Abstract
We have identified a family of resistin-like molecules (RELMs) in rodents and humans. Resistin is a hormone produced by fat cells. RELMα is a secreted protein that has a restricted tissue distribution with highest levels in adipose tissue. Another family member, RELMβ, is a secreted protein expressed only in the gastrointestinal tract, particularly the colon, in both mouse and human. RELMβ gene expression is highest in proliferative epithelial cells and is markedly increased in tumors, suggesting a role in intestinal proliferation. Resistin and the RELMs share a cysteine composition and other signature features. Thus, the RELMs together with resistin comprise a class of tissue-specific signaling molecules.
Resistin is a newly described circulating protein with no homology to any known hormone, cytokine, or other intercellular signaling molecule (1). Resistin is secreted specifically by adipocytes and has actions that antagonize insulin action (1). Because many polypeptide signaling molecules are members of multigene families, we used a functional genomic strategy to discover resistin homologs based on the unique cysteine-rich C terminus of mouse and human resistin. This analysis led to the discovery of a family of resistin-like molecules (RELMs) that are secreted proteins with unique tissue distributions and likely to be signaling molecules.
Materials and Methods
Northern and Western analyses were performed by standard methods (2, 3). Human multiple tissue and mouse embryonic Northern blots were obtained from CLONTECH. In situ hybridization was performed as described (4). Sequences were analyzed by using the National Center for Biotechnology Information blast server, dnastar (DNAstar, Madison, WI), psort, and signalp algorithms. Flag epitope-tagged RELMβ and RELMα were generated in the same manner as resistin (1). Immunoblot analysis was performed by using a mouse monoclonal FLAG antibody (Research Diagnostics, Flanders, NJ) at a dilution of 1:2,000. C57BL/6J-Apc (Min)/+ mice were killed at 14–20 wk of age. The entire length of the intestine was stained with 0.25% methylene blue (Sigma) to help visualize polyps. Tumors were scored blindly by a single observer. The polyps were at the noninvasive adenomatous stage. The average number of polyps detected per small intestine was 72 ± 10.
Results and Discussion
RELMα.
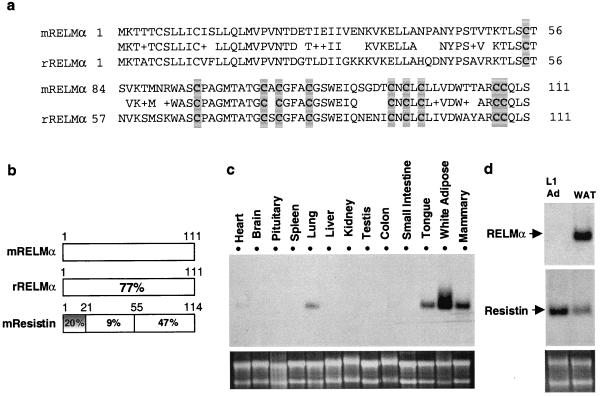
A search of the National Center for Biotechnology Information mouse expressed sequence tag (EST) database uncovered a series of cDNAs in mammary tissue, pancreas, and tongue libraries that encode a RELM, which we refer to as RELMα (Fig. 1a). The N terminus of RELMα contains a sequence that is predicted by the signalp algorithm to be a signal sequence cleavable between amino acids 23 and 24. Rat RELMα, identified as ESTs in lung and placental libraries, is 77% identical to the predicted mouse protein (Fig. 1 a and b). The C terminus of RELMα is highly related to resistin, particularly in the last 38 aa, where the identity is 63% with no gaps in the sequence and complete conservation of the cysteine residues (Fig. 1b, and see below). The N terminus of RELMα is only distantly related to resistin (Fig. 1b).
Figure 1.
Identification and characterization of RELMα. (a) Predicted amino acid sequences of murine and rat RELMα. Identical amino acids are indicated, conservative substitutions are indicated by +, and conserved cysteine residues are shaded. (b) Schematic comparison of murine RELMα, rat RELMα, and murine resistin predicted amino acid sequences. Percent identity is indicated. (c and d) Northern blot analysis of RELMα gene expression in various mouse tissues (c) and a comparison of 3T3-L1 adipocytes and mouse white adipose tissue (WAT) (d). Ethidium bromide staining of 28S and 18S RNA is shown in each case.
