Abstract
The rat zitter (zi) mutation induces hypomyelination and vacuolation in the central nervous system (CNS), which result in early-onset tremor and progressive flaccid paresis. By positional cloning, we found a marked decrease in Attractin (Atrn) mRNA in the brain of the zi/zi rat and identified zi as an 8-bp deletion at a splice donor site of Atrn. Atrn has been known to play multiple roles in regulating physiological processes that are involved in monocyte–T cell interaction, agouti-related hair pigmentation, and control of energy homeostasis. Rat Atrn gene encoded two isoforms, a secreted and a membrane form, as a result of alternative splicing. The zi mutation at the Atrn locus darkened coat color when introduced into agouti rats, as also described in mahogany (mg) mice, carrying the homozygous mutation at the Atrn locus. Transgenic rescue experiments showed that the membrane-type Atrn complemented both neurological alteration and abnormal pigmentation in zi/zi rats, but that the secreted-type Atrn complemented neither mutant phenotype. Furthermore, we discovered that mg mice exhibited hypomyelination and vacuolation in the CNS associated with body tremor. We conclude from these results that the membrane Atrn has a critical role in normal myelination in the CNS and would provide insights into the physiology of myelination as well as the etiology of myelin diseases.
The zitter rat was found in a colony of Sprague–Dawley rats as a tremorous mutant, and subsequent genetic analysis showed that the abnormality was caused by an autosomal recessive gene, zitter (zi) (1, 2). The tremor develops spontaneously at 3 weeks of age, and flaccid paresis of the hind limb is observed at around 6 months of age (3). The main pathological findings are progressive hypomyelination and vacuolation in the central nervous system (CNS) (4). Hypomyelination is characterized by a significant decrease in the density of myelinated fibers and the number of myelin lamellae and is accompanied by aberrant or elongated myelin sheath formation (4). Vacuoles consist mainly of swollen astrocytic processes and enlargement of extracellular space as well as periaxonal spaces. The vacuoles are first detected in the pons and the outer thalamus at 3 weeks of age. With increasing age, vacuoles extend into the deep cortex, hippocampus, cervical spinal gray matter, and the granular layer and white matter of the cerebellum (5). However, the initiation of myelination and the fundamental structures of myelin sheaths are normal in the zitter rat. The biochemical components of myelin, such as myelin basic protein, proteolipid protein, and myelin-associated glycoprotein, are also normally expressed (5, 6). Therefore, the zitter rat is expected to provide useful tools for the study of axon–glia interaction and the assembly of myelin sheaths in the complex process of CNS myelination.
The zi gene has been mapped to a genomic region between IL-1β (Il1b) and prion protein (Prnp) on rat chromosome (Chr) 3q35 (3, 7). Prnp is known as a causative gene for spongiform encephalopathy, and it was considered as a strong candidate for zi. However, there were no differences in mRNA expression levels and nucleotide sequences of Prnp between the zitter and normal control rats. Thus, Prnp was excluded as a candidate for zi (7, 8). A comparative map between rat, mouse, and human showed that rat 3q35 corresponds to mouse Chr 2(70–80 cM) and human Chr 2q14 and Chr 20pter-p13 (9), but no obvious candidate genes are present in these regions.
Here we will describe the identification of zi by positional cloning and subsequent transgenic rescue experiments and assessment of the neurological phenotypes of Attractin (Atrn) mutant, mahogany (mg) mice. Our results show that the Atrn locus product has multiple functions not only in immune response, hair pigmentation, and energy control, but also in myelination in the CNS.
Materials and Methods
Genetic Fine Mapping.
(WTC × WTC.ZI-zi/zi)F1(zi/+) female rats were backcrossed to male WTC.ZI-zi/zi homozygote rats and intercrossed to male zi/+ F1 rats to produce progeny for fine mapping of zi. Homozygote zi/zi animals were identified on the basis of appearance of tremor at 3–4 weeks of age. Simple sequence length polymorphisms (SSLPs) at Il1b and Prnp were genotyped as described (3, 7). Three gene-specific SSLP markers, D3Kur2/Ptpns1, D3Kur7/Ptpra, and D3Kur55/Sn, were developed from the published sequences (GenBank accession numbers D85183, Z36293, and L01702), and two SSLP markers, D3Kur1/Pdyn and D3Kur9/Oxt, were developed from the cosmid clones as described (10). While the physical map was assembled (see below), seven anonymous SSLP markers, D3Kur4, D3Kur20, D3Kur24, D3Kur30, D3Kur34, D3Kur37, and D3Kur40, were developed from rat cosmid and P1 clones.
Physical Mapping.
