Abstract
The mechanisms of mitochondrial proton (H+) leak under various pathophysiological conditions are poorly understood. In the present study it was hypothesized that different mechanisms underlie H+ leak in cardiac IR (ischaemia/reperfusion) injury and IPC (ischaemic preconditioning). Potential H+ leak mechanisms examined were UCPs (uncoupling proteins), allosteric activation of the ANT (adenine nucleotide translocase) by AMP, or the PT (permeability transition) pore. Mitochondria isolated from perfused rat hearts that were subjected to IPC exhibited a greater H+ leak than did controls (202±27%, P<0.005), and this increased leakage was completely abolished by the UCP inhibitor, GDP, or the ANT inhibitor, CAT (carboxyattractyloside). Mitochondria from hearts subjected to IR injury exhibited a much greater amount of H+ leak than did controls (411±28%, P<0.001). The increased leakage after IR was weakly inhibited by GDP, but was inhibited, >50%, by carboxyattractyloside. In addition, it was inhibited by cardioprotective treatment strategies including pre-IR perfusion with the PT pore inhibitors cyclosporin A or sanglifehrin A, the adenylate kinase inhibitor, AP5A (diadenosine pentaphosphate), or IPC. Together these data suggest that the small increase in H+ leak in IPC is mediated by UCPs, while the large increase in H+ leak in IR is mediated by the ANT. Furthermore, under all conditions studied, in situ myocardial O2 efficiency was correlated with isolated mitochondrial H+ leak (r2=0.71). In conclusion, these data suggest that the modulation of H+ leak may have important implications for the outcome of IR injury.
Keywords: adenine nucleotide translocase (ANT), H+ leak, permeability transition (PT), ischaemic preconditioning (IPC), sanglifehrin A (SfA), uncoupling proteins (UCPs)
Abbreviations: Δψm, transmembrane electrochemical gradient; AP5A, diadenosine pentaphosphate; CAT, carboxyattractyloside; CsA, cyclosporin A; IR, ischaemia/reperfusion; IPC, ischaemic preconditioning; UCPs, uncoupling proteins; ANT, adenine nucleotide translocase; MVO2, myocardial O2 consumption; PT, permeability transition; ROS, reactive oxygen species; RPP, rate pressure product; SfA, sanglifehrin A; TPMP+, triphenylmethylphosphonium
INTRODUCTION
Mitochondrial proton (H+) leak is a basal or induced permeability of the mitochondrial inner membrane, resulting in partial dissipation of the Δψm (transmembrane electrochemical gradient) and uncoupling of substrate oxidation from ATP synthesis [1,2]. Since mitochondrial-derived ATP is essential for cellular functions, such uncoupling is often regarded as being detrimental. However, recent evidence has suggested that a cytoprotective role exists for low level H+ leak [3–10], possibly because mild Δψm dissipation appears to diminish ROS (reactive oxygen species) generation by the organelle, and may also prevent mitochondrial Ca2+ overload [2,11,12].
The potential mechanisms of mitochondrial H+ leakage fall into four partially overlapping groups: (i) leak via the novel UCPs (uncoupling proteins) [1,13], (ii) allosteric stimulation of a H+ leakage function in the ANT (adenine nucleotide translocase) by AMP [14], (iii) transmembrane cycling of protonated/unprotonated non-esterified fatty acids [15,16], and (iv) a H+ sub-conductance state of the mitochondrial PT (permeability transition) pore [17–19]. Several of these mechanisms may be activated by mitochondrial events during cardiac IR (ischaemia/reperfusion) injury or IPC (ischaemic preconditioning), including the generation of ROS, PT pore opening, or AMP generation [20,21]. However, the role of H+ leak as an injurious or protective phenomenon in IR injury and IPC is poorly understood.
An increased H+ leak has been reported after cardiac ischaemia alone, with non-esterified fatty acid cycling as the proposed mechanism [22]. However, most of the damage in IR injury occurs during reperfusion, and thus a more extensive analysis is required. In addition, while it has been shown that IPC can decrease Δψm [3], or that IPC and IR can increase the rate of state 4 respiration [23,24], such changes can occur due to the altered activity of the respiratory chain, and thus the measurement of Δψm or respiration alone is insufficient to determine H+ leak. Strictly defined, H+ leak is the state 4 respiration rate required to maintain a given Δψm, and the only genuine method to determine H+ leak between experimental groups is to simultaneously measure both respiration and Δψm [1,2].
In the present investigation, a comprehensive study of mitochondrial H+ leak in IR injury and IPC was undertaken hypothesizing that the mechanism of H+ leak in these two conditions are different. Overall, a small reversible elevation of H+ leak was found in IPC, mediated by UCPs, and a large irreversible elevation of H+ leak was found in IR injury, mediated by the ANT.
MATERIALS AND METHODS
Male Sprague–Dawley rats (approx. 200 g) were from Harlan (Indianapolis, IN, U.S.A.). All procedures were in accordance with the National Institutes of Health, Guide for the Care and Use of Laboratory Animals. SfA (sanglifehrin A) was a gift from Novartis (Basel, Switzerland). All other chemicals were from Sigma (St. Louis MO, U.S.A.) unless otherwise stated.
Perfused rat hearts
Isolated rat hearts were retrograde (Langendorff) perfused with Krebs–Henseleit buffer containing, in mM: NaCl (118), KCl (4.7), MgSO4 (1.2), NaHCO3 (24), KH2PO4 (1.2), CaCl2 (2.5), D-glucose (11). A physiological pH of 7.4 was maintained with 95% O2 and 5% CO2 at 37 °C. Perfusion was carried out in constant-flow mode, at 12 ml/min per g of wet weight [25]. The venous outflow was cannulated for measurement of [O2] with an in-line O2 electrode (Microelectrodes, Bedford, NH, U.S.A.). MVO2 (myocardial O2 consumption) that was determined by integrating upstream [O2] and downstream [O2] with perfusion flow rate. After 20 min of equilibration, hearts were subjected to one of the following seven protocols: (i) control, consisting of a further 45 min of normoxic perfusion, (ii) IPC alone, consisting of 3×5 min ischaemia, interspersed with 5 min of reperfusion, (iii) IR, consisting of 25 min of global ischaemia followed by 30 min of reperfusion, (iv) IPC followed by IR, (v) IR with perfusion with the adenylate kinase inhibitor AP5A (diadenosine pentaphosphate; 50 μM) for 10 min prior to ischaemia, (vi) IR with perfusion with the PT pore inhibitor CsA (cyclosporin A; 0.2 μM) for 10 min prior to ischaemia, (vii) IR with perfusion with the PT pore inhibitor SfA (1 μM) for 10 min prior to ischaemia. At the end of all protocols, except (vi), mitochondria were isolated and their H+ leak was measured.
Isolated mitochondrial measurements
Heart mitochondria were isolated and their protein concentrations determined as previously described [25,26]. Total mitochondrial yields for the control, IR and IPC groups were 15.6±0.9, 15.2±1.1, and 14.5±1.2 mg mitochondrial protein per g wet cardiac tissue respectively. Mitochondrial H+ leak was determined by simultaneous measurement of state 4 respiration (O2 consumption) and Δψm, in a chamber equipped with electrodes sensitive to O2 and TPMP+ (triphenylmethylphosphonium) essentially as previously described [27,28], but omitting nigericin from the medium to eliminate artifactual H+ leak secondary to K+ fluxes. Briefly, mitochondria (0.5 mg/ml) were suspended in buffer containing KCl (120 mM), KH2PO4 (3 mM), Tris (50 mM), rotenone (5 μM), oligomycin (1 μg/ml) and fat-free BSA (0.25%, w/v), at pH 7.35 and 37 °C. After calibrating the TPMP+ electrode, mitochondria were energized and state 4 respiration initiated by the addition of succinate (5 mM). Complex II activity was titrated with malonate (50 μM–5 mM), and at the end of each incubation FCCP (carbonyl cyanide p-trifluoromethoxyphenylhydrazone; 1 μM) was added to uncouple mitochondria and to correct for electrode drift. The calculation of Δψm was obtained from the Nernst equation using a TPMP+-binding correction factor of 0.4, and an intra-mitochondrial volume of 0.65 μl/mg of protein [29]. To determine the relative contribution from UCPs or ANT to H+ leak, measurements were performed in the presence of GDP (1 mM) or CAT (carboxyattractyloside, 5 μM) respectively.
Immunoprecipitation and Western blotting
UCPs were immunoprecipitated from 2 mg of freshly isolated mitochondrial protein, using protein-A/G agarose (Calbiochem, San Diego, CA, U.S.A.), and polyclonal antibodies (1:20 dilution) against UCP2 or UCP3 (ADI, San Antonio, TX, U.S.A.). Samples were resolved on an SDS-PAGE (15%) gel, transferred to nitrocellulose, and probed with anti-UCP2/UCP3 antibodies at a 1:500 dilution. A horse radish peroxidase-linked secondary antibody (GE Biosciences, Piscataway, NJ, U.S.A.) was used at a 1:2500 dilution, with enhanced chemiluminescence detection (Pierce, Rockford, IL, U.S.A.).
Statistics
All data are representative of 4–7 separate experiments in seven experimental groups. Statistical differences between groups were determined by ANOVA [30].