The tissue distribution of RELMα gene expression is quite interesting. RELMα mRNA was most abundant in white adipose tissue (Fig. 1c). White adipose tissue is where resistin is expressed (1). Expression of RELMα also was observed in mammary tissue, which contains a significant fat pad. However, unlike resistin, RELMα was not expressed in 3T3-L1 adipocytes (Fig. 1d), nor in preadipocytes (data not shown), suggesting that RELMα might be produced by the stromal vascular constituents of adipose tissue. Also unlike resistin, RELMα mRNA was detectable in heart, lung, and tongue (Fig. 1c).
RELMβ.
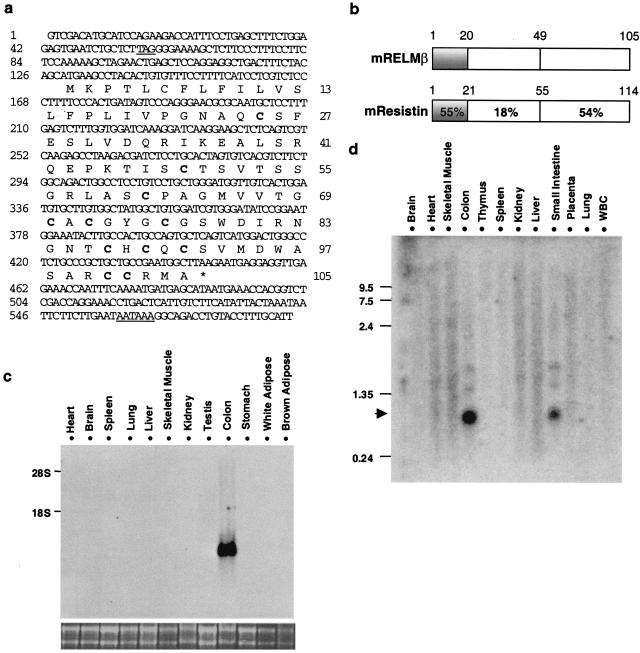
An additional RELM cDNA, referred to as RELMβ, was discovered in mouse colon EST databases. The mouse RELMβ cDNA and deduced amino acid sequence are shown in Fig. 2a. RELMβ is highly related to resistin, especially in its conserved cysteine-containing C terminus (Fig. 2b). The N terminus of RELMβ is predicted by the psort and signalp algorithms to contain a signal sequence that cleaves after the 20th amino acid. The predicted molecular mass of the processed form of RELMβ is 9,003 Da.
Figure 2.
RELMβ is an intestine-specific resistin-like molecule. (a) Murine RELMβ cDNA and protein sequences. In-frame upstream stop codon and putative polyadenylation sequence are underlined. Cysteine residues are indicated in bold, and the downstream stop codon is indicated by *. (b) Comparison of murine RELMβ and murine resistin predicted amino acid sequences. Percent identity is indicated. (c) Northern blot analysis of RELMβ gene expression in the mouse. 28S and 18S RNA is shown below to demonstrate loading of similar amounts of RNA (20 μg per lane). (d) Northern analysis of human RELMβ mRNA in multiple human tissues.
To assess the potential biological role of RELMβ, we performed Northern analysis of multiple mouse tissues. Remarkably, RELMβ mRNA was abundant in colon but not in multiple other tissues, including white adipose tissue, where resistin is uniquely expressed (Fig. 2c). Indeed, the vast majority of RELMβ ESTs derive from colon libraries. We also located multiple overlapping ESTs in human colon libraries. Human and mouse RELMβ are highly conserved, especially in the cysteine-rich C terminus that is most similar to resistin (see Fig. 4b). Importantly, similar to mouse RELMβ, the extreme N terminus also is predicted to contain a signal sequence that cleaves after the 20th amino acid. Northern analysis of multiple human tissues using the human RELMβ probe revealed that human RELMβ is also a small mRNA species detected only in colon and, to a lesser extent, small intestine (Fig. 2d).