A rat P1 library was screened at IncyteGenomics (Palo Alto, CA) by hybridizing with DNA fragments amplified by D3Kur7/Ptpra, D3Kur12/Oxt, D3Kur14, D3Kur18, D3Kur21, D3Kur27, and D3Kur33 (Fig. 1b). A rat P1-derived artificial chromosome (PAC) library was screened by hybridizing with DNA fragments amplified by D3Kur39, D3Kur44, and D3Kur50. Isolated clones were subjected to sequencing of their ends, and the sequences were used to design the PCR primers for sequence tagged site (STS) markers after repeat sequences were masked. To determine the precise physical positions of STS and SSLP markers as well as clone overlap and orientation, STS content mapping was performed by PCR with genomic clone DNA templates. Comparative mapping was performed by searching the database for genes or expressed sequence tags (ESTs) mapped to human Chr 20pter-p12 and mouse Chr 2, and by mapping them in the contig. blast homology searches were made by using the repeat-masked end sequences.
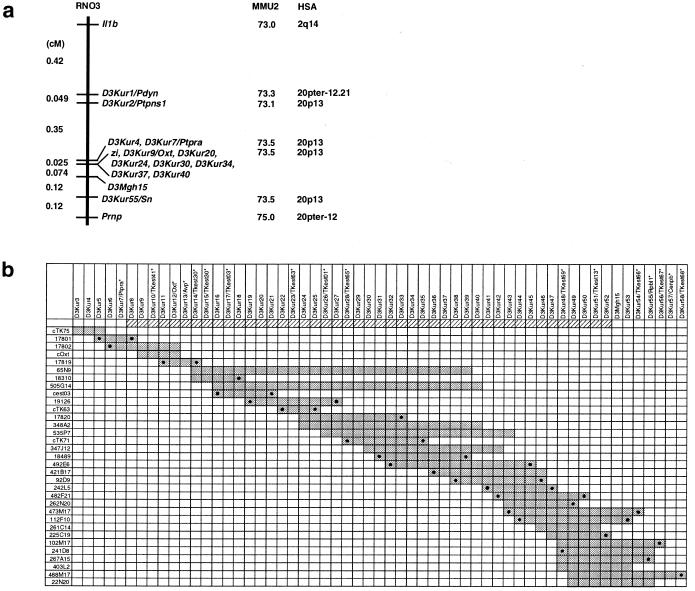
Figure 1.
Genetic linkage map and a contig of the zi-critical region. (a) The genetic linkage map of the region surrounding the zi locus. For loci also mapped in mouse and human, their locations in the corresponding chromosomes are indicated on their right. (b) The contig spanning the zi-critical region. Cosmid, P1, and PAC clones are listed on the left; STS markers are listed at the top. PCR signals on clones are indicated by dark gray boxes. The zi-critical region is depicted by oblique lines. PCR signals of the markers derived from end sequences are indicated by closed circles on the corresponding clones. Asterisks at the top show transcribed sequences. Cosmid clones are indicated by the addition of “c” to their names. P1 clones are indicated by five-digit numbers given by IncyteGenomics.
Cloning of Rat Atrn cDNA.
A rat brain cDNA library was constructed in λMOSElox (Amersham Pharmacia) and screened with a rat probe corresponding to nucleotides 50–437 of KIAA0548, the partial sequence of the human attractin (ATRN) gene (11). To obtain the 5′ part of the cDNA and the 3′ part of the short form of the rat Atrn gene, rapid amplifications of cDNA ends were conducted with the use of Marathon-Ready cDNA (CLONTECH).
Determination of Exon–Intron Boundaries of the Atrn Gene.
A shotgun library was constructed from PAC 473M17 containing the entire rat Atrn gene and screened with the rat Atrn cDNA. Intron–exon boundaries were defined either by sequencing of the isolated clones or by direct sequencing of the PAC with the use of the Atrn cDNA-specific primers. Introns were amplified by PCR with LA-Taq DNA polymerase (Takara Shuzo, Kyoto, Japan), with the PAC as a template, and their lengths were assessed by electrophoresis. The exon–intron boundaries were verified by direct sequencing of the PCR products.
Genotyping of Agouti and Atrn.
Genotypes at loci agouti (a) and Atrn of the F2 animals were determined by an SSLP marker in intron 2 of rat agouti (5′-GGGAAAGACCATAGCTGCTA-3′ and 5′-AATAACAGCCTGGGAAAATG-3′) and by the marker detecting the 8-bp deletion of the zi-allele of Atrn (5′-GGAGTTGGCAACTGAAGAAC-3′ and 5′-GAAAGGGCAGAGACAAAGTT-3′).
Transgenic Rescue Experiment.
