Abstract
The macrophage mannose receptor is the prototype for a family of receptors each having an extracellular region consisting of an N-terminal cysteine-rich domain related to the R-type carbohydrate-recognition domain of ricin, a fibronectin type II domain and eight to ten domains related to C-type carbohydrate-recognition domains. The mannose receptor acts as a molecular scavenger, clearing harmful glycoconjugates or micro-organisms through recognition of their defining carbohydrate structures. Cell-adhesion assays, as well as collagen-binding assays, have now been used to show that the mannose receptor can also bind collagen and that the fibronectin type II domain mediates this activity. Neither of the two types of sugar-binding domain in the receptor is involved in collagen binding. Fibroblasts expressing the mannose receptor adhere to type I, type III and type IV collagens, but not to type V collagen, and the adherence is inhibited by isolated mannose receptor fibronectin type II domain. The fibronectin type II domain shows the same specificity for collagen as the whole receptor, binding to type I, type III and type IV collagens. This is the first activity assigned to the fibronectin type II domain of the mannose receptor. The results suggest additional roles for this multifunctional receptor in mediating collagen clearance or cell–matrix adhesion.
Keywords: carbohydrate-recognition domain, cell adhesion, collagen, ELISA, fibronectin type II domain, mannose receptor
Abbreviations: CRD, carbohydrate-recognition domain; DMEM, Dulbecco's modified Eagle's medium; FNII, fibronectin type II; IPTG, isopropyl β-D-thiogalactoside; MMP, matrix metalloproteinase; MMR1–8, portion of the macrophage mannose receptor consisting of the eight CRDs; MMR-S, extracellular region of the macrophage mannose receptor; Ni-NTA, Ni2+-nitriloacetate; TBS, Tris-buffered saline
INTRODUCTION
The macrophage mannose receptor plays a well-documented role in mediating the clearance of pathogenic micro-organisms and potentially harmful glycoproteins by recognition of their defining carbohydrate structures [1]. The extracellular region of the receptor contains multiple domains that allow recognition of a diverse range of ligands (Figure 1A) [2]. Several of the eight C-type CRDs (carbohydrate-recognition domains) are involved in Ca2+-dependent recognition of mannose, N-acetylglucosamine or fucose residues [3,4]. This activity allows the receptor to mediate binding and internalization of micro-organisms, such as Pneumocystis carinii, and endogenous glycoproteins, such as tissue plasminogen activator and lysosomal enzymes [5–7]. An N-terminal cysteine-rich domain, which is homologous with the R-type CRD of ricin, binds sulphated N-acetylgalactosamine residues by a mechanism distinct from that of the C-type CRDs [8,9]. This activity allows the receptor to mediate the clearance of glycoprotein hormones, such as lutropin and thyrotropin, which bear oligosaccharides that terminate in GalNAc-4-SO4 [10,11].
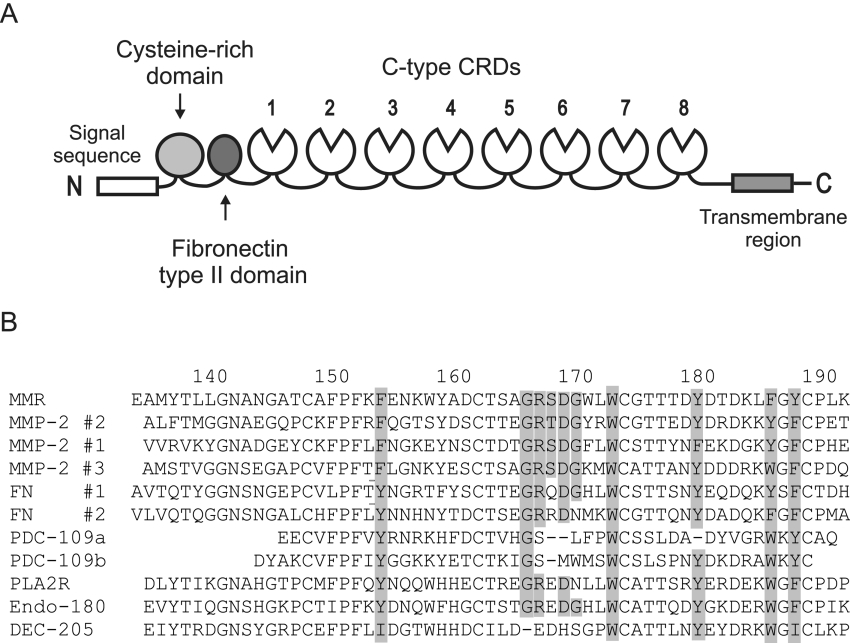
Figure 1. Domain organization of the mannose receptor.
(A) Diagrammatic representation of the mannose receptor. (B) Sequence alignment of selected FNII domains. MMP-2 #1, #2 and #3, three domains from MMP-2; FN #1 and #2, first and second domains from fibronectin; PDC-109a and b, two domains from bovine seminal plasma protein PDC109; PLA2R, phospholipase A2 receptor. Residues predicted to form the collagen-binding region in MMP-2 #2 [23,24] are shaded in MMP-2 #2 and where they are conserved in other domains. Numbers at the top correspond to the residues of the mannose receptor.
The mannose receptor contains an FNII (fibronectin type II) domain between the cysteine-rich domain and the first of the eight C-type CRDs. Although the C-type CRDs of the receptor and the N-terminal R-type CRD have been well studied, little is known about the function of the FNII domain. The FNII domain is not necessary for recognition of either mannose/N-acetylglucosamine/fucose-terminated glycoconjugates or the glycoprotein hormones [3,8] and no binding activity has been assigned to this domain in the mannose receptor. Binding activity of the FNII domain has also not been studied directly in the other members of the mannose receptor family, the phospholipase A2 receptor, DEC-205 and Endo180, which all share the same domain organization as the mannose receptor [12].
As well as being found in fibronectin and the members of the mannose receptor family, FNII domains are found in a relatively small number of other proteins. These include MMP (matrix metalloproteinase)-2 and -9, coagulation Factor XII, the cation-independent mannose 6-phosphate receptor and several proteins that are present in seminal fluid [13–17]. In fibronectin, the two type II domains are located in the collagen-binding region of the molecule, which binds to gelatin and α-chains of collagen [18,19]. The FNII domains of MMP-2 and -9 are also involved in binding of collagen [20,21]. These two enzymes, which bind and degrade components of the extracellular matrix, each have three FNII domains inserted in the catalytic domain near the active site. The bovine seminal fluid protein PDC-109 and its homologues, which each consist of two FNII domains, also bind gelatin and collagen, although it seems likely that the physiological ligands of these proteins are phospholipids [22].
Collagen/gelatin binding by FNII domains has been best studied in MMP-2. Each of the three FNII domains of MMP-2 can bind gelatin [20]. NMR binding assays with a collagen peptide as well as mutagenesis studies with the second MMP-2 FNII domain have identified a series of amino acids, including several solvent-exposed aromatic residues, that are likely to be involved in collagen/gelatin binding [23,24]. The solution structure of the second MMP-2 FNII domain, as well as the crystal structure of the whole enzyme, show that these residues are part of a hydrophobic pocket that is formed between two double-stranded antiparallel β-sheets [23,25].