RESULTS
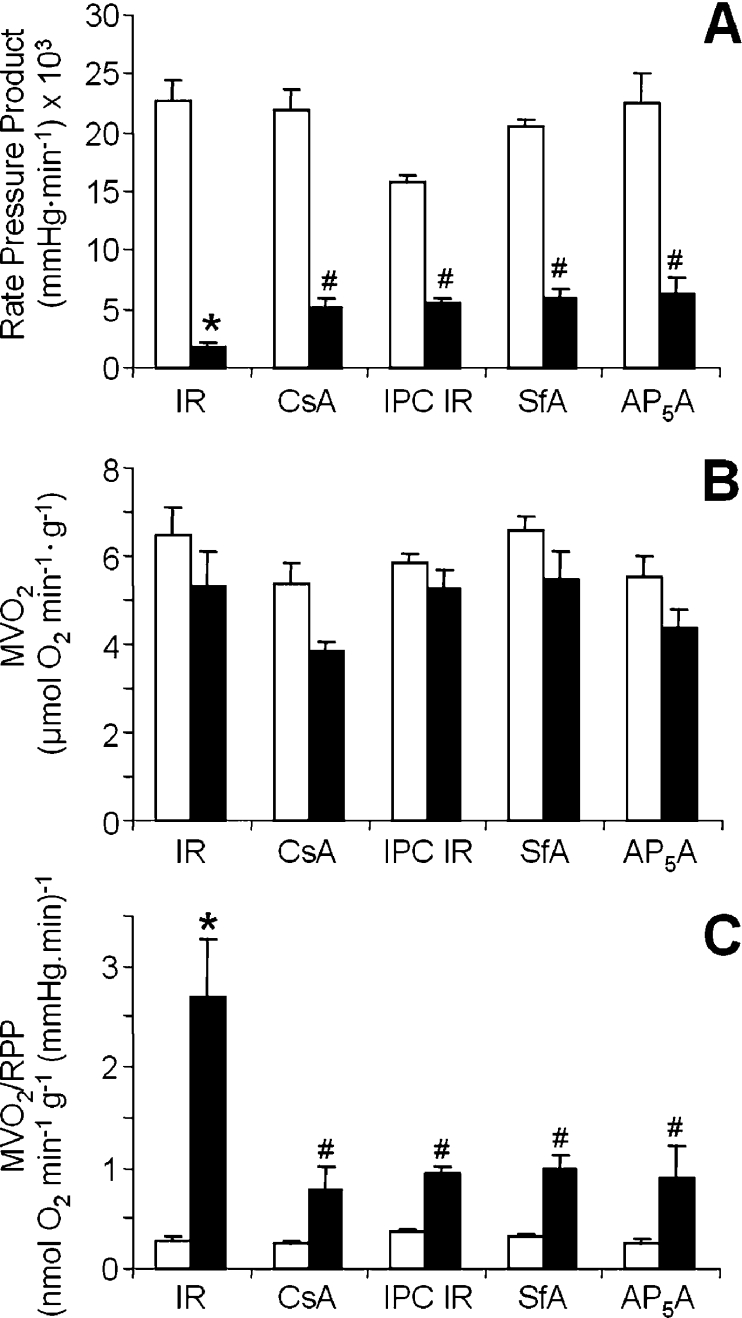
Rate pressure product (RPP) and O2 consumption data from the various heart perfusion protocols are shown in Figure 1. Severe left ventricular dysfunction occurred after IR injury, as shown by a post-IR RPP that was decreased to 8±2% of the pre-IR value (Figure 1A). Notably, pretreatment with CsA, IPC, SfA or AP5A significantly ameliorated this damage (post-IR recovery of RPP 23±3%, 35±3%, 29±5%, or 28±6% respectively). Figure 1(B) shows that MVO2 did not significantly change with any of the treatments. Thus expressing MVO2/RPP (Figure 1C) shows a large post-IR increase in the amount of O2 required for a given amount of cardiac functionality, and this increase was inhibited by the four cardioprotective treatments (CsA, IPC, SfA or AP5A). There are many potential causes of inefficiency between O2 consumption and contractile function that could occur in IR injury, including excitation-contraction coupling defects, ion cycling, ATP hydrolysis, adenine nucleotide loss or mitochondrial H+ leak. In the present study we chose to focus on mitochondrial H+ leak, since it is relatively unstudied in the context of IR injury and IPC.
Figure 1. Effects of IR on cardiac function.
(A) RPP calculated from heart rate (beats/min) and left ventricular developed pressure (systolic pressure−diastolic pressure). Data shown are: before the onset of ischaemia (open bars); after 30 min of reperfusion (filled bars). Experimental groups are: IR alone, IR+CsA, IR+IPC, IR+SfA and IR+AP5A (as described in the Methods and methods section). (B) MVO2 before and after IR. Experimental groups and labels are as shown in (A). (C) MVO2/RPP, as calculated from the data in (A) and (B). All data are the means±S.E.M. for 4–7 independent experiments, and were analysed for statistical differences by ANOVA. *, P<0.05 compared with pre-ischaemic values. #, P<0.05 compared with the IR-only group.
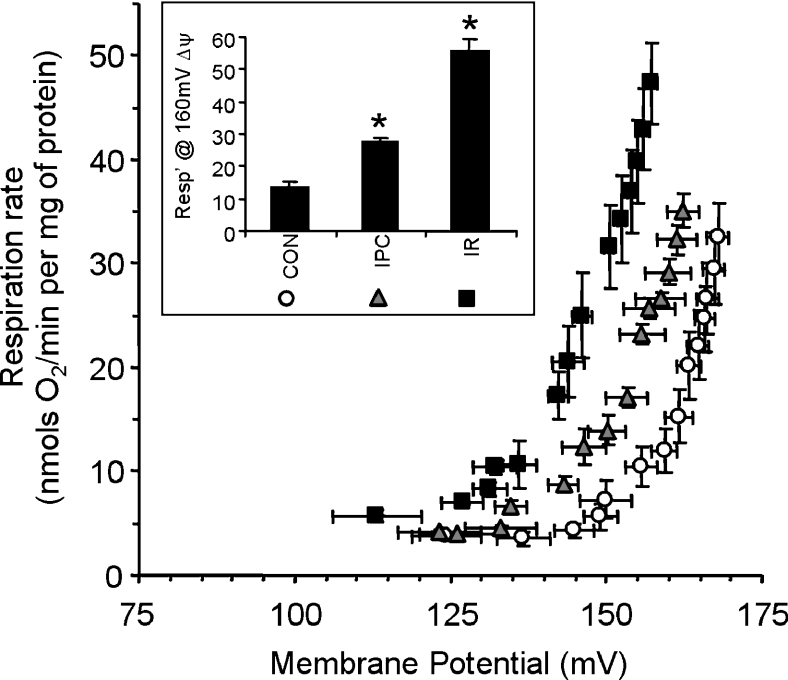
Figure 2 shows the H+ leak of mitochondria isolated from control, IPC- and IR-treated hearts. The curves appear to be similar to classical determinations of the rate of mitochondrial H+ leak in other models [1,22,27], with an upward/leftward shift representing increased leakage. To compare differences in H+ leak between experimental groups, the respiration rate required to maintain a common value of Δψm (160 mV) was calculated, and is shown in the panel inset. A large elevation in H+ leak was observed after IR (411±28% compared with control, P<0.001), with a smaller but nonetheless highly significant elevation after IPC alone (202±27% compared with control, P<0.005). Note that fatty-acid-free BSA was present in all mitochondrial incubations, and thus H+ leak was not mediated by non-esterified fatty acid cycling, as has been previously suggested [15,16,22].
Figure 2. H+ leak in control, IR- and IPC-mitochondria.
Measurement of the H+ leak was as described in the Materials and methods section in mitochondria isolated after a control perfusion (open circles), IPC (grey triangles) or IR (black squares). Inset: the respiration rate required to sustain a Δψm of 160 mV, extrapolated from individual H+ leak curves. For ease of comparison, matching symbols for the H+ leak curves are shown beneath each category. Data are the means±S.E.M. for at least six independent experiments. *, P<0.05 compared with control values (ANOVA).