A 4.4-kb cDNA fragment containing the coding sequence for a membrane type of Atrn and a 3.9-kb cDNA fragment for a secreted type were subcloned into pBluescriptII (Stratagene). Wild-type Atrn transgenes were constructed with the use of the cDNA fragment, the mouse metallothionein (MMT) promoter, or the modified chicken β-actin (CAG) promoter with cytomegalovirus immediate early enhancer (12), and a ≈500-bp fragment containing the simian virus 40 poly(A) signal derived from pEVBHis (Invitrogen). The transgene was microinjected into the pronuclei of fertilized oocytes collected from Wistar rats, and the eggs subsequently were transferred to recipients. The (Founder rats × WTC.ZI-zi/zi)F1 (zi/+, tg/−) rats were backcrossed with WTC.ZI-zi/zi rats to obtain zi/zi homozygous rats carrying the transgene (zi/zi, tg/−). Transgenic rats were identified by PCR analysis of genomic DNA isolated from tail biopsies. The copies of the transgene on autoradiograph normalized relative to endogenous Atrn gene were quantified with computerized image display system BAS2000 (Fuji Film, Tokyo).
Histopathological and Electromyogram (EMG) Analysis of mg Mice.
Histopathological analysis of C3H/HeJ-mg3J/mg3J mice was carried out at 40 days of age. Light microscopic observations of brain and spinal cord (cervical enlargement) and electron microscopic observations of thalamus and midbrain were performed as described (13). EMG recordings were performed at 3 weeks of age. Intramuscular EMG electrodes were acutely implanted in the quadriceps and hamstring muscles of the right hind limbs. EMGs were recorded within a bandwidth of 100–1,500 Hz and analyzed with a Signal Averager (Cambridge Electronic Design, Cambridge, U.K.).
Results
Genetic and Physical Maps of the zi-Critical Region.
A total of 3,104 backcross and 474 intercross animals were produced, and their genotype at the zi locus was determined by examining the presence of tremor. Thirty-five backcross and 12 intercross animals carried a recombinant chromosome between Il1b and Prnp. Genetic linkage study of these informative animals, using the five gene-specific and seven anonymous markers developed in this study, narrowed down zi to a 0.1-cM interval between D3Kur7/Ptpra and D3Mgh15, which corresponded to human Chr 20p13 and mouse Chr 2(73.5) (Fig. 1a). The zi gene showed no recombination with D3Kur9/Oxt, D3Kur20, D3Kur24, D3Kur30, D3Kur34, D3Kur37, and D3Kur40 in 4,052 informative meioses.
A rat P1 library was initially screened with D3Kur7/Ptpra and D3Kur12/Oxt. After the subsequent chromosomal walking with the P1 library and a rat PAC library and aligning them by STS content mapping with the use of 57 markers developed in this study, a contig that comprised five cosmid, seven P1, and 21 PAC clones and covered D3Kur3-D3Kur7/Ptpra-D3Mgh15-D3Kur58 was constructed. Transcripts in the contig were identified by comparative mapping and end sequencing of the constituent large-insert clones. Taking advantage of the large conserved syntenic region between rat Chr 3 and human Chr 20p, 62 genes/ESTs known to be located on human chromosome 20pter-p12 were tested, and four known genes (Avp, Cenpb, Oxt, and Ptpra) and four ESTs were localized in the contig (Table 1). With a blast homology search using 56 end sequences, one gene, Rpl12, and eight ESTs were found in the contig. Because an EST (TKest30) was commonly identified by the comparative mapping and the end sequencing, a total of 16 genes/ESTs were mapped in the contig (Fig. 1b and Table 1). Among the 16 genes/ESTs identified in the contig, 10 were localized in the zi-critical region.
Table 1.