Sequence comparisons show that the MMP-2 residues that are predicted to be involved in collagen binding are well conserved in the mannose receptor FNII domain (Figure 1B). Thus it is reasonable to propose that the mannose receptor FNII domain might be capable of binding to collagen. The MMP-2 collagen-binding residues are also quite well conserved in the FNII domains of Endo180 and the phospholipase A2 receptor. Endo180 binds collagen and it is likely that this activity is mediated by the FNII domain, although binding of collagen by this domain has not been demonstrated directly [26,27]. Collagen is likely to be a physiologically important ligand for Endo180 because fibroblasts from mice with a targeted deletion of the Endo180 gene show defects in adhesion and migration on collagen [28,29]. Cell-adhesion assays have also provided indirect evidence for collagen binding by the FNII domain of the phospholipase A2 receptor [30]. Transfected cells expressing the full-length receptor adhere to type I and type IV collagens, whereas cells expressing a truncated phospholipase A2 receptor, lacking the cysteine-rich domain and the FNII domain, do not.
In the present study, we provide direct evidence for collagen binding by the mannose receptor, and show that the FNII domain mediates this activity. The results suggest a new role for the mannose receptor in promoting cell–matrix adhesion.
MATERIALS AND METHODS
Materials
Gelatin–agarose, non-enzymatic cell dissociation solution, human placental type I, type III, type IV and type V collagens, mouse laminin, Toluidine Blue O, paraformaldehyde, Ni-NTA (Ni2+-nitrilotriacetate)–agarose, BSA, monoclonal anti-polyhistidine antibody and horseradish-peroxidase-conjugated sheep anti-mouse IgG were purchased from Sigma–Aldrich. Restriction enzymes and DNA-modifying enzymes were obtained from New England Biolabs. DMEM (Dulbecco's modified Eagle's medium) was obtained from Invitrogen. Bolton and Hunter reagent (2000 Ci/mmol) and IPTG (isopropyl β-D-thiogalactoside) were obtained from Amersham Biosciences. Tetramethylbenzidine and H2O2 were obtained from Pharmingen.
Adhesion of fibroblast cell lines to collagen
Stably transfected fibroblast cell lines expressing either the full-length human mannose receptor or a truncated mannose receptor consisting of the eight CRDs without the two N-terminal domains have been described previously [2,3]. Cell-adhesion assays were based on those described for studies with the phospholipase A2 receptor [30]. Human placental collagens were dissolved to a concentration of 2.5 mg/ml in 0.1 M ethanoic (acetic) acid and were diluted into PBS before use. Analysis of the collagen stock solutions by SDS/PAGE on 10% gels indicated that each type of collagen was pure and undegraded, and that the concentrations of the different stock solutions were approximately equal. Tissue-culture-treated 96-well plates (Costar) were coated with collagen (100 μl of 20 μg/ml in PBS per well) for 1 h at 37 °C. Wells were washed twice with TBS (Tris-buffered saline: 10 mM Tris/HCl, pH 7.4, and 0.15 M NaCl) and were blocked with 0.1% (w/v) BSA in TBS for 1 h at 37 °C, followed by two further TBS washes. Fibroblasts were harvested using a non-enzymatic cell dissociation solution following the manufacturer's protocol, centrifuged at 500 g for 2 min, and resuspended in DMEM containing 5 mM CaCl2 and 0.1% (w/v) BSA and buffered with 10 mM Hepes, pH 7.55. Cells were diluted to a concentration of 2×106/ml, and 100 μl aliquots were added to wells in triplicate and were left to attach for 15 min at 37 °C. Non-adherent cells were removed by gentle washing once with TBS containing 5 mM CaCl2. Adherent cells were fixed and stained by incubation overnight with 50 μl of PBS containing 1% (w/v) Toluidine Blue O and 3% (w/v) paraformaldehyde. After extensive washing with PBS, cells were dissolved in 50 μl of 2 M HCl, and the absorbance of each well was measured at 570 nm.
Binding of mannose receptor fragments to gelatin
Expression and purification of the whole extracellular region of the human macrophage mannose receptor (MMR-S), and the portion consisting of the eight CRDs (MMR1–8) have been described previously [31,32]. For gelatin-binding experiments, MMR-S and MMR1–8 samples were used at concentrations of 0.15 mg/ml and 0.2 mg/ml respectively. Mannose receptor cysteine-rich domain, produced using a bacterial expression system and purified on lutropin–agarose [33], was used at a concentration of 0.5 mg/ml. Protein samples (1 ml) were loaded on to 2 ml columns of gelatin–agarose that were pre-equilibrated in loading buffer (50 mM Tris/HCl, pH 7.4, and 5 mM CaCl2). Columns were washed with eight 1 ml aliquots of loading buffer and were eluted with 50 mM Tris/HCl, pH 7.4, containing 0.5 M NaCl. Fractions were analysed by SDS/PAGE.
Expression of mannose receptor FNII domain in bacteria
The portion of the human macrophage mannose receptor cDNA [2] coding for the FNII domain (nucleotides 563–739) was cloned into the expression vector pIN-IIIompA-2 [34] using standard recombinant DNA techniques. Synthetic oligonucleotides were used to fuse the 5′ end of the cDNA to the codons specifying the ompA signal sequence and to add codons specifying six histidine residues and a stop codon at the 3′ end. The integrity of the final expression plasmid was checked by DNA sequencing using an ABI prism 310 Genetic Analyser.
Luria–Bertani medium (1 litre), containing 50 μg/ml ampicillin was inoculated with 30 ml of an overnight culture of Escherichia coli strain JA221 containing the FNII expression plasmid. The culture was grown with shaking at 25 °C to a D550 of approx. 1 and IPTG was added to a final concentration of 50 μM. After growth for a further 18 h at 25 °C, cells were harvested by centriugation at 3300 g for 15 min in a Beckman JS-4.2 rotor. Bacterial pellets were resuspended in cold 10 mM Tris/HCl, pH 7.8, followed by centrifugation at 13800 g for 15 min at 4 °C in a Beckman JA14 rotor. The supernatants were discarded and the pellets were frozen. After thawing, cells were resuspended in 100 ml of N1 buffer (25 mM Tris/HCl, pH 7.8, and 0.5 M NaCl) containing 20 mM imidazole, and were lysed by sonication. The lysate was centrifuged at 45000 rev./min for 1 h in a Beckman Ti55.2 rotor. The supernatant was passed down a 1 ml column of Ni-NTA–agarose that was pre-loaded with 5 ml of 50 mM NiSO4 and equilibrated in N1 buffer containing 20 mM imidazole. The column was washed with 10 ml of N1 buffer containing 50 mM imidazole and eluted with N1 buffer containing 150 mM imidazole. Elution fractions were analysed by SDS/PAGE and Western blotting using an anti-polyhistidine monoclonal antibody. In addition, FNII domain was identified by N-terminal sequencing on a Beckman LF3000 protein sequencer following transfer to PVDF membranes [35]. For further purification, 2 ml samples of FNII domain were loaded on to a 2 ml gelatin–agarose column that was pre-equilibrated in 50 mM Tris/HCl, pH 7.4. The column was washed with 10 ml of 50 mM Tris/HCl, pH 7.4, and eluted with ten 1 ml aliquots of 50 mM Tris/HCl, pH 7.4, containing 8 M urea. Urea was removed from the pure protein samples by dialysis.