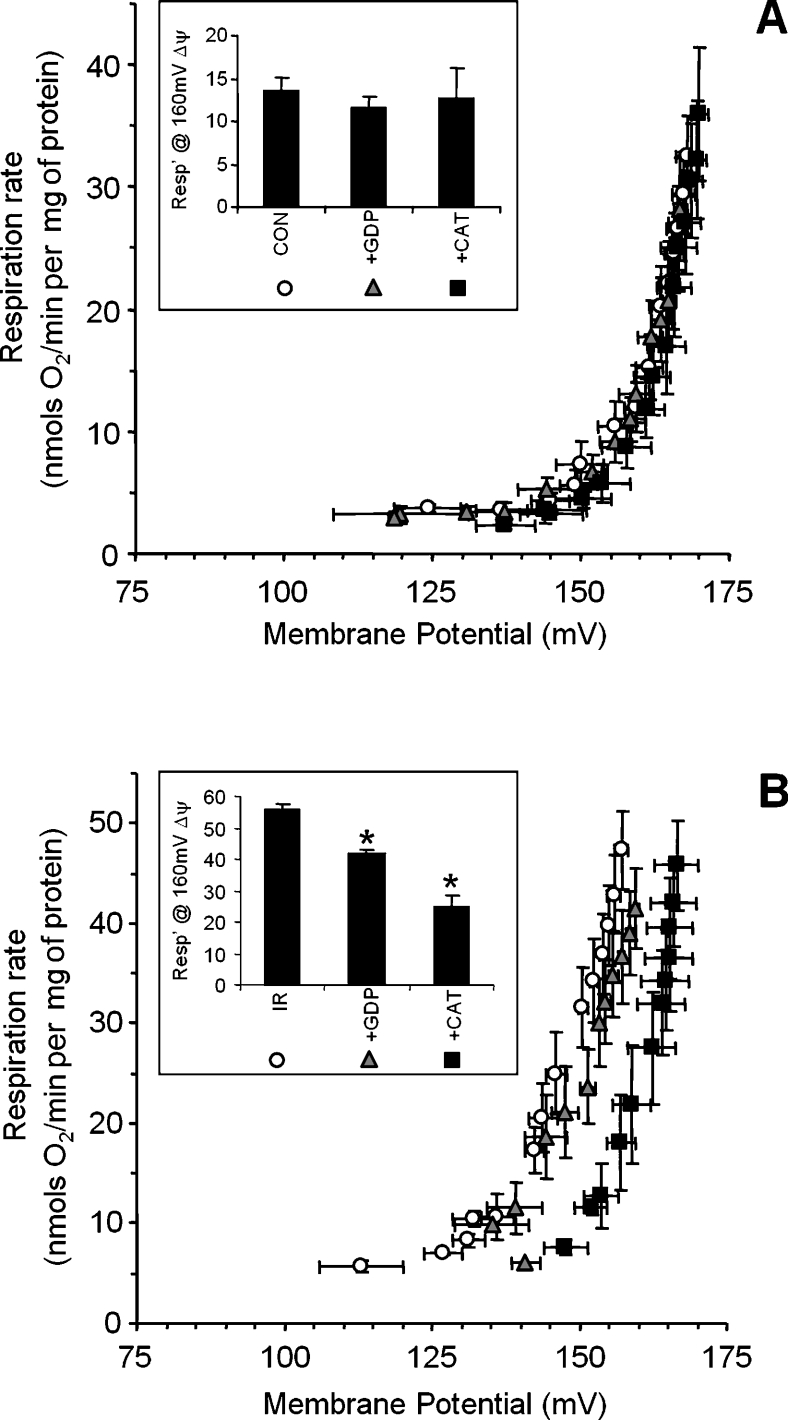
To determine the relative contribution of either UCPs or the ANT to H+ leak, measurements were performed in the presence of GDP or CAT respectively. Figure 3(A) shows that neither compound affected the H+ leak of control mitochondria, suggesting that baseline H+ leak is not mediated by UCPs or ANT stimulation, and in agreement with previous observations [1,2]. By contrast, Figure 3(B) shows that the increased H+ leak after IR injury was partially inhibited by GDP, suggesting a minor contribution from UCPs. Remarkably, the post-IR increase in H+ leak was inhibited approx. 60% by CAT, suggesting a major role for the ANT.
Figure 3. Effects of GDP or CAT on the H+ leak in control or IR-mitochondria.
The measurement of H+ leak was as described in the Materials and methods section, under normal conditions (open circles), in the presence of GDP (1 mM, grey triangles) or CAT (5 μM, black squares). (A) Controls, (B) IR-treated mitochondria. Insets: respiration at 160 mV, as in Figure 2. Data shown are the means±S.E.M. for at least five independent experiments. *, P<0.05 ccompared with regular H+ leak assay conditions (without GDP or CAT), as determined by ANOVA.
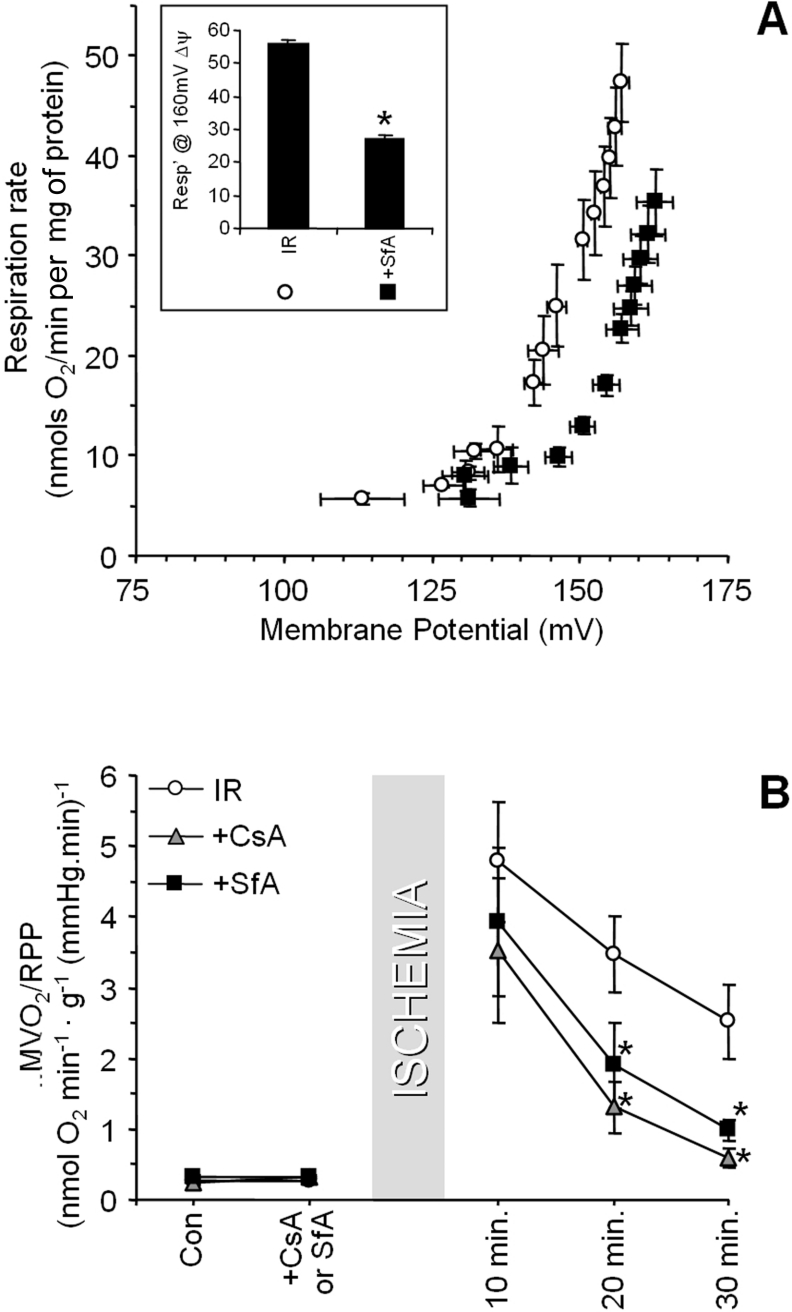
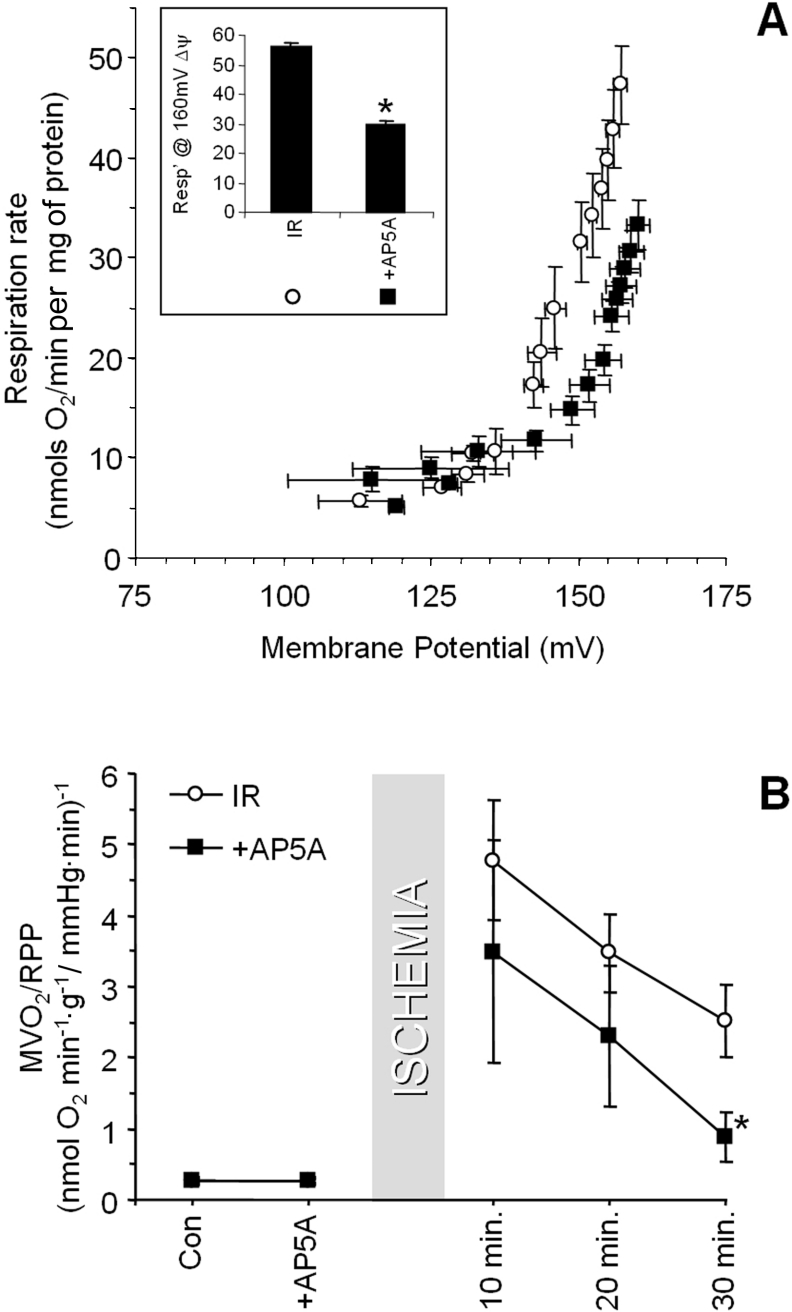
The ANT could mediate H+ leak by two distinct mechanisms, participation in the PT pore [17–19], or through its allosteric activation by AMP [14]. To investigate the former, hearts were perfused prior to IR with the PT pore inhibitors CsA or SfA [17,31,32]. Figure 4(A) shows that the H+ leak was lower in mitochondria isolated from SfA-treated IR hearts, compared with those from regular IR hearts. Figure 4(B) demonstrates that CsA or SfA also largely inhibited the post-IR increase in MVO2/RPP. Together, the data in Figure 4 indicate a potential role for PT pore opening in the increased H+ leak and decreased cardiac O2 efficiency after IR injury.