Genes/ESTs mapped to the contig constructed in this study
| Locus symbol* | STS maker | Method† | Origin | UniGene | Description |
|---|---|---|---|---|---|
| Ptpra | D3Kur7 | C | STS-WI-8798 | Hs.26045 | Protein-tyrosine phosphatase, receptor-type, alpha (OMIM#176884) |
| TKest41 | D3Kur10 | E | BAC179P17-M13F‡ | Mm.30057 | ESTs |
| Oxt | D3Kur12 | C | Hs.113216 | Oxytocin (OMIM#167050) | |
| Avp | D3Kur13 | C | Hs.89648 | Arginine vasopressin (OMIM#192340) | |
| TKest30 | D3Kur15 | C and E | STS-stSG4244 and P1-17819-T7 | Hs.26009 | KIAA0860 protein, complete cds |
| TKest03 | D3Kur17 | C | STS-A005R07 | Hs.90232 | KIAA0552 protein, complete cds |
| TKest63 | D3Kur23 | E | BAC173P11-M13F‡ | Mm.21399 | ESTs, Highly similar to HAM1 protein (Saccharomyces cerevisiae) |
| TKest01 | D3Kur26 | C | STS-stSG3058 | Rn.6943 | ESTs |
| TKest65 | D3Kur28 | E | Cosmid-TK71-M13F | Mm.35506 | ESTs |
| TKest69 | D3Kur48 | E | PAC241D8-SP6 | Rn.22428 | ESTs |
| TKest13 | D3Kur51 | C | STS-A005O05 | Hs.194019 | KIAA0548 protein, partial cds |
| TKest66 | D3Kur54 | E | PAC473M17-SP6 | Not assigned | EST from normalized rat spleen, cDNA clone RSPBR15 (AI013648) |
| Rpl12 | D3Kur55 | E | PAC267A15-T7 | Hs.182979 | Ribosomal protein L12 (OMIM#180475) |
| TKest67 | D3Kur56 | E | PAC102M17-T7 | Rn.39027 | ESTs |
| Cenpb | D3Kur57 | C | STS-Bdyc4e10 | Hs.85004 | Centromeric protein B (OMIM#117140) |
| TKest68 | D3Kur58 | E | PAC488M17-SP6 | Rn.41637 | EST |
Ordered according to map position from proximal (top) to distal (bottom).
C, comparative mapping; E, end sequence and BLAST homology search.
Derived from a bacterial artificial chromosome contig spanning the mouse syntenic region of the zi locus (T.K., unpublished result).
Reduction of Atrn Expression in Zitter Rats.
Expression levels of the 10 genes/ESTs in the zi-critical region were analyzed by Northern blot analysis. It was revealed that the expression of an EST, TKest13, was markedly reduced in WTC.ZI-zi/zi rats, although faint multiple bands of 8–10 kb were observed (Fig. 2a). The other nine genes/ESTs showed the same levels of expression in WTC and WTC.ZI-zi/zi rats, and no nucleotide differences resulted in amino acid changes specific to zi/zi rats. By a database search, TKest13 proved to be the KIAA0548, which is a part of the membrane type of the human ATRN gene.
Figure 2.
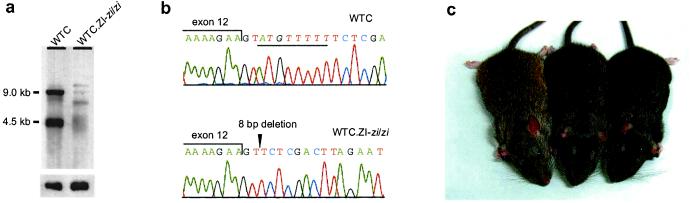
Zi mutation at rat Atrn gene. (a) Northern blot analysis of Atrn in the brains of WTC and WTC.ZI-zi/zi rats. A marked reduction of Atrn expression was observed in zi/zi rats. Hybridization signals of β-actin on the same blot are shown below. (b) Sequencing analysis of the splice donor site of the Atrn intron 12 in WTC (Upper) and WTC.ZI-zi/zi (Lower). An 8-bp deletion was identified in zi/zi rats (underlined in the Upper and shown with an arrowhead in the Lower) and was expected to result in an abnormal splicing. (c) Effect of the zi allele on coat color in rats. The agouti rat homozygous for the zi allele of Atrn (center) was more darkly pigmented than the agouti rat without the zi allele (Left) but less pigmented than nonagouti rat homozygous for the zi alleles (Right).
Isoforms and Exon–Intron Organization of the Rat Atrn Gene.
In the brain of WTC rats, two kinds of transcripts with sizes of 9.0 kb and 4.5 kb were observed. We cloned the two transcripts and determined their nucleotide sequences (GenBank accession numbers AB038387 and AB038388). We also determined the exon–intron boundaries and the lengths of introns of the rat Atrn gene. It spanned over 120 kb and contained at least 29 exons (Fig. 3a). The 4.5-kb transcript was found to be generated by an alternative splicing, skipping a splice donor site in intron 24, and using a stop codon and a polyadenylation signal in the intron (Fig. 3b).
Figure 3.
Rat Atrn gene and its transcripts. (a) Exon–intron organization of the rat Atrn gene. The gene comprises at least 29 exons and 28 introns, which are represented as boxes and lines. The length of intron 4 could not be determined. (b) Alternative splicing at exon 24 of the rat Atrn gene: genome organization around intron 24 (Top). The secreted-type Atrn transcript used the stop codon (TAA) and the poly(A) addition signal located within intron 24 (Middle). The membrane-type Atrn transcript was spliced at the potential donor site located 15 bp upstream from the stop codon of the secreted-type Atrn (Bottom). (c) Atrn transcripts for the secreted type (Upper) and membrane type (Lower). Five epidermal growth factor-related domains (green), a CUB domain (yellow), and c-type lectin (blue) are common in the two isoforms, but a transmembrane domain (red) is found only in the membrane type.
