in D. simulans males leading to reduced mating success with D. mauritiana females (Moehring et al. 2004). We can thus compare numbers, effects, locations, and coincidence of QTL across both studies to gain insight regarding the genetic basis of interspecific sexual isolation. In both cases, relatively few QTL, with moderate to large effects, contribute to behavioral isolation between species. It is thus possible that a few genes with relatively large effects generally account for sexual isolation. This statement must be tempered with the usual caveat that more QTL could be detected with larger numbers of backcross individuals and a greater density of markers.
In contrast to predictions of some models of sexual isolation via sexual selection, which postulate preferential accumulation of genes affecting sexual isolation on the X chromosome (Rice 1984; Charlesworth et al. 1987), we observed that autosomal loci had the greatest effects on most traits involved in prezygotic isolation for both the D. santomea/D. yakuba and D. simulans/D. mauritiana hybridizations. Similar results were also obtained in studies of sexual isolation between D. simulans and D. sechellia (Coyne 1992) and between D. pseudoobscura and D. persimilis (Noor 1997).
Another emerging theme from several interspecific hybridizations is that genes affecting sexual isolation are not the same in males and females. None of the QTL detected for female and male mating success overlapped in the D. santomea/D. yakuba hybridizations reported here, a finding previously observed for sexual isolation between D. simulans/D. mauritiana (Coyne 1989, 1993, 1996a,b; Moehring et al. 2004), D. arizonensis/D. mojavensis (Zouros 1981), and two “races” of D. melanogaster (Ting et al. 2001). This is also often true for sexual isolation in other species (Butlin and Ritchie 1989; Ritchie and Phillips 1998) and indicates that the complex genetic architecture underpinning the many morphological, behavioral, and chemical signals used in courtship does not typically overlap the genetic architecture required to perceive and evaluate these signals. There are, however, some instances in which male traits and female preference for the traits are genetically coupled (Hoy et al. 1977; Ritchie 1992; Marcillac et al. 2005).
Is it possible that the same genetic mechanisms contribute to sexual isolation in independent speciation events? We can address this question by comparing the locations of QTL affecting female and male mating success in the D. simulans/D. mauritiana and D. santomea/D. yakuba hybridizations. If QTL affecting sexual isolation do not colocalize, then we can rule out a common genetic basis. On the other hand, colocalization does not necessarily correspond to common genetic mechanisms, which must be addressed by further high-resolution mapping in both species pairs. One of the two QTL affecting female sexual isolation in the D. santomea/D. yakuba hybridization (82A–88B) does overlap with one of the seven QTL affecting female sexual isolation in the D. simulans/D. mauritiana hybridization (88B–93F), although the QTL peaks do not coincide (85E vs. 91C, respectively). In addition, two of the three QTL affecting male sexual isolation between D. santomea and D. yakuba (68A–73E, peak LR at 69A; and 95C–97D, peak LR at 96E) also affect male sexual isolation between D. simulans and D. mauritiana (69A–71B, peak LR at 70C; and 95D–100E, peak LR at 97B). However, the male traits are not identical in the two experiments. Here, we mapped QTL affecting reluctance of D. yakuba males to court D. santomea females; whereas Moehring et al. (2004) mapped QTL affecting male D. simulans traits against which female D. mauritiana discriminated. Nevertheless, we note that the 95–100 region on chromosome 3 has been repeatedly implicated in studies mapping QTL for sexual behavior in Drosophila (Moehring and Mackay 2004; Moehring et al. 2004; this article). A positional candidate gene in this region, E(Spl), both fails to complement QTL affecting variation in male mating behavior (Moehring and Mackay 2004) and exhibits altered transcript abundance between lines selected for increased and decreased copulation latency (Mackay et al. 2005).
Finally, we can ask to what extent QTL affecting sexual isolation overlap those affecting the large morphological difference in abdominal pigmentation between D. santomea and D. yakuba (Lachaise et al. 2000; Llopart et al. 2002; Carbone et al. 2005). D. santomea is completely devoid of any pigmentation while D. yakuba shows the sexually dimorphic pattern typical of the D. melanogaster group: females' yellow abdomens are striped with black, while those of males have black tips. In addition to this being a major morphological difference between these two species, pigmentation has also been shown previously to have an impact on mating success (e.g., Sturtevant 1915; Bastock 1956). We find that the single QTL at 15F4–20E affecting pigmentation in (D. yakuba/D. santomea F1 ♀ × D. santomea ♂) BC hybrid females (Carbone et al. 2005) overlaps the single QTL at 7D–16E affecting copulation latency for these females paired with D. yakuba males mapped in this study. In addition, the two autosomal QTL at 34B4–57E6 and 69A1–83C4 affecting pigmentation in (D. yakuba/D. santomea F1 ♀ × D. yakuba ♂) BC hybrid males (Carbone et al. 2005) overlap two of the QTL at 48D–50D and 68A–73E, affecting copulation success of these males paired with D. santomea females mapped in this study. These observations raise the interesting hypothesis that common genes may underlie the evolution of the morphological and behavioral differences between these species. Llopart et al. (2002) have shown that any effect of pigmentation on sexual isolation in this species pair is not due to a visual cue, since there was no difference in measures of sexual isolation between these species determined under light and dark conditions. It remains possible, however, that genes affecting pigmentation may have pleiotropic effects on other behavioral traits, such as locomotion, that could be a component of mating behavior. Alternatively, it is possible that selective sweeps at pigmentation loci resulted in fixation of chromosomally linked regions containing genes affecting mating preference. These hypotheses can be distinguished by jointly mapping QTL affecting pigmentation and mating behavior in backcross hybrids.