Figure 4. Effects of PT pore inhibitors on H+ leak and MVO2/RPP in IR injury.
Hearts were perfused with either SfA or CsA prior to ischaemia, as detailed in the Materials and methods section. (A) H+ leak in mitochondria isolated from hearts subjected to IR-only (open circles), or IR with SfA (black squares). Inset: respiration at 160 mV, as in Figure 2. (B) MVO2/RPP measured during IR protocols. Data shown are from the control (Con) condition (before CsA/SfA infusion), the period immediately prior to ischaemia (after CsA/SfA infusion), and for various reperfusion times. Open circles, regular IR protocol; grey triangles, IR with CsA; black squares, IR with SfA. Data are the means±S.E.M. for at least four independent experiments. *, P<0.05 compared with the regular IR group (ANOVA).
To investigate a role for allosteric ANT activation by AMP [14] in post-IR H+ leak, hearts were perfused with the adenylate kinase inhibitor, AP5A, prior to ischaemia, to prevent AMP formation. Figure 5(A) shows that the H+ leak in mitochondria isolated from AP5A-treated IR hearts is significantly lower that of mitochondria from regular IR hearts. Figure 5(B) shows that AP5A almost completely inhibited the post-IR increase in MVO2/RPP after 30 min of reperfusion. Together these data suggest that a significant proportion of the increased H+ leak in IR injury may also be mediated by an AMP-ANT allosteric effect. Overall, the data in Figures 4 and 5 suggest that an increased H+ leak after IR injury occurs via the ANT, with contributions from both PT pore opening and AMP allosteric activation, and a minimal role for UCPs.
Figure 5. Effects of AP5A on H+ leak and MVO2/RPP in IR injury.
Hearts were perfused with AP5A prior to ischaemia, as detailed in the Materials and methods section. (A) H+ leak in mitochondria isolated from hearts subjected to IR only, or IR with AP5A. Inset: respiration at 160 mV, as in Figure 2. (B) MVO2/RPP measured during IR protocols. Data shown are from the control (Con) condition (before AP5A infusion), the period immediately prior to ischaemia (after AP5A infusion), and various reperfusion times. In both panels, open circles represent regular IR data, and black squares represent IR with AP5A. All data are the means±S.E.M. for at least four independent experiments. *, P<0.05 compared with the regular IR group (ANOVA).
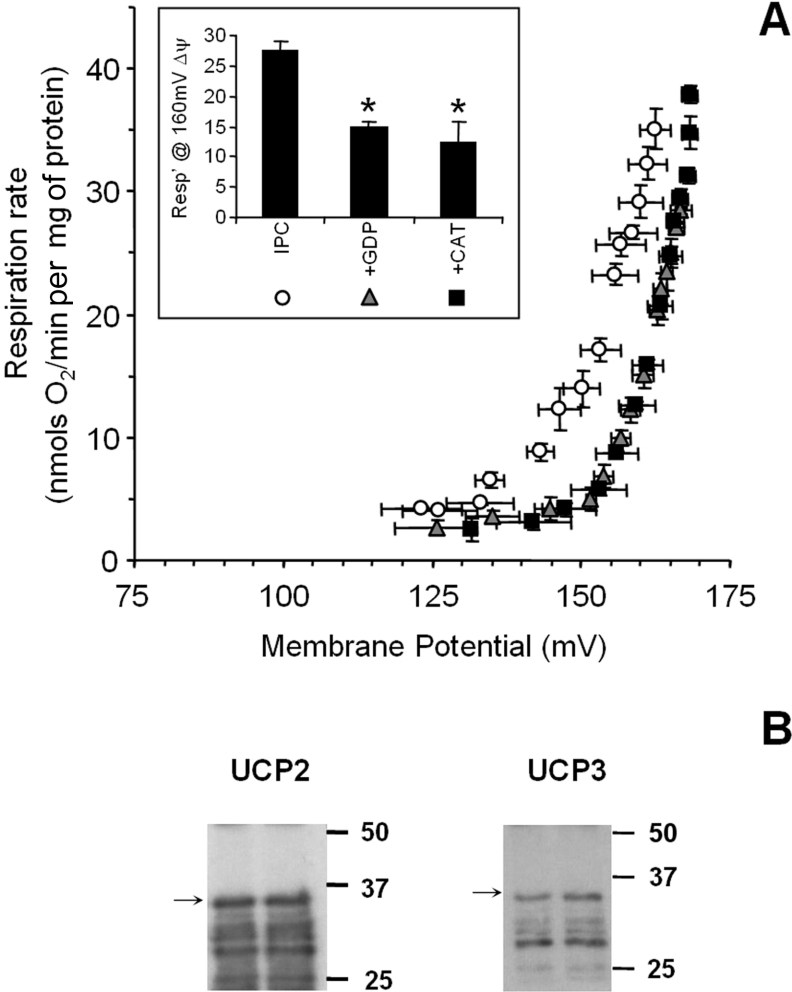
In addition to the large elevation in the H+ leak observed after IR injury, we next examined the mechanism of the small but significant increase in H+ leak after IPC (Figure 2). Figure 6(A) shows that the IPC-induced elevation in the H+ leak was completely inhibited by both the UCP inhibitor, GDP, and the ANT inhibitor, CAT. While these data suggest a role for UCPs in H+ leak, the existence of UCPs in the heart is still debated, owing to the reliance of many studies on mRNA evidence alone, and to discrepancies that exist between UCP mRNA and protein levels [2]. Indeed, preliminary Western blotting (results not shown) failed to detect UCPs in isolated rat heart mitochondria. Thus an immunoprecipitation approach was adopted, and the results, shown in Figure 6(B), confirm the existence of both UCP2 and UCP3 proteins in rat heart mitochondria. This is in agreement with a single report that these proteins are present in human heart mitochondria [33].
Figure 6. H+ leak in IPC-treated mitochondria, and UCP expression.
(A) H+ leak measurements were performed as described in the Materials and methods section, under regular conditions (without GDP or CAT; open circles), or in the presence of GDP (grey triangles), or CAT (black squares). Inset: respiration at 160 mV, as in Figure 2. Data are the means±S.E.M. for four independent experiments. *, P<0.05 compared with regular H+ leak assay conditions, determined by ANOVA. (B) Immunoprecipitation and Western blotting of UCP2 and UCP3 proteins was performed with freshly isolated control rat heart mitochondria as described in the Materials and methods section. Western blots are representative of at least three independent experiments. Molecular masses (kDa) are shown on the right of the blots. The arrows point to intact UCP, with lower bands assumed to be proteolytic fragments.