The 9.0-kb transcript was deduced to encode a 1,432-aa polypeptide, which corresponded to the membrane-type human ATRN locus product. Rat membrane type showed 93% and 97% homology with human and mouse Atrn product, respectively. The 4.5-kb transcript was deduced to encode a 1,276-aa polypeptide, which shared 1,271 N-terminal amino acid residues with the product of the 9.0-kb transcript, had five unique C-terminal residues, and did not have the transmembrane domain. It corresponded to the secreted type of the human ATRN locus product, although this secreted type is not observed in mice. Both polypeptides were predicted to contain four epidermal growth factor domains, a CUB domain, and C-type lectin domain (Fig. 3c).
A Splice Site Mutation in Atrn in Zitter Rats.
The entire coding region and all of the exon-intron boundaries of Atrn were sequenced in WTC.ZI-zi/zi rats. An 8-bp deletion at the splice donor site of intron 12 was identified (Fig. 2b), which was expected to result in aberrant and unstable transcripts. There were no other nucleotide differences between WTC and WTC.ZI-zi/zi rats.
Effect of the zi Mutation on Coat Color.
To examine the effect of the zi-allele of Atrn on coat color (see Discussion), the mutant allele was brought into a nonalbino background by producing F2 intercross rats from ACI/N (A/A, +/+) and WTC.ZI-zi/zi (a/a, zi/zi, albino). Ten genotyped nonalbino agouti rats homozygous for the zi allele of Atrn were recovered from the 115 F2 rats, and all of them showed a darker coat color than the agouti rats without the zi allele (Fig. 2c).
Transgenic Rescue.
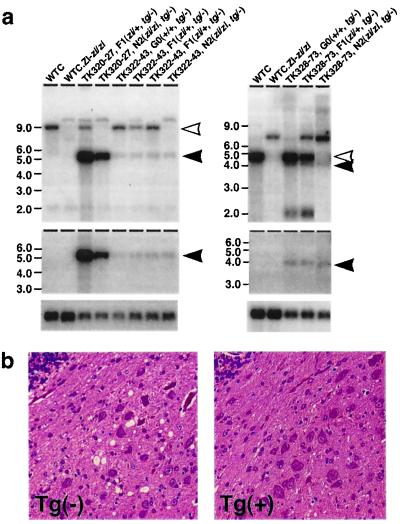
To confirm that Atrn is zi itself, and to clarify which transcript is responsible for the neuropathological phenotypes in zitter rats, we carried out transgenic rescue experiments. The wild-type transcript for the membrane type was placed under the control of a CAG promoter or MMT promoter (CAG + M, MMT + M), and the secreted type was placed under the control of MMT promoter (MMT + S). Six independent transgenic lines, two lines for each construct, were established (Table 2). Southern blot analysis demonstrated that five of the six lines carried a single copy of the transgene, and one line carried approximately 100 copies. Northern blot analysis with a probe specific for the membrane-type transcript detected 9.0-kb endogenous and 4.9-kb transgene-specific transcripts in the brain of CAG + M and MMT + M lines (Fig. 4a). A probe specific for the secreted-type transcript detected 4.5-kb endogenous and 4.4-kb transgene-specific transcripts in the brain of CAG + S lines (Fig. 4a).
Table 2.
Phenotypes of zi/zi rats in Atrn-transgenic lines
| Line | Transgene
|
Number of N2 animals examined | Phenotypes
|
|||
|---|---|---|---|---|---|---|
| Name | Number of copy | Symptoms | CNS pathology | Coat color | ||
| TK320-14 | CAG+M | ∼1 | 6 | Normal | Normal | Agouti |
| TK320-27 | CAG+M | ∼1 | 10 | Normal | Normal | Agouti |
| TK322-43 | MMT+M | ∼1 | 8 | Normal | Normal | Agouti |
| TK322-48 | MMT+M | ∼1 | 8 | Normal | Normal | Agouti |
| TK328-73 | MMT+S | ∼100 | 5 | Tremor, paresis | Vacuolation, hypomyelination | Mahogany |
| TK328-76 | MMT+S | ∼1 | 5* | Tremor, paresis | Vacuolation, hypomyelination | Mahogany |
N3 animals were included.
Figure 4.