Acknowledgments
We thank Mary Anna Carbone and Ted Morgan for helpful discussions. This work was funded by National Institutes of Health research grants to J.A.C. (GM 58260) and T.F.C.M. (GM45344 and GM 58260). This is a publication of the W. M. Keck Center for Behavioral Biology.
References
- Ashburner, M., 1989. Drosophila: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Basten, C. J., B. S. Weir and Z-B. Zeng, 1999. QTL Cartographer: Version 1.13. Department of Statistics, North Carolina State University, Raleigh, NC.
- Bastock, M., 1956. A gene mutation which changes a behavior pattern. Evolution 10: 421–439. [Google Scholar]
- Butlin, R. K., and M. G. Ritchie, 1989. Genetic coupling in mate recognition systems: What is the evidence? Biol. J. Linn. Soc. 37: 237–246. [Google Scholar]
- Carbone, M. A., A. Llopart, M. deAngelis, J. A. Coyne and T. F. C. Mackay, 2005. Quantitative trait loci affecting the difference in pigmentation between Drosophila yakuba and D. santomea. Genetics 171: 211–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cariou, M.-L., J.-F. Silvain, V. Daubin, J.-L. Dalage and D. Lachaise, 2001. Divergence between Drosophila santomea and allopatric or sympatric populations of D. yakuba using paralogous amylase genes and migration scenarios along the Cameroon volcanic line. Mol. Ecol. 10: 649–660. [DOI] [PubMed] [Google Scholar]
- Charlesworth, B., J. A. Coyne and N. H. Barton, 1987. The relative rates of evolution of sex chromosomes and autosomes. Am. Nat. 130: 113–146. [Google Scholar]
- Churchill, G. A., and R. W. Doerge, 1994. Empirical threshold values for quantitative trait mapping. Genetics 138: 963–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civetta, C., and E. J. F. Cantor, 2003. The genetics of mating recognition between Drosophila simulans and D. sechellia. Genet. Res. 82: 117–126. [DOI] [PubMed] [Google Scholar]
- Coyne, J. A., 1989. The genetics of sexual isolation between two sibling species, Drosophila simulans and Drosophila mauritiana. Proc. Natl. Acad. Sci. USA 86: 5464–5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne, J. A., 1992. Genetics of sexual isolation in females of the Drosophila simulans species complex. Genet. Res. 60: 25–31. [DOI] [PubMed] [Google Scholar]
- Coyne, J. A., 1993. The genetics of an isolating mechanism between two sibling species of Drosophila. Evolution 47: 778–788. [DOI] [PubMed] [Google Scholar]
- Coyne, J. A., 1996. a Genetics of a difference in male cuticular hydrocarbons between two sibling species, Drosophila simulans and D. sechellia. Genetics 143: 1689–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne, J. A., 1996. b Genetics of sexual isolation in male hybrids of Drosophila simulans and D. mauritiana. Genet. Res. 68: 211–220. [DOI] [PubMed] [Google Scholar]
- Coyne, J. A., and H. A. Orr, 2004. Speciation. Sinauer Associates, Sunderland, MA.