Analytical ultracentrifugation
Equilibrium sedimentation experiments were carried out on purified mannose receptor FNII domain in a Beckman Optima XL-A analytical ultracentrifuge using an An55Ti rotor at 35000 rev./min and 20 °C. Equilibrium distributions from three different loading concentrations were analysed using the non-linear curve-fitting program Nonlin supplied with the instrument.
Detection of specific binding of the FNII domain to collagen using an ELISA
Before the assay, 96-well plates (Maxisorp; Nunc) were coated with collagen (100 μl of a 100 μg/ml solution in PBS) for 1 h at 37 °C. Wells were washed three times with tap water and were blocked with 200 μl of 3% (w/v) BSA in PBS for 1 h at room temperature (21 °C), followed by three further washes with water. Mannose receptor FNII domain was diluted to the desired concentration in 3% (w/v) BSA and 50 mM Tris/HCl, pH 7.4, and 100 μl aliquots were added to wells in duplicate. After incubation for 2 h at 37 °C, assay plates were washed three times with tap water. To detect specific FNII domain binding, an anti-polyhistidine monoclonal antibody was used. Antibody (100 μl of a 1:1000 dilution in PBS) was added to each well for 1 h at room temperature. Wells were washed five times with water before addition of horseradish-peroxidase-conjugated anti-mouse IgG (100 μl of a 1:5000 dilution in PBS). After incubation at room temperature for 1 h, wells were washed five times with tap water, followed by addition of 100 μl of a 1:1 mixture of H2O2 and tetramethylbenzidine. Following 30 min of incubation in the dark at room temperature, 50 μl of 2 M HCl was added to each well, and the absorbance was measured at 450 nm.
Preparation of 125I-labelled FNII domain
FNII domain (100 μg in 100 μl of 25 mM Hepes, pH 7.4) was added to Bolton and Hunter reagent (0.1 mCi, dried from benzene). After incubation at room temperature for 10 min, TBS was added to stop the reaction. Labelled protein was separated from labelling reagent on a 1 cm×18 cm Sephadex G-25 column that was pre-equilibrated in TBS.
Direct collagen-binding assays
Plastic microtitre plates with removable wells (Immulon 4; Dynex Technologies) were coated with 100 μl of collagen (100 μg/ml in PBS) at 4 °C overnight. After coating, wells were washed three times with cold wash buffer (50 mM Tris/HCl, pH 7.4) and blocked with 200 μl of 3% (w/v) BSA in wash buffer for 1 h at 4 °C. Blocked wells were washed three times with wash buffer before addition of ligand. FNII domain ligand was prepared by mixing non-radioactive domain with radio-iodinated domain (concentration ratio 100:1) in wash buffer containing 3% (w/v) BSA. Duplicate serial 2-fold dilutions were prepared and added (75 μl/well) to the collagen-coated wells. Following incubation at 4 °C for 2 h, wells were washed five times with wash buffer, dried and counted on a Wallac 1470 Wizard Gamma Counter. Data were fitted to the following equation for saturable binding superimposed on a linearly increasing background of non-specific binding:
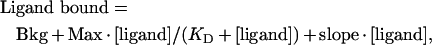 |
where Bkg is the background binding, Max is the saturation level for specific binding, slope is the linear increase in non-specific binding and KD is the concentration of ligand at which half-maximal specific saturable binding is attained [36,37].
RESULTS
Mannose-receptor-mediated adhesion of cells to collagen
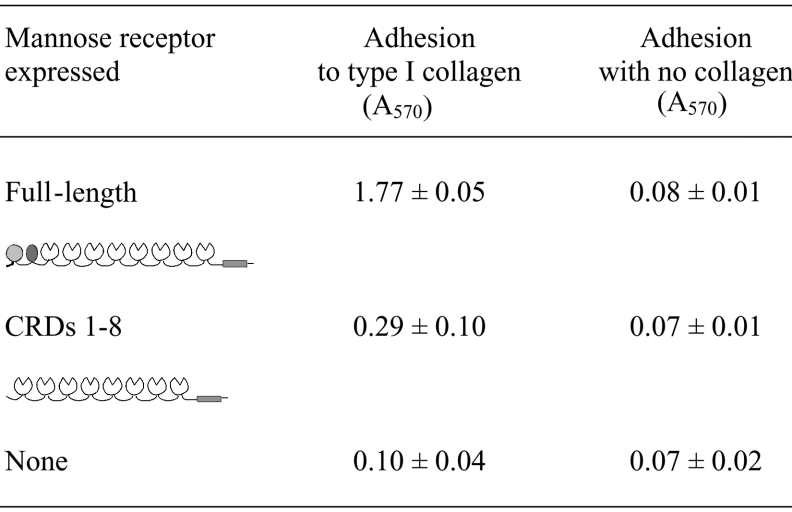
The ability of the mannose receptor to mediate adhesion to collagen was tested using stably transfected fibroblasts expressing the full-length mannose receptor or a truncated receptor consisting of the eight C-type CRDs, but lacking the R-type CRD and the FNII domain. These cell lines have been used previously to demonstrate that the two N-terminal domains are not required for mediating binding and endocytosis of mannosylated ligands. Cells that express the truncated receptor consisting of the eight C-type CRDs bind and endocytose mannosylated ligands as efficiently as cells expressing the full-length receptor [3,4]. Initially, binding of the transfected fibroblasts to type I collagen was investigated. Adherence assays were carried out in the absence of serum to eliminate adhesion to tissue culture plates mediated by fibronectin in foetal calf serum. Fibroblasts expressing the full-length mannose receptor adhered to type I collagen (Table 1). Comparison of the absorbance value obtained for these fibroblasts with a standard curve generated using cells adhering in serum indicated that essentially all of the cells adhere. In contrast, when the truncated form of the receptor lacking the cysteine-rich and FNII domains was expressed, few cells adhered to collagen. Fibroblasts transfected with empty vector did not adhere to collagen, and no adherence of any fibroblasts was seen in the absence of collagen. The results indicate that the mannose receptor can mediate adhesion of cells to type I collagen and that one or both of the two N-terminal domains are required for this activity. Similar results were obtained in the presence of EDTA (results not shown) providing confirmation that Ca2+-dependent sugar binding by the C-type CRDs of the receptor does not play a role in mediating adherence to collagen.
Table 1. Adhesion of fibroblasts expressing the mannose receptor to type I collagen.
Adherent cells were quantified using a colorimetric assay as described in the Materials and methods section. Results are means±S.D. for three experiments.