Transgenic rescue from zitter neuropathological phenotypes by introduction of the wild-type Atrn gene. (a) Expression of transgene assessed by Northern blot analysis. Poly(A) RNAs from WTC, WTC.ZI-zi/zi, TK320-27, TK322-43, and TK328-73 are probed with membrane-type Atrn (Top, Left) and secreted-type Atrn (Top, Right). The membrane-type Atrn-specific probe detected 9.0-kb endogenous (open arrowhead) and 4.9-kb transgene (closed arrowhead) transcripts (Left). The secreted-type Atrn-specific probe detected 4.5-kb endogenous (open arrowhead) and 4.4-kb transgene (closed arrowhead) transcripts (Right). A DNA fragment containing simian virus 40 poly(A) additional signal only detected the transgene-specific transcripts indicated by closed arrowheads (Middle). Hybridization signals of β-actin on the same blot are shown at the bottom. (b) Sagittal section of cerebellum nuclei of zi/zi rats from a TK320–27 transgenic line with the transgene (Right) or without the transgene (Left). Note that the Tg(−) cerebellum exhibits vacuolation, whereas the Tg(+) cerebellum is restored.
All of the 38 transgenic zi/zi rats in N2 progeny of TK320-14, TK320-27, TK322-43, and TK322-48 lines were completely rescued from body tremor, aberrant myelination, and vacuolations in the CNS (Fig. 4b). They showed no flaccid paresis at 6 months of age. On the other hand, TK328-73 and TK328-76 lines failed to be rescued from tremor, hypomyelination, or vacuolation of the CNS. Thus, the zitter neuropathological phenotypes were complemented in all lines expressing the membrane-type transgene but not the secreted-type transgene (Table 2). Furthermore, the membrane type also rescued darkly pigmented coat color phenotypes but not the secreted type (Table 2).
Neurological Alterations in mg Mice.
Light microscopic observation of the CNS of mg3J/mg3J mice revealed vacuole formation that was widely distributed in the brainstem, cerebral cortex, cerebellum, and spinal cord. The vacuoles were well delineated and circular, and their sizes were variable, some reaching up to 10–20 μm in diameter. In Epok 812 sections stained with toluidine blue, aberrant myelin sheaths and vacuoles surrounded by myelin were identified (Fig. 5a). Electron microscopic observation revealed that some vacuoles contained vacuolar membranous structures, which were considered to be degenerate organelles. Such vacuoles were regarded as swollen processes of neurons or glial cells. In addition, elongated redundant myelin sheaths and scroll-like structures were identified among the myelinated axons (Fig. 5b). The distribution and morphology of vacuoles and aberrant myelin sheaths are very similar to those observed in the zitter rats (4–6).
Figure 5.
Neurological abnormalities of C3H/HeJ-mg3J/mg3J mice. (a) An Epok 812 semithin section of the mesencephalic tegmentum. Vacuoles were formed in neuropil. They appeared to be empty, and some of them were surrounded by myelin (arrow) (toluidine blue staining, ×600). (b) An electron micrograph of the thalamus. Aberrant myelin sheaths, such as scroll-like structures (white arrows), were evident among the myelinated axons (×4,500). (c) EMGs of C3H/HeJ-mg3J/mg3J mice. The horizontal bar over the EMGs indicates a tremor pattern: nearly reciprocal activation of the quadriceps and hamstring muscles at a fairly constant rate. Background irregularities presumably exhibit motor units that fired near their threshold.
Examination of mg3J/mg3J mice allowed us to detect slight body tremor after weaning that occurred sporadically at rest and, more often, a few seconds before locomotion. The tremor was confirmed by EMGs showing strong and reciprocal bursts of activity in both the quadriceps and the hamstring muscles at a fairly constant rate (Fig. 5c). The tremor frequency was approximately 17 Hz.
Discussion
In this study, we found a marked reduction of secreted- and membrane-type Atrn transcripts in the brain of the zitter rat. The 8-bp deletion we found at the splice donor site of intron 12 of Atrn was expected to result in unstable transcripts, and the deletion was considered to be the causative mutation for the abnormalities observed in the zitter rat. Transgenic study demonstrated that only the membrane-type transgene can rescue the neurological abnormalities and coat color of the zitter rat. We also demonstrated that the mg3J/mg3J mice, which carried a 5-bp deletion in the coding sequence of Atrn, showed body tremor and histopathological abnormalities similar to those of the zitter rat. These results conclude that the membrane-type Atrn locus product, but not the secreted type, is responsible for the zitter mutant phenotypes observed in this study, including hypomyelination and vacuolation in the CNS and hair pigmentation.