- Coyne, J. A., S. Y. Kim, A. S. Chang, D. Lachaise and S. Elwyn, 2002. Sexual isolation between two species with overlapping ranges: Drosophila santomea and Drosophila yakuba. Evolution 56: 2424–2434. [DOI] [PubMed] [Google Scholar]
- Coyne, J. A., S. Elwyn and E. Rolan-Alvarez, 2005. Sexual isolation between Drosophila yakuba and D. santomea: effects of environment and experimental design. Evolution 59: 2588–2601. [PubMed] [Google Scholar]
- Doerge, R. W., and G. A. Churchill, 1996. Permutation tests for multiple loci affecting a quantitative character. Genetics 142: 285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer, D. S., and T. F. C. Mackay, 1996. Introduction to Quantitative Genetics, Ed. 4. Longman, London. [DOI] [PMC free article] [PubMed]
- Gleason, J. M., and M. G. Ritchie, 2004. Do quantitative trait loci for a courtship song difference between Drosophila simulans and D. sechellia overlap with candidate genes and intraspecific QTL? Genetics 166: 1303–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy, R. R., J. Hahn and R. C. Paul, 1977. Hybrid cricket auditory behavior: evidence for genetic coupling in animal communication. Science 195: 82–84. [DOI] [PubMed] [Google Scholar]
- Lachaise, D., M. Harry, M. Solignac, F. Lemeunier, V. Bénassi et al., 2000. Evolutionary novelties in islands: Drosophila santomea, a new melanogaster sister species from São Tomé. Proc. R. Soc. Lond. Ser. B 267: 1487–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemeunier, F., and M. Ashburner, 1976. Relationships within the melanogaster species subgroup of the genus Drosophila (Sophophora). II. Phylogenetic relationships between six species based upon polytene chromosome banding sequences. Proc. R. Soc. Lond. B. 193: 275–294. [DOI] [PubMed] [Google Scholar]
- Llopart, A., S. Elwyn, D. Lachaise and J. A. Coyne, 2002. Genetics of a difference in pigmentation between Drosophila yakuba and Drosophila santomea. Evolution 56: 2262–2277. [DOI] [PubMed] [Google Scholar]
- Llopart, A., D. Lachaise and J. A. Coyne, 2005. An anomalous hybrid zone in Drosophila. Evolution 59: 2602–2607. [PubMed] [Google Scholar]
- Liu, J., J. M. Mercer, L. F. Stam, G. C. Gibson, Z-B. Zeng et al., 1996. Genetic analysis of a morphological shape difference in the male genitalia of Drosophila simulans and D. mauritiana. Genetics 142: 1129–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay, T. F. C., S. L. Heinsohn, R. F. Lyman, A. J. Moehring, T. J. Morgan et al., 2005. Genetics and genomics of Drosophila mating behavior. Proc. Natl. Acad. Sci. USA 102: 6622–6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald, S. J., and D. B. Goldstein, 1999. A quantitative genetic analysis of male sexual traits distinguishing the sibling species Drosophila simulans and D. sechellia. Genetics 153: 1683–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcillac, F., Y. Grosjean and J.-F. Ferveur, 2005. A single mutation alters production and discrimination of Drosophila sex pheromones. Proc. R. Soc. Biol. 272: 303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehring, A. J., and T. F. C. Mackay, 2004. The quantitative genetic basis of male mating behavior in Drosophila melanogaster. Genetics 167: 1249–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehring, A. J., J. Li, M. D. Schug, S. Smith, M. DeAngelis et al., 2004. Quantitative trait loci for sexual isolation between Drosophila simulans and D. mauritiana. Genetics 167: 1265–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noor, M. A. F., 1997. Genetics of sexual isolation and courtship dysfunction in male hybrids of Drosophila pseudoobscura and D. persimilis. Evolution 51: 809–815. [DOI] [PubMed] [Google Scholar]
- Rice, W. R., 1984. Sex chromosomes and the evolution of sexual dimorphism. Evolution 38: 735–742. [DOI] [PubMed] [Google Scholar]
- Ritchie, M. G., 1992. Behavioral coupling in tettigoniid hybrids. Behav. Genet. 22: 369–379. [DOI] [PubMed] [Google Scholar]
- Ritchie, M. G., and S. D. F. Phillips, 1998. The genetics of sexual isolation, pp. 291–308 in Endless Forms: Species and Speciation, edited by D. J. Howard and S. H. Berlocher. Oxford University Press, London/New York/Oxford.
- Sturtevant, A. H., 1915. Experiments on sex recognition and the problem of sexual selection in Drosophila. J. Anim. Behav. 5: 351–366. [Google Scholar]
- Takahashi, A., and C. T. Ting, 2004. Genetic basis of sexual isolation in Drosophila melanogaster. Genetica 120: 273–284. [DOI] [PubMed] [Google Scholar]
- Ting, C. T., A. Takahashi and C.-I Wu, 2001. Incipient speciation by sexual selection: concurrent evolution at multiple loci. Proc. Natl. Acad. Sci. USA 98: 6709–6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, C. I, H. Hollocher, D. J. Begun, C. F. Aquadro and Y. Xu, 1995. Sexual isolation in Drosophila melanogaster: a possible case of incipient speciation. Proc. Natl. Acad. Sci. USA 92: 2519–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, S., and W. R. Atchley, 1996. Mapping quantitative trait loci for complex binary diseases using line crosses. Genetics 143: 1417–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, M. A., A. G. Blouin and M. A. F. Noor, 2001. Courtship songs of Drosophila pseudoobscura and D. persimilis II. Genetics of species differences. Heredity 86: 68–77. [DOI] [PubMed] [Google Scholar]
- Zeng, Z-B., 1994. Precision mapping of quantitative trait loci. Genetics 136: 1457–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, Z-B., J. Liu, L. F. Stam, C. H. Kao, J. M. Mercer et al., 2000. Genetic architecture of a morphological shape difference between two Drosophila species. Genetics 154: 299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouros, E., 1981. The chromosomal basis of sexual isolation in two sibling species of Drosophila: D. arizonensis and D. mohavensis. Genetics 97: 703–718. [DOI] [PMC free article] [PubMed] [Google Scholar]