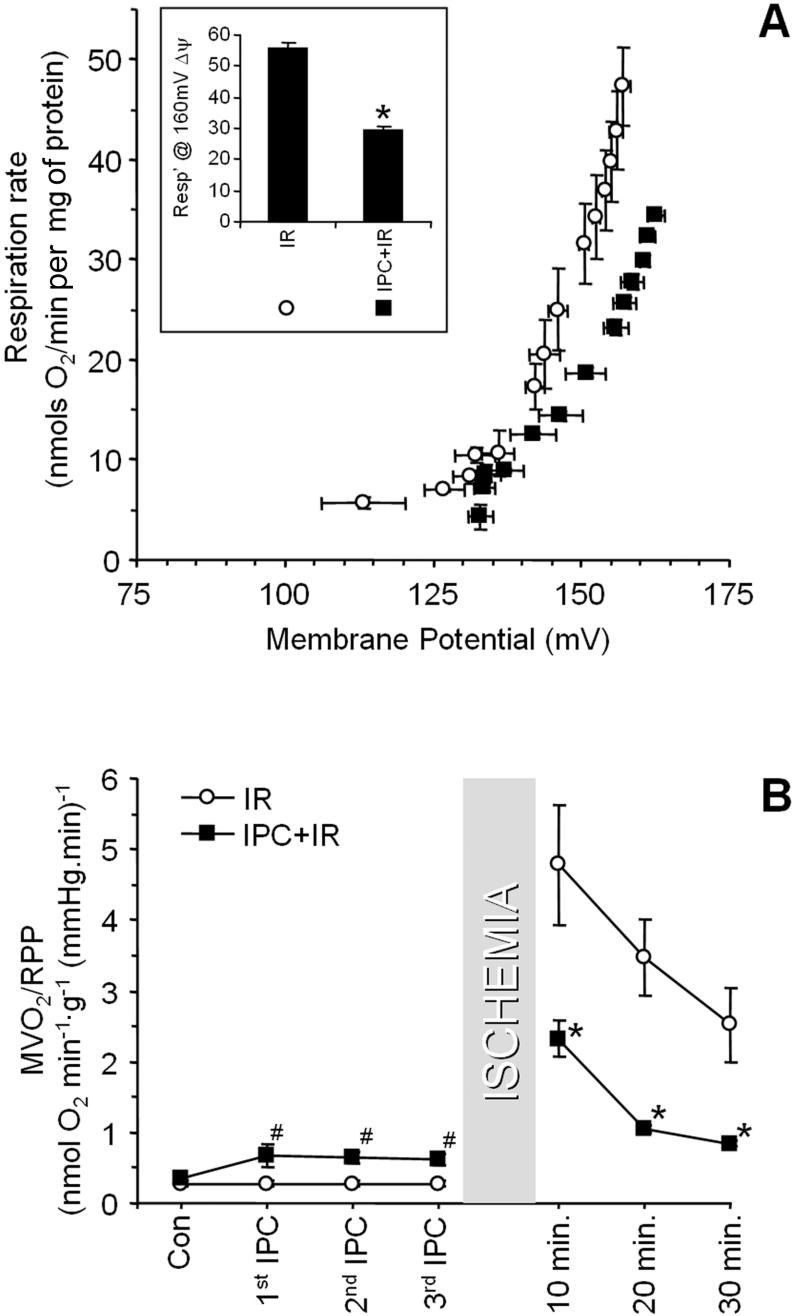
Having established that the H+ leak was slightly increased after IPC, and to a greater extent after IR, we next investigated the connection between these two events. As expected, IPC protected the heart from IR injury (Figure 1). Figure 7(A) shows that IPC before IR inhibited the IR-induced increase in mitochondrial H+ leak. In agreement with these data, Figure 7(B) shows that the post-IR elevation in MVO2/RPP was largely prevented in hearts subjected to IPC. In addition, Figure 7(B) shows that MVO2/RPP was significantly increased during the IPC development protocol (i.e. measured during the first, second and third reperfusion periods between transient ischaemia). This is consistent with the increased H+ leak observed in mitochondria isolated from hearts subjected to IPC alone (Figure 2).
Figure 7. Protective effects of IPC on H+ leak in IR injury.
Hearts were subject to IR alone, or with prior administration of IPC, as detailed in the Materials and methods section. (A) H+ leak curves from mitochondria isolated from hearts subjected to IR-only or IPC+IR. Inset: respiration at 160 mV, as in Figure 2. (B) MVO2/RPP (see Figure 4) during the development of IPC (i.e. measured during the first, second and third reperfusion periods between transient ischaemia), and after subsequent IR. In both panels, open circles represent regular IR data, and black squares represent IPC+IR. Data are the means±S.E.M. for at least five independent experiments. *, P<0.05 compared with the regular IR group (ANOVA).
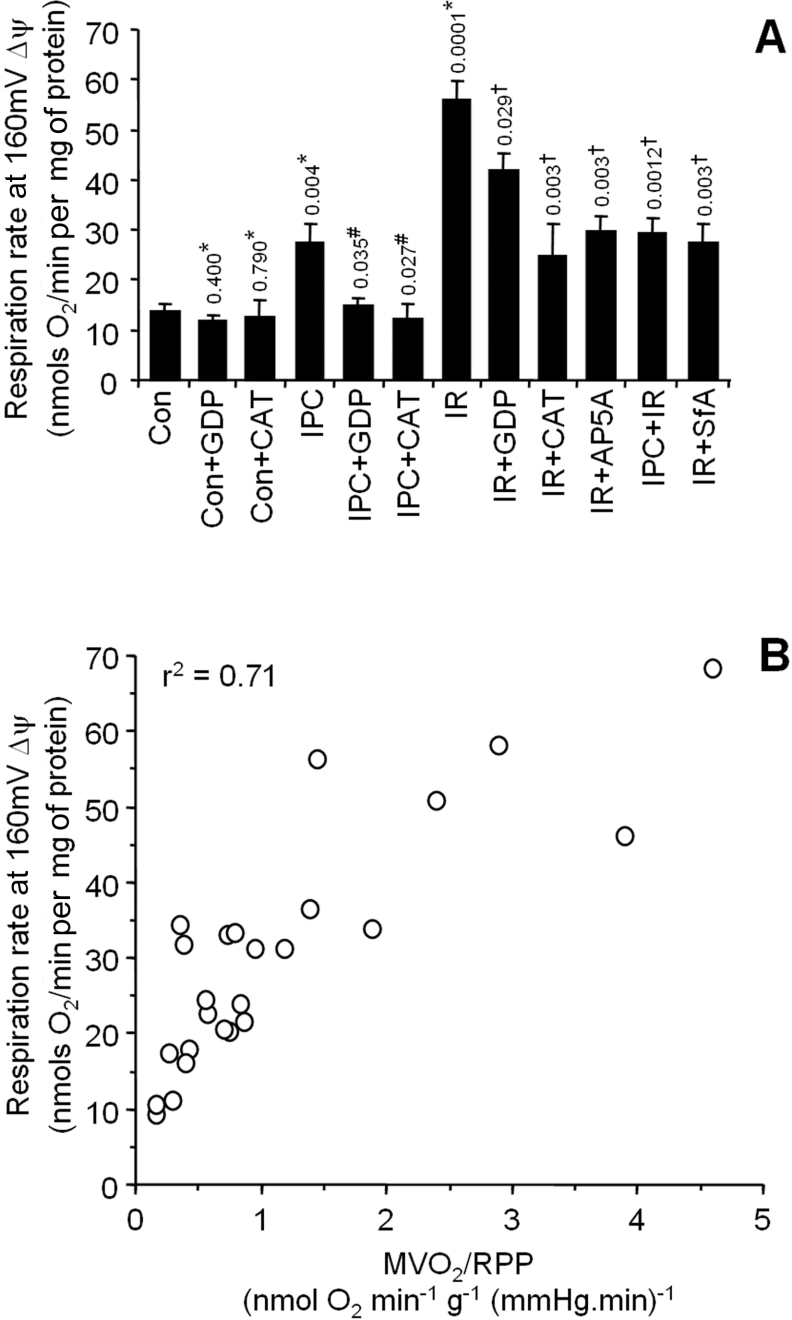
The combined mitochondrial H+ leak data for all conditions studied are shown in Figure 8(A), and were analysed by ANOVA. Since it had previously been assumed (but not determined experimentally) that changes in MVO2/RPP may originate partly from changes in the H+ leak [8], we next sought to examine the relationship between MVO2/RPP and H+ leak. Paired data (i.e. from the same heart) for isolated mitochondrial H+ leak and whole heart MVO2/RPP were plotted. The resulting plot in Figure 8(B) shows a strong correlation between MVO2/RPP and H+ leak (r2=0.71). While several factors may underlie the increased MVO2/RPP observed in IR injury or IPC, this plot suggests that the mitochondrial H+ leak may partially contribute to such an increase.
Figure 8. Overall H+ leak and its correlation with MVO2/RPP.
(A) Combined H+ leak data (respiration at 160 mV Δψm) under all conditions, taken from panel insets in Figures 2–7. Numbers and symbols above the bars represent P values for between group ANOVA, as follows: *, relative to control; #, relative to IPC; †, relative to IR. (B) The relationship between MVO2/RPP and H+ leak. Individual mitochondrial H+ leak data points from six experimental groups (control, IPC, IR, IPC+IR, SfA+IR and AP5A+IR) were plotted against MVO2/RPP data from corresponding hearts. r2 was obtained by linear regression analysis. Note that both parameters (H+ leak and MVO2/RPP) were not available for all experiments performed, and thus the total number of points on this plot is less than the total n value for the entire study.
DISCUSSION
The main findings of this study are as follows. (i) A large elevation in the mitochondrial H+ leak after IR, is mediated by the ANT, with a minimal contribution from UCPs. (ii) A small elevation in the H+ leak after IPC is mediated primarily by UCPs, both UCP2 and UCP3 proteins being present in rat heart mitochondria. (iii) Under all conditions in which changes in the H+ leak were observed, corresponding changes in MVO2/RPP were also observed.