Functions of the Atrn locus products have been investigated from immunological studies in human and analysis of the mouse coat color mutant mahogany (mg). The secreted-type ATRN locus product Attractin (ATRN) is a glycosylated serum protein and is known as a mediator of monocyte spreading and T cell clustering (14). Recently, the presence of membrane-type ATRN has been demonstrated in humans (15). In mice, only membrane-type ATRN has been observed so far, and its loss-of-function mutations are known to be the cause of mg mutants. The mg mutant mice display darkened coat color that is agouti-dependent and increased basal metabolic rate that is agouti-independent (16–18). Both the secreted-type and membrane-type ATRN contain two epidermal growth factor domains, a CUB domain, a C-type lectin domain, and two laminin-like epidermal growth factor domains (14, 17, 19). The membrane-type ATRN is thought to have these functional domains in its extracellular portion.
This study demonstrated that ATRN has a novel function in myelination, in addition to those functions in the immune response, hair pigmentation, and energy metabolism. Overlap of the CNS regions showing high Atrn expression with those showing severe hypomyelination and vacuolation also supports our conclusion (20). The myelination requires the coordinated synthesizing of various structural proteins and enzymes, the initial axon–glia interaction, the assembly process of the myelin sheath, and the long-term interaction of axons with myelinating glia (21). Extracellular functional domains in the membrane-type ATRN indicate that it is involved in cell adhesion and receptor–ligand interactions (22–24). These strongly suggest that membrane-type ATRN is anchored on the surface of neurons or glial cells and mediates the myelination signal through its extracellular domains.
Although the role of the membrane-type ATRN in energy metabolism has been known in mice (16), it would be difficult to evaluate its roles in rats, because basal metabolic rate cannot be measured precisely because of the presence of conspicuous tremor in zitter rats. Conversely, mg3J/mg3J mice exhibited slight tremor and hypomyelination and vacuolation in their CNS. Because even minimal increases in muscle tone significantly increase basal metabolic rate, an increase of basal metabolic rate in the mg mutant mice could be partially due to the neurological abnormalities. Although it is proposed that inhibitors of the membrane-type ATRN would be attractive candidates for drugs to prevent or reverse the most common forms of human obesity (25–27), the critical role of ATRN in myelination disclosed in this study raises great concern about this idea.
As for positional cloning in this study, the comparative mapping and end sequencing complemented each other and efficiently identified the transcription units in the zi-critical region. The potential of the comparative mapping approach will be appreciably expanded when the entire sequence of the human genome is determined (9). Therefore, we expect that the approach described here would be a prototype for identifying transcripts in the rat contig.
Acknowledgments
We are grateful to Dr. T. Sugimura for critical reading of the manuscript. We also thank M. Sugiyama, T. Nomoto, C. Okumura, S. Nakanishi, and K. Yamasaki for their technical assistance. This work was supported by a grant-in-aid for the second-term Cancer Control Strategy from the Ministry of Health and Welfare; a grant-in-aid from the Ministry of Education, Science, Sports, and Culture; a grant for Research on Health Sciences focusing on Drug Innovation from the Japan Health Sciences Foundation; and a grant from the Sankyo Foundation of Life Science.
Abbreviations
- Atrn
Attractin
- zi
zitter
- mg
mahogany
- CNS
central nervous system
- Chr
chromosome
- EST
expressed sequence tag
- EMG
electromyogram
- Prnp
prion protein gene
- SSLP
simple sequence length polymorphism
- PAC
P1-derived artificial chromosome
- STS
sequence tagged site
- MMT
mouse metallothionein
- CAG
chicken β-actin
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Data deposition: The rat Atrn cDNA and exon sequences reported in this paper have been deposited in the GenBank database (accession nos. AB038387, AB038388, and AB049222–AB049248). The oligonucleotide sequences for the STS markers developed in this study are available at http://www.ncc.go.jp/research/rat-genome.