Figure 4.
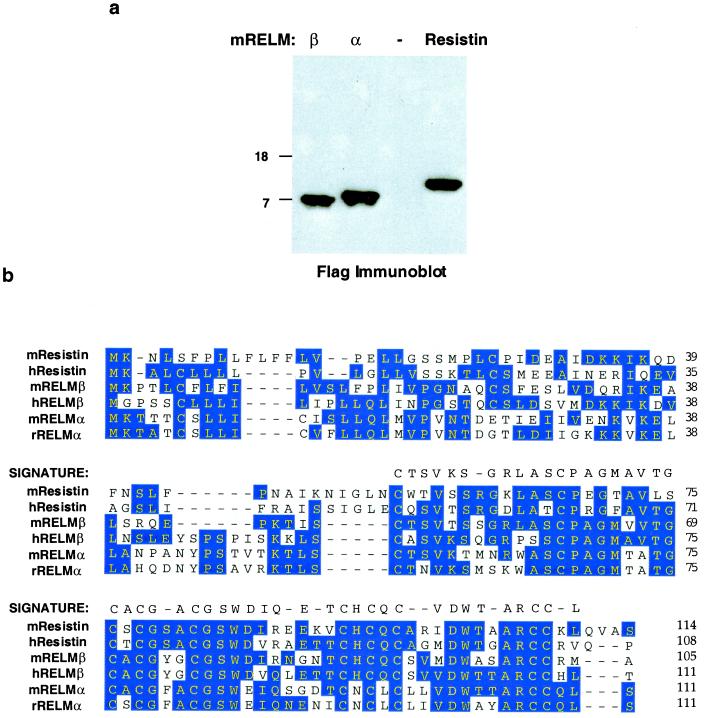
The RELM family of secreted proteins. (a) RELMs are secreted proteins. RELMβ, RELMα, and resistin were fused to Flag epitope at C terminus and expressed in 293T cells. Media was sampled and analyzed by immunoblot using Flag antibody. − indicates control vector. (b) Consensus sequence of the RELM family. Blue shading indicates amino acid identity of two or more family members, aligned by dnastar megalign program using the Jotun Hein method. The signature sequence characteristic of the RELMs is indicated.
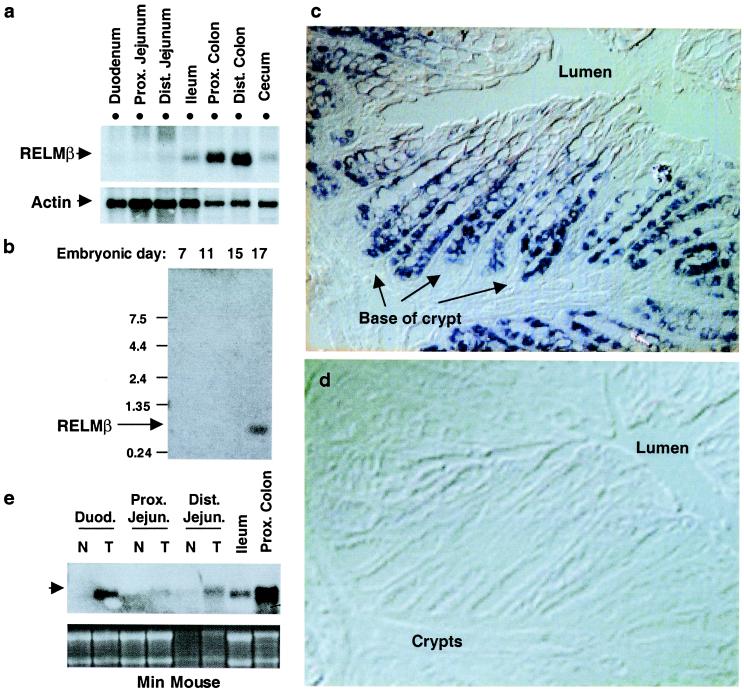
Within the mouse intestine, RELMβ gene expression was by far most abundant in proximal and distal colon (Fig. 3a). RELMβ mRNA was also detected in the cecum, and the ileum, but at much lower levels (Fig. 3a). During mouse embryogenesis, RELMβ gene expression is not detected until day 17 (Fig. 3b). Interestingly, this is the approximate stage at which rodent colon undergoes significant remodeling with a transition from an undifferentiated stratified epithelium to a simple columnar epithelium (5). As the colon consists of multiple cell types, including epithelial cells, lamina propria cells, and the muscularis propria, we performed in situ hybridization to precisely define where RELMβ is expressed. RELMβ mRNA is abundant in proliferative epithelia at the bases of the crypts and is dramatically extinguished in the nonproliferating differentiated epithelia that have migrated up from the crypt base to the luminal surface (Fig. 3c). The specificity of the in situ hybridization signal was revealed by lack of signal using a sense probe (Fig. 3d).