Adhesion experiments with other types of collagen indicate that the mannose receptor displays specificity for certain collagen types. Fibroblasts expressing the full-length mannose receptor adhered to type III and type IV collagens as well as to type I collagen, but not to type V collagen (Table 2). Fibroblasts expressing the truncated mannose receptor and mock-transfected cells did not adhere to any of the collagen types, and no adherence to laminin was seen with any of the transfectants (results not shown).
Table 2. Adhesion of fibroblasts expressing the full-length mannose receptor to various collagen types.
Adherent cells were quantified using a colorimetric assay as described in the Materials and methods section. Results are means±S.D. for three experiments.
| Collagen type | Adhesion (A570) |
|---|---|
| I | 1.77±0.05 |
| IV | 1.30±0.02 |
| III | 0.87±0.03 |
| V | 0.14±0.03 |
| No collagen | 0.08±0.01 |
Binding of the mannose receptor to gelatin
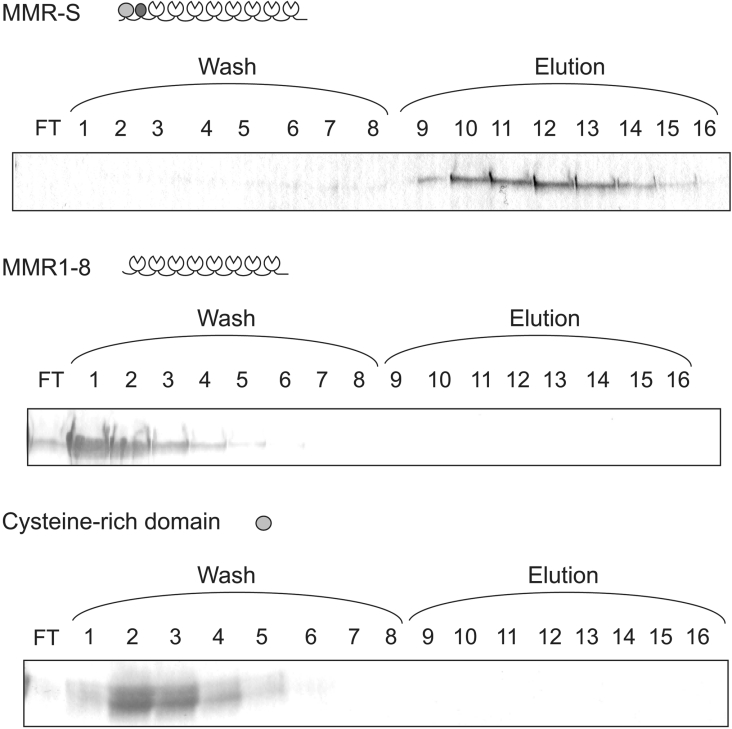
Further evidence for collagen binding by the mannose receptor was obtained by testing the ability of soluble fragments of the receptor to bind to immobilized gelatin (denatured type I collagen). A soluble fragment of the receptor consisting of the whole extracellular domain binds to a gelatin–agarose column (Figure 2, top panel). Binding appears to be relatively weak, because the protein could be eluted, albeit not very efficiently, by increasing the salt concentration in the buffer. Identical conditions were used previously to demonstrate binding of Endo180 to a gelatin column, and this protein also eluted with high salt [27]. In contrast, a soluble fragment of the mannose receptor consisting of just the eight C-type CRDs (MMR1–8) appeared in the flowthrough and wash fractions of the column eluate, indicating that this fragment of the receptor does not bind to gelatin (Figure 2, middle panel). These experiments provide a direct demonstration of the ability of the mannose receptor to bind to collagen, and, like the cell-adhesion assays, they indicate that one or both of the two N-terminal domains are required for this activity. Using the same approach, it was found that an expressed form of the mannose receptor cysteine-rich domain does not bind to the gelatin–agarose column, providing indirect evidence that the FNII domain must mediate the observed collagen-binding activity (Figure 2, bottom panel).
Figure 2. Binding of mannose receptor fragments to gelatin.
Mannose receptor fragments were loaded on to a gelatin–agarose column (2 ml). Wash and elution fractions were analysed by SDS/PAGE (15% gels for MMR-S and MMR1–8, and 17.5% for the cysteine-rich domain). Gels were stained with Coomassie Blue. FT, flowthrough.
Demonstration of collagen binding by the mannose receptor FNII domain
The FNII domain of the mannose receptor was expressed in bacteria so that its collagen-binding activity could be assessed directly. The expression construct consists of DNA encoding amino acid residues 135–193 of the mannose receptor fused to codons specifying the ompA signal sequence at the 5′ end and to codons specifying six histidine residues at the 3′ end. The ompA signal sequence directs the expressed domain to the periplasmic space where folding and correct formation of disulphide bonds occurs [34,38]. The histidine tag at the C-terminus of the domain allowed easy purification and detection of the expressed protein. The expressed domain (molecular mass 7.4 kDa) was eluted from a Ni-NTA column with 150 mM imidazole (Figure 3). N-terminal sequencing confirmed the identity of the protein and showed that the ompA signal sequence is processed correctly. Further purification to remove remaining bacterial proteins was carried out by passing material that was eluted from the Ni-NTA–agarose column through a gelatin–agarose column. All of the FNII domain that was eluted from the Ni-NTA–agarose column bound to the gelatin–agarose column, and could be eluted by addition of urea to the wash buffer (Figure 3). The isolated FNII domain appears to bind to gelatin more strongly than the whole extracellular domain of the mannose receptor, because it was not eluted with high salt concentrations, but was eluted by urea, as is the case for FNII domains from other proteins [23]. The fact that the FNII domain could be purified on gelatin–agarose confirms that it has collagen-binding activity.
Figure 3. Expression and purification of mannose receptor FNII domain.
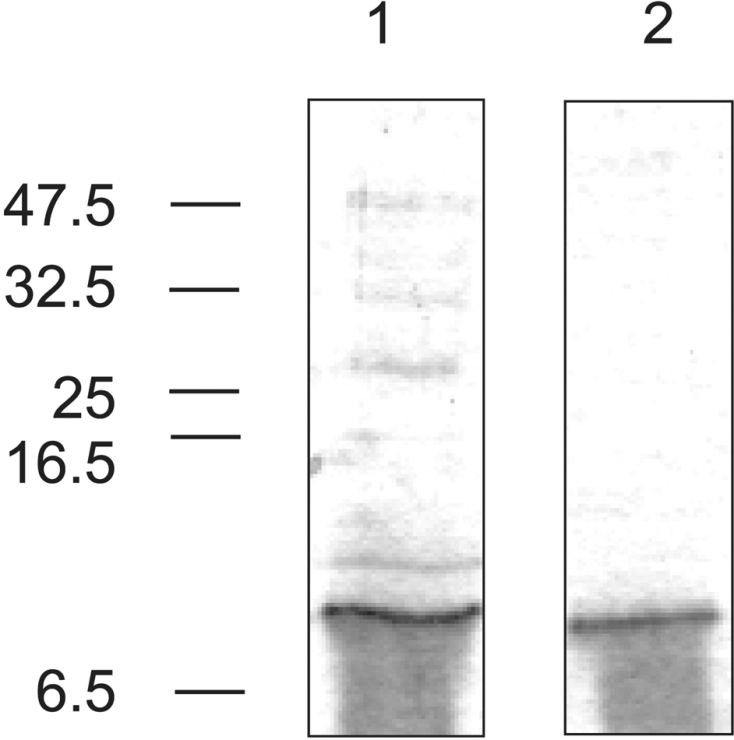
Expressed FNII domain purified by Ni-NTA–agarose affinity chromatography (lane 1) and gelatin–agarose affinity chromatography (lane 2). Gels (20% polyacrylamide) were stained with Coomassie Blue. The positions of molecular-mass markers are indicated on the left (sizes in kDa). The smearing seen under the band is a gel artefact that is often seen when running very small proteins using the Tris/glycine buffer system. N-terminal sequencing of material taken from the smear indicates that it is intact FNII domain.