It should be noted that a cause-and-effect relationship cannot be inferred from the correlation between H+ leak and MVO2/RPP shown in Figure 8(B). This is partly because the Langendorff-perfused hearts in the present study were in non-working mode, in which RPP is not a true measure of cardiac work. Despite this, numerous studies have used both RPP to represent work, and MVO2/RPP as a measure of energetic efficiency in non-working hearts [8,34–38]. In this context it is important to emphasize that regardless of the mode of perfusion (working or non-working), any relationship between MVO2/RPP and H+ leak is purely correlative. This will remain the case until tools facilitating the manipulation of H+ leak in situ become available. While UCP2, and UCP3 knockout mice have been generated [39,40], each exhibits compensatory over-expression of other UCPs, and a dual knockout has not yet been created. Similarly, there are no specific UCP inhibitors currently available commercially.
In support of a pathological role for H+ leak in IR injury, all of the treatment strategies that inhibited the post-IR elevation in H+ leak (CsA, IPC, SfA and AP5A) also significantly improved post-IR cardiac functional recovery (Figure 1). A potential role for the PT pore in the post-IR increase in the H+ leak is supported by data in Figure 4. However, this interpretation is subject to the specificity of the pharmacological agents used to inhibit the PT pore. In particular, CsA can also inhibit calcineurin, although the repetition of these results when using the novel PT pore inhibitor, SfA, which does not inhibit calcineurin [32], suggests that CsA is acting on the PT pore in this case. While the mechanism and nature of the PT pore are beyond the scope of this discussion (reviewed in [17,41,42]) it is noteworthy that the PT pore can exist in several ion-selective flickering states that are unrelated to full-scale PT pore opening and cytochrome c release [18,19,43]. We do not consider it to be likely that large-scale PT pore opening accounts for the H+ leak observed in IR injury, since this would be accompanied by cytochrome c release resulting in the inhibition of respiration, which was not observed (Figure 2). The presence of EGTA in the mitochondrial isolation medium also makes it unlikely that PT pore opening occurred during this isolation. Furthermore, large-scale PT pore opening would result in mitochondrial swelling and altered centrifugal buoyancy, but the similar yields and purity [44] of mitochondria from control compared with IR hearts suggests that this is not the case. The inhibition of IR-induced H+ leak by CAT (Figure 3B) strongly implicates ANT, but it should be noted that CAT stabilizes the c-conformation of ANT, which promotes PT pore opening. Thus ANT probably mediates H+ leakage via a mechanism that is related, but is not identical, to that of the PT pore.
A continuum of mitochondrial pores and channels are known to involve the ANT, and while large-scale PT pore opening is at one end of this spectrum, a H+-selective channel induced in the ANT via allosteric stimulation by AMP [14] is at the other end. The latter may be particularly relevant to ischaemia, in which AMP levels are increased. In addition, ANT content has recently been demonstrated as a significant determinant of basal H+ leakage [45]. In order to investigate the possible role of the AMP-induced H+ leak in IR-mitochondria, hearts were treated with the adenylate kinase inhibitor, AP5A, in order to limit AMP formation, and this inhibited the IR-induced elevation in both cardiac MVO2/RPP and mitochondrial H+ leak (Figure 5). Notably, the downstream effects of AMP may not be limited to allosteric activation of ANT, since AMP can also promote PT pore opening [17,41,42]. Thus some of the observed cardioprotection from AP5A (Figure 1) may be due to PT pore inhibition. Overall, these data undoubtedly suggest a role for the ANT in IR-induced H+ leak. What is less clear, is the exact conformation and mode of action of the ANT. Better experimental tools are required to distinguish between the various intermediate conductance states of the ANT–PT pore complex.
A multi-factorial origin for increased H+ leak in IR-mediated injury is also supported by data showing that the UCP inhibitor, GDP, decreased H+ leak by a similar amount in IR- and IPC-treated mitochondria (Figure 8A). While GDP treatment completely returned the level of H+ leakage to baseline in IPC-treated mitochondria, it failed to do so in IR-mitochondria, owing to additional non-UCP-mediated H+ leak mechanisms, as described above. The assignment of the small elevation in H+ leak in IPC (Figure 6) to UCPs, hinges on the specificity of the inhibitors used. While CAT is known to inhibit UCPs [46], it also inhibits ANT (as described above). In isolated mitochondria GDP is a reasonably selective UCP inhibitor. However, preliminary studies in perfused hearts failed to show that GDP had any effect on cardiac function in IPC (results not shown). Thus although MVO2/RPP is increased during the development stages of IPC (Figure 7B), the lack of pharmacological tools to specifically inhibit UCPs inside cells precludes the assignment of this increase to a UCP-mediated H+ leak. The mechanism of UCP activation in IPC also remains to be determined, although in this context it is known that ROS can activate UCPs [47–49] and ROS are critical for cell signalling in IPC [21,50]. Furthermore, it was recently shown that cardiac UCP2 mRNA expression is increased during the delayed second window of IPC [6]. This is the first study to suggest the involvement of endogenously expressed baseline levels of UCPs in short-term IPC signalling.
Mild mitochondrial uncoupling via over-expression of UCPs or by treatment with chemical uncouplers, has previously been advocated as a cytoprotective strategy in a wide range of cellular and whole-organ models of IR injury [3–10]. The downstream protective effects of such uncoupling are thought to be mediated via lowering of mitochondrial ROS generation or inhibition of mitochondrial Ca2+ overload [2,11,12,21]. However, recent reports have shown that UCP transfection can sensitize cells to apoptosis [51] and accelerate the progression of atherosclerosis [52], suggesting that the utility of transgenic systems for modulating UCP activity may be limited.
Opening of the mitochondrial K+ATP channel has been implicated in the mechanism of IPC [53,54], although the existence of this channel is still debated [55–57]. In considering the contribution from mitochondrial K+ fluxes to the uncoupling observed in IPC, several factors should be noted: nigericin was absent from our incubations [27], and thus a fast K+-mediated H+ leak would require a rapid outwards K+ flux through the K+/H+ exchanger, balanced by a rapid K+ influx through a channel such as K+ATP. While the incubation of mitochondria in K+-based solutions causes an initial (<30 s) saturated K+ uptake (of which approx. 20% is accounted for by the K+ATP channel [58]), this uptake is not sustained. Maximal sustained K+ entry rates into isolated heart mitochondria are approx. 5 nmol/min per mg [59], whereas H+ leak rates (as described above) are approx. 200–300 nmol H+/min per mg. Thus K+ cycling can account for approx. 2% of the H+ leak. Consistent with this observation, previous studies have estimated that the contribution from K+/H+ cycling fluxes to mitochondrial H+ leak is ‘small or non-existent’ [1,60].
Overall, the current data highlight a bi-modal behaviour of H+ leakage, that is either cardioprotective in IPC or pathological in IR, and is dependent upon its magnitude. In this regard it is interesting to draw a parallel with the PT pore. The pathological role of the PT pore in IR injury is well established [17,41,42] and is paralleled by a large increase in H+ leak in response to IR. It is also known that IPC prevents large-scale PT pore opening upon reperfusion [61], and similarly IPC prevents a large-scale H+ leak upon reperfusion. Notably, our data demonstrating a small increase in H+ leak in IPC appear to be consistent with a recent proposal that transient PT pore flickering is implicated in the mechanism of IPC [62], although the latter has recently been challenged [63]. Overall, while PT pore flickering is implicated in IPC, and large-scale PT pore opening occurs in IR, whether the former mechanistically prevent the latter remains to be proven. Similarly, whether a small H+ leak in IPC is involved in the mechanism of preventing a large H+ leak in response to IR, is unknown.
In summary, the line between what does and does not constitute the ‘PT pore’ requires refinement, not only to understand the current data set, but also in resolving the controversial role of the PT pore in IPC. The identification of the upstream regulators of both the H+ leak and the PT pore will be essential to further our understanding of the complex roles that these phenomena play in IPC, and IR injury. Furthermore, the development of drugs to modulate the H+ leak should afford avenues for pharmacological preconditioning and the amelioration of cardiac IR injury [64].
Acknowledgments
The present study was funded by a grant from the National Institutes of Health (RO1 HL71158).