Figure 3.
RELMβ is expressed in proliferative intestinal epithelia. (a) Northern analysis of RELMβ mRNA levels in mouse intestine. β-actin hybridization is shown as control for loading. (b) Northern analysis of RELMβ mRNA during mouse embryogenesis. (c and d) In situ hybridization of serial sections of mouse colon probed with RELMβ antisense (c) and sense (d) probes. Positive staining is dark blue. (e) Northern analysis of RELMβ in normal appearing small intestine (N) and adjacent tumors (T) in a min mouse. 28 and 18S RNA is shown as loading control. A representative of three independent experiments is shown.
The robust expression of RELMβ in the proliferative compartment led us to study min mice, which harbor a mutation of the APC gene and thus develop intestinal tumors similar to humans with familial adenomatous polyposis (6). As in wild-type mice, RELMβ expression was modest in normal duodenum and jejunum. However, in the min mouse, RELMβ mRNA was markedly increased in tumors immediately adjacent to the normal tissue (Fig. 3e). Thus, RELMβ expression is greatest in intestinal epithelial cells whose proliferative rate is increased because of normal as well as pathological mechanisms. Specialized intestinal epithelial cells, such as columnar, goblet, enteroendocrine, and Paneth cells, express secreted proteins that have diverse functional roles (7–10). In particular, transforming growth factor type α expression has been localized to colonic crypt cells, where it may regulate cell proliferation. Although the function of RELMβ remains to be established, expression of a secreted protein restricted to the colonic epithelium is unique.
The RELM Family of Secreted Proteins.
To determine whether RELMα and RELMβ are secreted proteins, their cDNAs as well as that of resistin were fused in-frame to the flag epitope at the C-terminal end of the putative ORF and transfected into 293T human embryonic kidney cells. As predicted by analysis of the primary amino acid sequences, RELMα and RELMβ, like resistin, were secreted into the media by these cells (Fig. 4a). Although various intestinal epithelial cell types secrete proteins with diverse functional roles (7–10), RELMβ is unique both in its structure and its pattern of expression. In this regard, it will be critical to determine whether RELMβ secretion is directed apically into the gut lumen or basolaterally into the lamina propria. Future studies will determine whether RELMα is a circulating hormone like resistin or a paracrine, autocrine, or exocrine signaling molecule. The finding of RELMα in addition to resistin in fat cells raises the possibility of interactions between these two related molecules.
The consensus RELM is thus a protein of 105–114 aa in length with three domains: (i) an N-terminal signal sequence, (ii) a variable middle portion, and (iii) a highly conserved C-terminal signature sequence that constitutes nearly half of the molecule (Fig. 4b). The signature region of the RELMs contains a unique and invariant spacing of the cysteine residues: C-X11-C-X8-C-X-C-X3-C-X10-C-X-C-X-C-X9-CC-X3–6-END. This is reminiscent of but clearly distinct from so-called “EGF repeats” that are characteristic of a number of signaling molecules (11). Presumably, the unique cysteine pattern contributes to folding and multimerization of the RELMs. We speculate that the highly conserved signature region contributes to binding to a related family of receptors that are yet to be discovered. Each RELM is likely to have distinct biological functions consistent with its unique pattern of expression.