Selectivity of collagen binding by the mannose receptor FNII domain
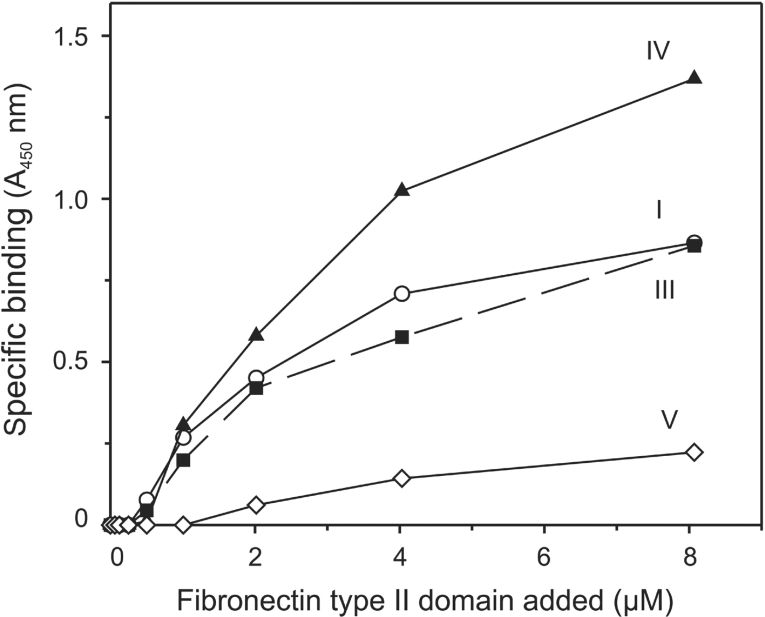
The ability of the FNII domain to bind to different types of collagen was assessed using an ELISA. Specific binding of the FNII domain to collagen-coated plates was detected with an anti-polyhistidine antibody that recognizes the histidine tag at the C-terminus of the expressed protein. The FNII domain bound well to type I, type III and type IV collagens, but very poorly to type V collagen (Figure 4). Thus the FNII domain alone shows the same specificity for collagen as the whole mannose receptor.
Figure 4. Specificity of collagen binding by mannose receptor FNII domain.
Specific binding of purified FNII domain to various collagen types detected using an ELISA. A450 values obtained in the absence of FNII domain were subtracted from those obtained in the presence of the domain to give specific binding. Results shown are representative of three experiments.
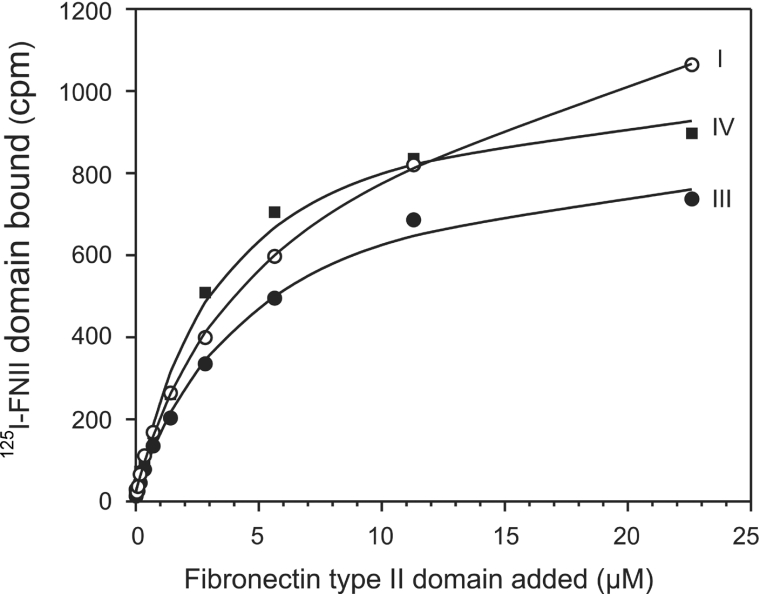
Values of KD for FNII domain binding to collagen estimated from the ELISA were in the micromolar range. However, the concentration range at which binding can be detected in this assay was not large. In order to allow more reliable determination of dissociation constants, a direct binding assay was used. The FNII domain was radio-iodinated to allow direct detection of binding to collagen. Radiolabelled domain mixed with unlabelled domain was allowed to bind to collagen-coated microtitre plates. The FNII domain showed saturable binding to collagen types I, III and IV (Figure 5). Dissociation constants obtained were (4.2±0.3)×10−6 M for type I collagen, (4.6±2.3)×10−6 M for type IV collagen and (6.0±1.4)×10−6 M for type III collagen (mean±S.D. of three determinations for type I and type IV collagens and of two determinations for type III collagen). The oligomeric state of the FNII domain was investigated in order to determine whether association of FNII domains contributed to the measured affinity for collagen. Equilibrium sedimentation analytical centrifugation gave a molecular mass of 7570±120 Da, which corresponded closely to the calculated molecular mass of 7439 Da and indicated that the FNII domain exists as a monomer. Thus the dissociation constants obtained represent those for interaction of FNII domain monomers with collagen.
Figure 5. Demonstration of saturable binding of mannose receptor FNII domain to collagen.
Results shown are representative of three assays for type I and type IV collagens and two for type III collagen.