References
- 1.Brand M. D., Chien L. F., Ainscow E. K., Rolfe D. F., Porter R. K. The causes and functions of mitochondrial proton leak. Biochim. Biophys. Acta. 1994;1187:132–139. doi: 10.1016/0005-2728(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 2.Brookes P. S. Mitochondrial H+ leak and ROS generation: an odd couple. Free Radical Biol. Med. 2004;38:12–23. doi: 10.1016/j.freeradbiomed.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 3.Minners J., Lacerda L., McCarthy J., Meiring J. J., Yellon D. M., Sack M. N. Ischemic and pharmacological preconditioning in Girardi cells and C2C12 myotubes induce mitochondrial uncoupling. Circ. Res. 2001;89:787–792. doi: 10.1161/hh2101.098372. [DOI] [PubMed] [Google Scholar]
- 4.Minners J., van den Bos E. J., Yellon D., Schwalb H., Opie L., Sack M. Dinitrophenol, cyclosporin A, and trimetazidine modulate preconditioning in the isolated rat heart: support for a mitochondrial role in cardioprotection. Cardiovasc. Res. 2000;47:68–73. doi: 10.1016/s0008-6363(00)00069-9. [DOI] [PubMed] [Google Scholar]
- 5.Ganote C. E., Armstrong S. C. Effects of CCCP-induced mitochondrial uncoupling and cyclosporin A on cell volume, cell injury and preconditioning protection of isolated rabbit cardiomyocytes. J. Mol. Cell. Cardiol. 2003;35:749–759. doi: 10.1016/s0022-2828(03)00114-7. [DOI] [PubMed] [Google Scholar]
- 6.McLeod C., Hoyt R., Sack M. UCP-2, a functional target in delayed preconditioning induced cardioprotection? Cardiovasc. J. S. Afr. 2004;15:S4. [Google Scholar]
- 7.Teshima Y., Akao M., Jones S., Marban E. Uncoupling protein-2 overexpression inhibits mitochondrial death pathway in cardiomyocytes. Circ. Res. 2003;93:192–200. doi: 10.1161/01.RES.0000085581.60197.4D. [DOI] [PubMed] [Google Scholar]
- 8.Hoerter J., Gonzales-Barroso M., Couplan E., Mateo P., Gelly C., Cassard-Doulcier A., Diolez P., Bouillaud F. Mitochondrial uncoupling protein 1 expressed in the heart of transgenic mice protects against ischemic-reperfusion damage. Circulation. 2004;110:528–533. doi: 10.1161/01.CIR.0000137824.30476.0E. [DOI] [PubMed] [Google Scholar]
- 9.Bienengraeber M., Ozcan C., Terzic A. Stable transfection of UCP1 confers resistance to hypoxia/reoxygenation in a heart-derived cell line. J. Mol. Cell. Cardiol. 2003;35:861–865. doi: 10.1016/s0022-2828(03)00147-0. [DOI] [PubMed] [Google Scholar]
- 10.Mattiasson G., Shamloo M., Gido G., Mathi K., Tomasevic G., Yi S., Warden C. H., Castilho R. F., Melcher T., Gonzalez-Zulueta M., et al. Uncoupling protein-2 prevents neuronal death and diminishes brain dysfunction after stroke and brain trauma. Nat. Med. 2003;9:1062–1068. doi: 10.1038/nm903. [DOI] [PubMed] [Google Scholar]
- 11.Negre-Salvayre A., Hirtz C., Carrera G., Cazenave R., Troly M., Salvayre R., Penicaud L., Casteilla L. A role for uncoupling protein-2 as a regulator of mitochondrial hydrogen peroxide generation. FASEB J. 1997;11:809–815. [PubMed] [Google Scholar]
- 12.Starkov A. A., Fiskum G. Regulation of brain mitochondrial H2O2 production by membrane potential and NAD(P)H redox state. J. Neurochem. 2003;86:1101–1107. doi: 10.1046/j.1471-4159.2003.01908.x. [DOI] [PubMed] [Google Scholar]
- 13.Stuart J. A., Cadenas S., Jekabsons M. B., Roussel D., Brand M. D. Mitochondrial proton leak and the uncoupling protein 1 homologues. Biochim. Biophys. Acta. 2001;1504:144–158. doi: 10.1016/s0005-2728(00)00243-7. [DOI] [PubMed] [Google Scholar]
- 14.Cadenas S., Buckingham J., St Pierre J., Dickinson K., Jones R., Brand M. AMP decreases the efficiency of skeletal-muscle mitochondria. Biochem. J. 2000;351:307–311. [PMC free article] [PubMed] [Google Scholar]
- 15.Garlid K., Jaburek M., Jezek P., Varecha M. How do uncoupling proteins uncouple? Biochim. Biophys. Acta. 2000;1459:383–389. doi: 10.1016/s0005-2728(00)00175-4. [DOI] [PubMed] [Google Scholar]
- 16.Jezek P., Zackova M., Ruzicka M., Skobisova E., Jaburek M. Mitochondrial uncoupling proteins–facts and fantasies. Physiol. Res. 2004;53:S199–S211. [PubMed] [Google Scholar]
- 17.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem. J. 1999;341:233–249. [PMC free article] [PubMed] [Google Scholar]
- 18.Broekemeier K. M., Klocek C. K., Pfeiffer D. R. Proton selective substate of the mitochondrial permeability transition pore: regulation by the redox state of the electron transport chain. Biochemistry. 1998;37:13059–13065. doi: 10.1021/bi980820c. [DOI] [PubMed] [Google Scholar]
- 19.Huser J., Blatter L. A. Fluctuations in mitochondrial membrane potential caused by repetitive gating of the permeability transition pore. Biochem. J. 1999;343:311–317. [PMC free article] [PubMed] [Google Scholar]
- 20.Weiss J. N., Korge P., Honda H. M., Ping P. Role of the mitochondrial permeability transition in myocardial disease. Circ. Res. 2003;93:292–301. doi: 10.1161/01.RES.0000087542.26971.D4. [DOI] [PubMed] [Google Scholar]
- 21.Zaugg M., Schaub M. C. Signaling and cellular mechanisms in cardiac protection by ischemic and pharmacological preconditioning. J. Muscle Res. Cell Motil. 2003;24:219–249. doi: 10.1023/a:1026021430091. [DOI] [PubMed] [Google Scholar]
- 22.Borutaite V., Morkuniene R., Budriunaite A., Krasauskaite D., Ryselis S., Toleikis A., Brown G. Kinetic analysis of changes in activity of heart mitochondrial oxidative phosphorylation system induced by ischemia. J. Mol. Cell. Cardiol. 1996;28:2195–2201. doi: 10.1006/jmcc.1996.0211. [DOI] [PubMed] [Google Scholar]
- 23.Bosetti F., Baracca A., Lenaz G., Solaini G. Increased state 4 mitochondrial respiration and swelling in early post-ischemic reperfusion of rat heart. FEBS Lett. 2004;563:161–164. doi: 10.1016/S0014-5793(04)00294-7. [DOI] [PubMed] [Google Scholar]
- 24.Muscari C., Bonafe F., Gamberini C., Giordano E., Lenaz G., Caldarera C. M. Ischemic preconditioning preserves proton leakage from mitochondrial membranes but not oxidative phosphorylation during heart reperfusion. Cell Biochem. Funct. 2005 doi: 10.1002/cbf.1294. in the Press. [DOI] [PubMed] [Google Scholar]
- 25.Brookes P. S., Digerness S. B., Parks D. A., Darley-Usmar V. M. Mitochondrial function in response to cardiac ischemia-reperfusion after oral treatment with quercetin. Free Radical Biol. Med. 2002;32:1220–1228. doi: 10.1016/s0891-5849(02)00839-0. [DOI] [PubMed] [Google Scholar]
- 26.Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 27.Brand M. Measurement of mitochondrial proton-motive force. In: Brown G. C., Cooper C. E., editors. Bioenergetics: a Practical Approach. Oxford, U.K.: IRL Press; 1995. pp. 39–62. [Google Scholar]
- 28.Brookes P., Salinas E., Darley-Usmar K., Eiserich J., Freeman B., Darley-Usmar V., Anderson P. Concentration-dependent effects of nitric oxide on mitochondrial permeability transition and cytochrome c release. J. Biol. Chem. 2000;275:20474–20479. doi: 10.1074/jbc.M001077200. [DOI] [PubMed] [Google Scholar]
- 29.Borutaite V., Mildaziene V., Brown G. C., Brand M. D. Control and kinetic analysis of ischemia-damaged heart mitochondria: which parts of the oxidative phosphorylation system are affected by ischemia? Biochim. Biophys. Acta. 1995;1272:154–158. doi: 10.1016/0925-4439(95)00080-1. [DOI] [PubMed] [Google Scholar]
- 30.Wallenstein S., Zucker C., Fleiss J. Some statistical methods useful in circulation research. Circ. Res. 1980;47:1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- 31.Griffiths E. J., Halestrap A. P. Protection by Cyclosporin A of ischemia/reperfusion-induced damage in isolated rat hearts. J. Mol. Cell. Cardiol. 1993;25:1461–1469. doi: 10.1006/jmcc.1993.1162. [DOI] [PubMed] [Google Scholar]
- 32.Clarke S. J., McStay G. P., Halestrap A. P. Sanglifehrin A acts as a potent inhibitor of the mitochondrial permeability transition and reperfusion injury of the heart by binding to cyclophilin-D at a different site from cyclosporin A. J. Biol. Chem. 2002;277:34793–34799. doi: 10.1074/jbc.M202191200. [DOI] [PubMed] [Google Scholar]
- 33.Murray A. J., Anderson R. E., Watson G. C., Radda G. K., Clarke K. Uncoupling proteins in human heart. Lancet. 2004;364:1786–1788. doi: 10.1016/S0140-6736(04)17402-3. [DOI] [PubMed] [Google Scholar]