References
- 1.Rehm S, Mehraein P, Anzil A P, Deerberg F. Lab Anim Sci. 1982;32:70–73. [PubMed] [Google Scholar]
- 2.Yamada T, Mori M, Hamada S, Serikawa T, Yamada J. J Hered. 1989;80:383–386. doi: 10.1093/oxfordjournals.jhered.a110879. [DOI] [PubMed] [Google Scholar]
- 3.Kuramoto T, Yamasaki K, Kondo A, Nakajima K, Yamada M, Serikawa T. Exp Anim. 1998;47:75–81. doi: 10.1538/expanim.47.75. [DOI] [PubMed] [Google Scholar]
- 4.Kondo A, Nagara H, Akazawa K, Tateishi J, Serikawa T, Yamada J. Brain. 1991;114:979–999. doi: 10.1093/brain/114.2.979. [DOI] [PubMed] [Google Scholar]
- 5.Kondo A, Sendoh S, Miyata K, Takamatsu J. J Neurocytol. 1995;24:533–544. doi: 10.1007/BF01179978. [DOI] [PubMed] [Google Scholar]
- 6.Kondo A, Sendoh S, Akazawa K, Sato Y, Nagara H. Brain Res Dev Brain Res. 1992;67:217–228. doi: 10.1016/0165-3806(92)90222-i. [DOI] [PubMed] [Google Scholar]
- 7.Kuramoto T, Mori M, Yamada J, Serikawa T. Biochem Biophys Res Commun. 1994;200:1161–1168. doi: 10.1006/bbrc.1994.1573. [DOI] [PubMed] [Google Scholar]
- 8.Gomi H, Ikeda T, Kunieda T, Itohara S, Prusiner S B, Yamanouchi K. Neurosci Lett. 1994;166:171–174. doi: 10.1016/0304-3940(94)90478-2. [DOI] [PubMed] [Google Scholar]
- 9.Serikawa T, Cui Z, Yokoi N, Kuramoto T, Kondo Y, Kitada K, Guenet J L. Exp Anim. 1998;47:1–9. doi: 10.1538/expanim.47.1. [DOI] [PubMed] [Google Scholar]
- 10.Kuramoto T, Mori M, Hirayama N, Saburi S, Yamada J, Serikawa T. Acta Histochem Cytochem. 1993;26:325–332. [Google Scholar]
- 11.Nagase T, Ishikawa K, Miyajima N, Tanaka A, Kotani H, Nomura N, Ohara O. DNA Res. 1998;5:31–39. doi: 10.1093/dnares/5.1.31. [DOI] [PubMed] [Google Scholar]
- 12.Niwa H, Yamamura K, Miyazaki J. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 13.Inui T, Yamamura T, Yuasa H, Kawai Y, Okaniwa A, Serikawa T, Yamada J. Brain Res. 1990;517:123–133. doi: 10.1016/0006-8993(90)91017-b. [DOI] [PubMed] [Google Scholar]
- 14.Duke-Cohan J S, Gu J, McLaughlin D F, Xu Y, Freeman G J, Schlossman S F. Proc Natl Acad Sci USA. 1998;95:11336–11341. doi: 10.1073/pnas.95.19.11336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duke-Cohan J S, Tang W, Schlossman S F. Adv Exp Med Biol. 2000;477:173–185. doi: 10.1007/0-306-46826-3_20. [DOI] [PubMed] [Google Scholar]
- 16.Dinulescu D M, Fan W, Boston B A, McCall K, Lamoreux M L, Moore K J, Montagno J, Cone R D. Proc Natl Acad Sci USA. 1998;95:12707–12712. doi: 10.1073/pnas.95.21.12707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagle D L, McGrail S H, Vitale J, Woolf E A, Dussault B J, Jr, DiRocco L, Holmgren L, Montagno J, Bork P, Huszar D, et al. Nature (London) 1999;398:148–152. doi: 10.1038/18210. [DOI] [PubMed] [Google Scholar]
- 18.Miller K A, Gunn T M, Carrasquillo M M, Lamoreux M L, Galbraith D B, Barsh G S. Genetics. 1997;146:1407–1415. doi: 10.1093/genetics/146.4.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gunn T M, Miller K A, He L, Hyman R W, Davis R W, Azarani A, Schlossman S F, Duke-Cohan J S, Barsh G S. Nature (London) 1999;398:152–156. doi: 10.1038/18217. [DOI] [PubMed] [Google Scholar]
- 20.Lu X, Gunn T M, Shieh K, Barsh G S, Akil H, Watson S J. FEBS Lett. 1999;462:101–107. doi: 10.1016/s0014-5793(99)01494-5. [DOI] [PubMed] [Google Scholar]
- 21.Kettenmann H, Ransom B R. Neuroglia. New York: Oxford Univ. Press; 1995. [Google Scholar]
- 22.Bork P, Beckmann G. J Mol Biol. 1993;231:539–545. doi: 10.1006/jmbi.1993.1305. [DOI] [PubMed] [Google Scholar]
- 23.Appella E, Weber I T, Blasi F. FEBS Lett. 1988;231:1–4. doi: 10.1016/0014-5793(88)80690-2. [DOI] [PubMed] [Google Scholar]
- 24.Weis W I, Taylor M E, Drickamer K. Immunol Rev. 1998;163:19–34. doi: 10.1111/j.1600-065x.1998.tb01185.x. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz M W. Nat Med. 1999;5:374–375. doi: 10.1038/7365. [DOI] [PubMed] [Google Scholar]
- 26.Jackson I J. Trends Genet. 1999;15:429–431. doi: 10.1016/s0168-9525(99)01824-7. [DOI] [PubMed] [Google Scholar]
- 27.Dinulescu D M, Cone R D. J Biol Chem. 2000;275:6695–6698. doi: 10.1074/jbc.275.10.6695. [DOI] [PubMed] [Google Scholar]