Inhibition of mannose-receptor-mediated cell adhesion to collagen by the isolated FNII domain
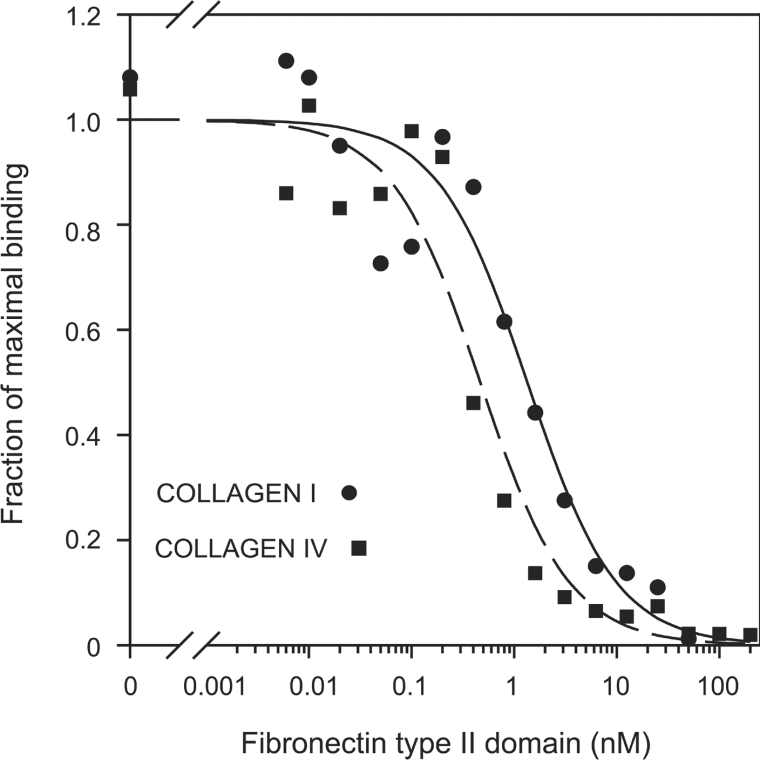
To provide further evidence that the FNII domain is predominantly responsible for mediating mannose receptor adhesion to collagen, inhibition assays were performed. Adhesion of fibroblasts expressing full-length mannose receptor to type I and type IV collagens was studied in the presence of a range of FNII domain concentrations. Mannose-receptor-mediated adhesion of fibroblasts to collagen can be completely inhibited by the FNII domain (Figure 6), confirming that collagen binding by the receptor can be attributed specifically to this domain.
Figure 6. Inhibition of mannose-receptor-mediated cell adhesion to collagen by mannose receptor FNII domain.
Adhesion of fibroblasts expressing the full-length mannose receptor to type I and type IV collagens was determined in the presence of increasing concentrations of purified FNII domain. Results shown are representative of three experiments.
DISCUSSION
The experiments described in the present study demonstrating collagen binding by the mannose receptor FNII domain provide the first information on the activity of this domain of the receptor. The receptor mediates collagen binding in addition to the Ca2+-dependent recognition of mannose and related sugars by the C-type CRDs, and recognition of sulphated N-acetylgalacto-samine by the cysteine-rich domain. Thus specific recognition functions have now been assigned to each of the three types of domain in the extracellular region of the mannose receptor.
Comparison with other collagen-binding FNII domains
Like other collagen-binding proteins with FNII domains, the mannose receptor binds gelatin. However, the specificity of the mannose receptor for native collagen types is different to specificities reported for other collagen-binding proteins containing FNII domains. Intact mannose receptor and the isolated FNII domain bind native type I, type III and type IV collagens, but not type V, while a recombinant fragment of MMP-2 containing all three FNII domains binds native type I collagen, but not native type IV collagen [39]. MMP-2 and fibronectin are thought to be able to bind native type I collagen owing to a slight relaxation of the triple helix at the collagenase cleavage site, where there is a low imino acid content leading to reduced hydrogen bonding [39]. Although the residues thought to be involved in collagen binding by the second FNII domain of MMP-2 are conserved in the mannose receptor FNII domain, there are obviously differences in the mechanism of collagen binding by these domains.
The micromolar dissociation constant for mannose receptor FNII domain binding to type I, type III and type IV collagens is similar to that for the three FNII domain constructs of MMP-2 binding to type I collagen [39], and for collagen binding by a construct consisting of the two type II domains of fibronectin [40]. However, the weaker binding of the whole extracellular domain of the mannose receptor to gelatin–agarose suggests that the binding affinity of the FNII domain may be modulated by the close proximity of the cysteine-rich domain and CRD-1 [32]. Nevertheless, the assays with transfected fibroblasts indicate that the affinity of the mannose receptor for collagen under physiological conditions is sufficient to mediate cell adhesion.
Physiological relevance of mannose receptor collagen-binding activity
The collagen-binding activity of the mannose receptor could allow it to play a role either in mediating clearance of collagen fragments or in mediating cell–matrix adhesion. The C-type CRDs of the mannose receptor bind a high-mannose oligosaccharide attached to the propeptide of type I procollagen and mediate clearance of this specific propeptide [7,41]. More general binding and clearance of fragments of type I and type III collagens by the mannose receptor would be a novel scavenging function of the receptor. Because type I and type III collagens are the major fibrillar collagens that are found in connective tissues throughout the body, scavenging of fragments of these collagens by the mannose receptor could play an important role in tissue remodelling and wound healing. However, endocytosis of collagen fragments following binding by the FNII domain remains to be demonstrated.
The fibroblast adhesion assays clearly indicate that the mannose receptor is able to mediate cell adhesion to collagen. Binding of the receptor to type IV collagen, which is the major sheet-forming collagen found in basal laminae, would be particularly important for a role of the receptor in mediating cell–matrix adhesion. Mannose-receptor-mediated adhesion to collagen could play a role in trafficking of macrophages to sites of inflammation and to lymph nodes. Expression of the mannose receptor on dendritic cells might also help facilitate their migration to lymph nodes. In the brain, where the receptor is expressed on microglial cells, astrocytes and neurons [42,43], mannose-receptor-mediated matrix adhesion might play a role in cell migration and axonal growth. It is interesting that the cysteine-rich domain of the mannose receptor has been shown to bind chondroitin sulphate residues that are found on the proteoglycans of the extracellular matrix [44]. Thus the two N-terminal domains of the mannose receptor could both play a part in promoting adhesion of macrophages and other cells expressing the mannose receptor to the extracellular matrix.
Neither of the two types of sugar-binding domain in the mannose receptor is involved in collagen binding. However, it is possible that binding of collagen by the FNII domain could modulate the binding of sugar ligands to the other domains. In addition, the mannose receptor FNII domain might have specificities for other types of ligand, as is seen for FNII domains from other proteins. Thus there may be some mannose receptor ligands that interact with each of the three types of domain in the receptor.
Comparison with other members of the mannose receptor family
Like the mannose receptor, the phospholipase A2 receptor is able to mediate adhesion of cells to type I and type IV collagens [30]. Other collagen types have not been tested, and it has not been shown conclusively that the FNII domain of the phospholipase A2 receptor mediates collagen binding. However, given the sequence similarity between the two receptors, it seems likely that collagen-binding activity is mediated by the FNII domain of the phospholipase A2 receptor as well as by that of the mannose receptor. As is the case with the mannose receptor, collagen is not the only ligand for the phospholipase A2 receptor, which mediates endocytosis of secretory phospholipases following binding of these enzymes by the domains related in sequence to C-type CRDs [30].