We have not detected RELMs in the nearly completed genomes of Caenorhabditis elegans or Drosophila melanogaster, suggesting that RELMs may be specific to higher organisms. Nevertheless, it is possible that additional RELMs will be discovered as the sequencing of the human and other genomes is completed. While this work was in progress, Holcomb et al. (12) detected RELMα (which they call FIZZ1) in the inflammatory zone of mice with allergic pulmonary inflammation. While those investigators focused on RELMα expression in the lung, we have found that the levels of RELMα gene expression in white adipose tissue, as well as mammary tissue and tongue, are higher than in lung. These independent studies also identi-fied RELMβ as FIZZ2 and corroborate our findings that RELMβ is highly abundant in colon. Clearly, additional work will be needed to understand the functions and mechanisms of action of each member of this family of tissue-specific, secreted proteins.
Acknowledgments
We thank Myles Brown and members of the Lazar lab for helpful discussions. This work was supported by grants to M.A.L. and G.D.W. from the National Institute of Diabetes and Digestive and Kidney Diseases and the National Institute of Allergy and Infectious Diseases. C.M.S. was supported by an unrestricted postdoctoral research fellowship from Pfizer. E.J.B. was supported by a student research fellowship from the American Diabetes Association. R.R.B. is a trainee of the Medical Scientist Training Program.
Abbreviations
- RELMs
resistin-like molecules
- EST
expressed sequence tag
Footnotes
References
- 1.Steppan, C. M., Bailey, S. T., Bhat, S., Wright, C. M., Brown, E. J., Banerjee, R. R., Ahima, R. S. & Lazar, M. A. (2001) Nature (London), in press. [DOI] [PubMed]
- 2.Chawla A, Schwarz E J, Dimaculangan D D, Lazar M A. Endocrinology. 1994;135:798–800. doi: 10.1210/endo.135.2.8033830. [DOI] [PubMed] [Google Scholar]
- 3.Huang E Y, Zhang J, Miska E A, Guenther M G, Kouzarides T, Lazar M A. Genes Dev. 2000;14:45–54. [PMC free article] [PubMed] [Google Scholar]
- 4.Yang H, Jiang W, Furth E E, Wen X, Katz J P, Sellon R K, Silberg D G, Antalis T M, Schweinfest C W, Wu G D. Am J Physiol. 1998;275:G1445–G1453. doi: 10.1152/ajpgi.1998.275.6.G1445. [DOI] [PubMed] [Google Scholar]
- 5.Williams L, Bell L. Embryology. 1991;183:573–578. doi: 10.1007/BF00187906. [DOI] [PubMed] [Google Scholar]
- 6.Su L-K, Kinzler K W, Vogelstein B, Preisinger A C, Moser A R, Luongo C, Gould K A, Dove W F. Science. 1992;256:668–670. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- 7.O'Neil D A, Porter E M, Elewaut D, Anderson G M, Eckmann L, Ganz T, Kagnoff M F. J Immunol. 1999;163:6718–6724. [PubMed] [Google Scholar]
- 8.Barnard J A, Beauchamp R D, Russell W E, Dubois R N, Coffey R J. Gastroenterology. 1995;108:564–580. doi: 10.1016/0016-5085(95)90087-x. [DOI] [PubMed] [Google Scholar]
- 9.Roth K A, Kim S, Gordon J I. Am J Physiol. 1992;263:G174–G180. doi: 10.1152/ajpgi.1992.263.2.G174. [DOI] [PubMed] [Google Scholar]
- 10.Sands B E, Podolsky D K. Annu Rev Physiol. 1996;58:253–273. doi: 10.1146/annurev.ph.58.030196.001345. [DOI] [PubMed] [Google Scholar]
- 11.Campbell I D, Bork P. Curr Biol. 1993;3:385–392. [Google Scholar]
- 12.Holcomb I N, Kabakoff R C, Chan B, Baker T W, Gurney A, Henzel W, Nelson C, Lowman H B, Wright B D, Skelton N J, et al. EMBO J. 2000;19:4046–4055. doi: 10.1093/emboj/19.15.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]