- 34.Cardenas G., Torres J. C., Zamora J., Banos G. Isolated heart function during ischemia and reperfusion in sucrose-fed rats: effect of insulin infusion. Cardiovasc. Pathol. 2005;14:256–226. doi: 10.1016/j.carpath.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Kupriyanov V. V., St Jean M., Xiang B., Butler K. W., Deslauriers R. Contractile dysfunction caused by normothermic ischaemia and KCL arrest in the isolated pig heart: a 31P NMR study. J. Mol. Cell. Cardiol. 1995;27:1715–1730. doi: 10.1016/s0022-2828(95)90854-4. [DOI] [PubMed] [Google Scholar]
- 36.Chen Q., Camara A. K., An J., Novalija E., Riess M. L., Stowe D. F. Sevoflurane preconditioning before moderate hypothermic ischemia protects against cytosolic [Ca2+] loading and myocardial damage in part via mitochondrial K(ATP) channels. Anesthesiology. 2002;97:912–920. doi: 10.1097/00000542-200210000-00025. [DOI] [PubMed] [Google Scholar]
- 37.Takeuchi K., Minakawa M., Otaki M., Odagiri S., Itoh K., Murakami A., Yaku H., Kitamura N. Hyperthyroidism causes mechanical insufficiency of myocardium with possibly increased SR Ca2+-ATPase activity. Jpn. J. Physiol. 2003;53:411–416. doi: 10.2170/jjphysiol.53.411. [DOI] [PubMed] [Google Scholar]
- 38.Shen W., Tian R., Saupe K. W., Spindler M., Ingwall J. S. Endogenous nitric oxide enhances coupling between O2 consumption and ATP synthesis in guinea pig hearts. Am. J. Physiol. Heart Circ. Physiol. 2001;281:H838–H846. doi: 10.1152/ajpheart.2001.281.2.H838. [DOI] [PubMed] [Google Scholar]
- 39.Arsenijevic D., Onuma H., Pecqueur C., Raimbault S., Manning B. S., Miroux B., Couplan E., Alves-Guerra M. C., Goubern M., Surwit R., et al. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat. Genet. 2000;26:435–439. doi: 10.1038/82565. [DOI] [PubMed] [Google Scholar]
- 40.Vidal-Puig A. J., Grujic D., Zhang C. Y., Hagen T., Boss O., Ido Y., Szczepanik A., Wade J., Mootha V., Cortright R., et al. Energy metabolism in uncoupling protein 3 gene knockout mice. J. Biol. Chem. 2000;275:16258–16266. doi: 10.1074/jbc.M910179199. [DOI] [PubMed] [Google Scholar]
- 41.Halestrap A. P., Clarke S. J., Javadov S. A. Mitochondrial permeability transition pore opening during myocardial reperfusion- a target for cardioprotection. Cardiovasc. Res. 2004;61:372–385. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 42.Di Lisa F., Canton M., Menabo R., Dodoni G., Bernardi P. Mitochondria and reperfusion injury. The role of permeability transition. Basic Res. Cardiol. 2003;98:235–241. doi: 10.1007/s00395-003-0415-x. [DOI] [PubMed] [Google Scholar]
- 43.Vergun O., Reynolds I. J. Distinct characteristics of Ca2+-induced depolarization of isolated brain and liver mitochondria. Biochim. Biophys. Acta. 2005;1709:127–137. doi: 10.1016/j.bbabio.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 44.Tompkins A. J., Burwell L. S., Digerness S. B., Zaragoza C., Holman W. L., Brookes P. S. Mitochondrial dysfunction in cardiac ischemia-reperfusion injury: ROS from complex I, without inhibition. Biochim. Biophys. Acta. 2006;1762:223–231. doi: 10.1016/j.bbadis.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 45.Brand M. D., Pakay J. L., Ocloo A., Kokoszka J., Wallace D. C., Brookes P. S., Cornwall E. J. The basal proton conductance of mitochondria depends on adenine nucleotide translocase content. Biochem. J. 2005;392:353–362. doi: 10.1042/BJ20050890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klingenberg M. Principles of carrier catalysis elucidated by comparing two similar membrane translocators from mitochondria, the ADP/ATP carrier and the uncoupling protein. Ann. N.Y. Acad. Sci. 1985;456:279–288. doi: 10.1111/j.1749-6632.1985.tb14877.x. [DOI] [PubMed] [Google Scholar]
- 47.Echtay K., Roussel D., St-Pierre J., Jekabsons M., Cadenas S., Stuart J., Harper J., Roebuck S., Morrison A., Pickering S., et al. Superoxide activates mitochondrial uncoupling proteins. Nature (London) 2002;415:96–99. doi: 10.1038/415096a. [DOI] [PubMed] [Google Scholar]
- 48.Echtay K., Esteves T., Pakay J., Jekabsons M., Lambert A., Portero-Otin M., Pamplona R., Vidal-Puig A., Wang S., Roebuck S., Brand M. A signalling role for 4-hydroxy-2-nonenal in regulation of mitochondrial uncoupling. EMBO J. 2003;22:4103–4110. doi: 10.1093/emboj/cdg412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Echtay K., Murphy M., Smith R., Talbot D., Brand M. Superoxide activates mitochondrial uncoupling protein 2 from the matrix side. Studies using targeted antioxidants. J. Biol. Chem. 2002;277:47129–47135. doi: 10.1074/jbc.M208262200. [DOI] [PubMed] [Google Scholar]
- 50.Da Silva M., Sartori A., Belisle E., Kowaltowski A. Ischemic preconditioning inhibits mitochondrial respiration, increases H2O2 release, and enhances K+ transport. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H154–H162. doi: 10.1152/ajpheart.00955.2002. [DOI] [PubMed] [Google Scholar]
- 51.Dejean L., Camara Y., Brigitte S., Solanes G., Villarroya F. Uncoupling protein-3 sensitizes cells to mitochondrial-dependent stimulus of apoptosis. J. Cell. Physiol. 2004;201:294–304. doi: 10.1002/jcp.20048. [DOI] [PubMed] [Google Scholar]
- 52.Bernal-Mizrachi C., Gates A. C., Weng S., Imamura T., Knutsen R. H., DeSantis P., Coleman T., Townsend R. R., Muglia L. J., Semenkovich C. F. Vascular respiratory uncoupling increases blood pressure and atherosclerosis. Nature (London) 2005;435:502–506. doi: 10.1038/nature03527. [DOI] [PubMed] [Google Scholar]
- 53.Liu Y., Sato T., O'Rourke B., Marban E. Mitochondrial ATP-dependent potassium channels: novel effectors of cardioprotection? Circulation. 1998;97:2463–2469. doi: 10.1161/01.cir.97.24.2463. [DOI] [PubMed] [Google Scholar]
- 54.Holmuhamedov E. L., Jahangir A., Oberlin A., Komarov A., Colombini M., Terzic A. Potassium channel openers are uncoupling protonophores: implication in cardioprotection. FEBS Lett. 2004;568:167–170. doi: 10.1016/j.febslet.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 55.Das M., Parker J. E., Halestrap A. P. Matrix volume measurements challenge the existence of diazoxide/glibencamide-sensitive KATP channels in rat mitochondria. J. Physiol. 2003;547:893–902. doi: 10.1113/jphysiol.2002.035006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ardehali H., Chen Z., Ko Y., Mejia-Alvarez R., Marban E. Multiprotein complex containing succinate dehydrogenase confers mitochondrial ATP-sensitive K+ channel activity. Proc. Natl. Acad. Sci. U.S.A. 2004;101:11880–11885. doi: 10.1073/pnas.0401703101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lacza Z., Snipes J. A., Miller A. W., Szabo C., Grover G., Busija D. W. Heart mitochondria contain functional ATP-dependent K+ channels. J. Mol. Cell. Cardiol. 2003;35:1339–1347. doi: 10.1016/s0022-2828(03)00249-9. [DOI] [PubMed] [Google Scholar]
- 58.Costa A., Garlid K., West I., Lincoln T., Downey J., Cohen M., Critz S. Protein kinase G transmits the cardioprotective signal from cytosol to mitochondria. Circ. Res. 2005;97:329–336. doi: 10.1161/01.RES.0000178451.08719.5b. [DOI] [PubMed] [Google Scholar]
- 59.Jung D., Chavez E., Brierley G. Energy-dependent exchange of K+ in heart mitochondria: K+ influx. Arch. Biochem. Biophys. 1977;183:452–459. doi: 10.1016/0003-9861(77)90380-0. [DOI] [PubMed] [Google Scholar]
- 60.Brown G., Brand M. On the nature of the mitochondrial proton leak. Biochim. Biophys. Acta. 1991;1059:55–62. doi: 10.1016/s0005-2728(05)80187-2. [DOI] [PubMed] [Google Scholar]
- 61.Hausenloy D., Yellon D., Mani-Babu S., Duchen M. Preconditioning protects by inhibiting the mitochondrial permeability transition. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H841–H849. doi: 10.1152/ajpheart.00678.2003. [DOI] [PubMed] [Google Scholar]
- 62.Hausenloy D., Wynne A., Duchen M., Yellon D. Transient mitochondrial permeability transition pore opening mediates preconditioning-induced protection. Circulation. 2004;109:1714–1717. doi: 10.1161/01.CIR.0000126294.81407.7D. [DOI] [PubMed] [Google Scholar]
- 63.Halestrap A. Does the mitochondrial permeability transition have a role in preconditioning? Circulation. 2004;110:303. doi: 10.1161/01.CIR.0000141458.28925.D2. [DOI] [PubMed] [Google Scholar]
- 64.Brookes P. S., Yoon Y., Robotham J. L., Anders M. W., Sheu S. S. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am. J. Physiol. Cell Physiol. 2004;287:C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]