In contrast, collagen binding is likely to be the major function of Endo180. Endo180 binds to collagens type I, II, IV and V and can mediate endocytosis of collagen as well as adhesion of cells to collagen [26–29]. Targeted deletion of exons 2–6 of the Endo180 gene in mice, which results in expression of a truncated receptor lacking the cysteine-rich domain, the FNII domain and the first C-type CRD, causes defects in collagen internalization by fibroblasts as well as impairment of fibroblast adhesion and migration on collagen [28,29]. Although Endo180 can bind glycoconjugates through recognition of sugars by the second C-type CRD [33], unlike the case for the mannose receptor, no physiological glycoprotein ligands for Endo180 have been identified.
No binding activity has been demonstrated for the fourth member of the mannose receptor family, DEC-205, which is thought to play a role in antigen uptake by dendritic cells [45]. Neither the N-terminal cysteine-rich domain nor the domains related in sequence to C-type CRDs in DEC-205 contain the residues necessary for sugar binding, indicating that this protein is unlikely to bind glycoproteins [12]. However, it is possible that the FNII domain will bind collagen. It is interesting that three of the four members of the mannose receptor family are now known to share one activity, i.e. binding of collagens. Although the sugar-binding activities of the C-type CRDs and the cysteine-rich domain are not conserved throughout the family, collagen binding by the FNII domain may be. Thus it is plausible that collagen binding represents the primordial function of this family of receptors.
Acknowledgments
This work was supported by grants 041845 and 075565 from the Wellcome Trust.
References
- 1.Weis W. I., Taylor M. E., Drickamer K. The C-type lectin superfamily in the immune system. Immunol. Rev. 1998;163:19–34. doi: 10.1111/j.1600-065x.1998.tb01185.x. [DOI] [PubMed] [Google Scholar]
- 2.Taylor M. E., Conary J. T., Lennarz M. R., Stahl P. D., Drickamer K. Primary structure of the mannose receptor contains multiple motifs resembling carbohydrate-recognition domains. J. Biol. Chem. 1990;265:12156–12162. [PubMed] [Google Scholar]
- 3.Taylor M. E., Bezouska K., Drickamer K. Contribution to ligand binding by multiple carbohydrate-recognition domains in the macrophage mannose receptor. J. Biol. Chem. 1992;267:1719–1726. [PubMed] [Google Scholar]
- 4.Taylor M. E., Drickamer K. Structural requirements for high affinity binding of complex ligands by the macrophage mannose receptor. J. Biol. Chem. 1993;268:399–404. [PubMed] [Google Scholar]
- 5.Ezekowitz R. A. B., Williams D. J., Koziel H., Armstrong M. Y. K., Warner A., Richards F. F., Rose R. M. Uptake of Pneumocystis carinii mediated by the macrophage mannose receptor. Nature (London) 1991;351:155–158. doi: 10.1038/351155a0. [DOI] [PubMed] [Google Scholar]
- 6.Otter M., Barrett-Bergshoeff M. M., Rijken D. C. Binding of tissue-type plasminogen activator by the mannose receptor. J. Biol. Chem. 1991;266:13931–13935. [PubMed] [Google Scholar]
- 7.Lee S. J., Evers S., Roeder D., Parlow A. F., Risteli J., Risteli L., Lee Y. C., Feizi T., Langen H., Nussenzweig M. C. Mannose receptor-mediated regulation of serum glycoprotein homeostasis. Science. 2002;295:1898–1901. doi: 10.1126/science.1069540. [DOI] [PubMed] [Google Scholar]
- 8.Fiete D., Beranek M. C., Baenziger J. U. A cysteine-rich domain of the “mannose” receptor mediates GalNAc-4-SO4 binding. Proc. Natl. Acad. Sci. U.S.A. 1998;95:2089–2093. doi: 10.1073/pnas.95.5.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y., Chirino A. J., Leteux C., Feizi T., Misulovin Z., Nussenzweig M. C., Bjorkman P. J. Crystal structure of the cysteine-rich domain of mannose receptor complexed with a sulfated carbohydrate ligand. J. Exp. Med. 2000;191:1105–1115. doi: 10.1084/jem.191.7.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiete D., Baenziger J. U. Isolation of the SO4-4-GalNAcβ1,4GlcNAcβ1,2Manα-specific receptor from rat liver. J. Biol. Chem. 1997;272:14629–14637. doi: 10.1074/jbc.272.23.14629. [DOI] [PubMed] [Google Scholar]
- 11.Fiete D., Beranek M. C., Baenziger J. U. The macrophage/endothelial cell mannose receptor cDNA encodes a protein that binds oligosaccharides terminating with SO4-4-GalNAcβ1,4GlcNAcβ or Man at independent sites. Proc. Natl. Acad. Sci. U.S.A. 1997;94:11256–11261. doi: 10.1073/pnas.94.21.11256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor M. E. Evolution of a family of receptors containing multiple C-type carbohydrate-recognition domains. Glycobiology. 1997;7:v–viii. doi: 10.1093/glycob/7.3.323. [DOI] [PubMed] [Google Scholar]
- 13.Collier I. E., Wilhelm S. M., Eisen A. Z., Marmer B. L., Grant G. A., Seltzer J. L., Kronberger A., He C. S., Bauer E. A., Goldberg G. I. H-ras oncogene-transformed human bronchial epithelial cells (TBE-1) secrete a single metalloprotease capable of degrading basement membrane collagen. J. Biol. Chem. 1988;263:6579–6587. [PubMed] [Google Scholar]
- 14.Wilhelm S. M., Collier I. E., Marmer B. L., Eisen A. Z., Grant G. A., Goldberg G. I. The SV40 transformed human lung fibroblasts secrete a 92-kDa type IV collagenase which is identical to that secreted by normal human macrophages. J. Biol. Chem. 1989;264:17213–17221. [PubMed] [Google Scholar]
- 15.Cool D. E., Edgell C. J., Louie G. V., Zoller M. J., Brayer G. D., MacGillivray R. T. Characterization of human blood coagulation factor XII cDNA: prediction of the primary structure of factor XII and the tertiary structure of β-factor XIIa. J. Biol. Chem. 1985;260:13666–13676. [PubMed] [Google Scholar]
- 16.Lobel P., Dahms N. M., Kornfeld S. Cloning and sequence analysis of the cation-independent mannose 6-phosphate receptor. J. Biol. Chem. 1988;263:2563–2570. [PubMed] [Google Scholar]
- 17.Esch F. S., Ling N. C., Bohlen P., Ying S. Y., Guillemin R. Primary structure of PDC-1-9, a major protein constituent of bovine seminal plasma. Biochem. Biophys. Res. Commun. 1983;113:861–867. doi: 10.1016/0006-291x(83)91078-1. [DOI] [PubMed] [Google Scholar]
- 18.Kornblihtt A. R., Umezawa K., Vibe-Pedersen K., Baralle F. E. Primary structure of human fibronectin: differential splicing may generate at least 10 polypeptides from a single gene. EMBO J. 1985;4:1755–1759. doi: 10.1002/j.1460-2075.1985.tb03847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guidry C., Miller E. J., Hook M. A second fibronectin-binding region is present in collagen α chains. J. Biol. Chem. 1990;265:19230–19236. [PubMed] [Google Scholar]
- 20.Banyai L., Tordai H., Patthy L. The gelatin-binding site of human 72 kDa type IV collagenase (gelatinase A) Biochem. J. 1994;298:403–407. doi: 10.1042/bj2980403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collier I. E., Krasnov P. A., Strongin A. Y., Birkedal-Hansen H., Goldberg G. I. Alanine scanning mutagenesis and functional analysis of the fibronectin-like collagen-binding domain from human 92-kDa type IV collagenase. J. Biol. Chem. 1992;267:6776–6781. [PubMed] [Google Scholar]
- 22.Desnoyers L., Manjunath P. Major proteins of bovine seminal plasma exhibit novel interactions with phospholipid. J. Biol. Chem. 1992;267:10149–10155. [PubMed] [Google Scholar]
- 23.Briknarova K., Grishaev A., Banyai L., Tordai H., Patthy L., Llinas M. The second type II module from human matrix metalloproteinase: structure, function and dynamics. Structure. 1999;7:1235–1245. doi: 10.1016/s0969-2126(00)80057-x. [DOI] [PubMed] [Google Scholar]
- 24.Tordai H., Patthy L. The gelatin-binding site of the second type-II domain of gelatinase A/MMP-2. Eur. J. Biochem. 1999;259:513–518. doi: 10.1046/j.1432-1327.1999.00070.x. [DOI] [PubMed] [Google Scholar]
- 25.Morgunova E., Tuuttila A., Bergmann U., Isupov M., Lindquist Y., Schnieder G., Tryggvason K. Structure of human pro-matrix metalloproteinase-2: activation mechanism revealed. Science. 1999;284:1667–1670. doi: 10.1126/science.284.5420.1667. [DOI] [PubMed] [Google Scholar]
- 26.Behrendt N., Jensen O. N., Engelholm L. H., Mortz E., Mann M., Dane K. A urokinase receptor-associated protein with specific collagen binding properties. J. Biol. Chem. 2000;275:1993–2002. doi: 10.1074/jbc.275.3.1993. [DOI] [PubMed] [Google Scholar]
- 27.Wienke D., MacFadyen J. R., Isacke C. M. Identification and characterization of the endocytic transmembrane glycoprotein Endo180 as a novel collagen receptor. Mol. Biol. Cell. 2003;14:3592–3604. doi: 10.1091/mbc.E02-12-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engelholm L. H., List K., Netzel-Arnett S., Cukierman E., Mitola D. J., Aaronson H., Kjoller L., Larsen J. K., Yamada K. M., Strickland D. K., et al. uPARAP/Endo180 is essential for cellular uptake of collagen and promotes fibroblast collagen adhesion. J. Cell Biol. 2003;160:1009–1015. doi: 10.1083/jcb.200211091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.East L., McCarthy A., Wienke D., Sturge J., Ashworth A., Isacke C. M. A targeted deletion in the endocytic receptor gene Endo180 results in a defect in collagen uptake. EMBO Rep. 2003;4:710–716. doi: 10.1038/sj.embor.embor882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ancian P., Lambeau G., Lazdunski M. Multifunctional activity of the extracellular domain of the M-type (180 kDa) membrane receptor for secretory phospholipases A2. Biochemistry. 1995;34:13146–13151. doi: 10.1021/bi00040a028. [DOI] [PubMed] [Google Scholar]
- 31.Simpson D. Z., Hitchen P. G., Elmhirst E. L., Taylor M. E. Multiple interactions between pituitary hormones and the mannose receptor. Biochem. J. 1999;343:403–411. [PMC free article] [PubMed] [Google Scholar]
- 32.Napper C. E., Dyson M. H., Taylor M. E. An extended conformation of the macrophage mannose receptor. J. Biol. Chem. 2001;276:14759–14766. doi: 10.1074/jbc.M100425200. [DOI] [PubMed] [Google Scholar]
- 33.East L., Rushton S., Taylor M. E., Isacke C. M. Characterization of sugar binding by the mannose receptor family member, Endo-180. J. Biol. Chem. 2002;277:50469–50475. doi: 10.1074/jbc.M208985200. [DOI] [PubMed] [Google Scholar]
- 34.Ghrayeb J., Kimura K., Takahara M., Hsuing H., Masui Y., Inouye M. Secretion cloning vectors in Escherichia coli. EMBO J. 1984;3:2437–2442. doi: 10.1002/j.1460-2075.1984.tb02151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsudaira P. Sequence from picomole quantities of protein electroblotted onto polvinylidene difluoride membranes. J. Biol. Chem. 1987;262:10035–10038. [PubMed] [Google Scholar]
- 36.Levitzki A. Ligand binding. In: Creighton T. E., editor. Protein Function: a Practical Approach. New York: Oxford University Press; 1997. pp. 101–129. [Google Scholar]
- 37.Iobst S. T., Wormald M. R., Weis W. I., Dwek R. A., Drickamer K. Binding of sugar ligands to Ca2+-dependent animal lectins. I. Analysis of mannose binding by site-directed mutagenesis and NMR. J. Biol. Chem. 1994;269:15505–15511. [PubMed] [Google Scholar]
- 38.Pines O., Inouye M. Expression and secretion of proteins in E. coli. Mol. Biotechnol. 1999;12:25–34. doi: 10.1385/MB:12:1:25. [DOI] [PubMed] [Google Scholar]
- 39.Steffensen B., Wallon U. M., Overall C. M. Extracellular matrix binding properties of recombinant fibronectin type II-like modules of human 72-kDa gelatinase/type IV collagenase: high affinity binding to native type I collagen but not native type IV collagen. J. Biol. Chem. 1995;270:11555–11566. doi: 10.1074/jbc.270.19.11555. [DOI] [PubMed] [Google Scholar]
- 40.Pickford A. R., Smith S. P., Staunton D., Boyd J., Campbell I. D. The hairpin structure of the 6F11F22F2 fragment from human fibronectin enhances gelatin binding. EMBO J. 2001;20:1519–1529. doi: 10.1093/emboj/20.7.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smedsrod B., Melkko J., Risteli L., Risteli J. Circulating C-terminal propeptide of type I procollagen is cleared mainly via the mannose receptor in liver endothelial cells. Biochem. J. 1990;271:345–350. doi: 10.1042/bj2710345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Linehan S. A., Martinez-Pomares L., Stahl P. D., Gordon S. Mannose receptor and its putative ligands in normal murine lymphoid and nonlymphoid organs: in situ expression of mannose receptor by selected macrophages, endothelial cells, perivascular microglia, and mesangial cells, but not dendritic cells. J. Exp. Med. 1999;189:1961–1972. doi: 10.1084/jem.189.12.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burudi E. M. E., Regnier-Vigouroux A. Regional and cellular expression of the mannose receptor in the post-natal developing mouse brain. Cell Tissue Res. 2001;303:307–317. doi: 10.1007/s004410000311. [DOI] [PubMed] [Google Scholar]
- 44.Leteux C., Chai W., Loveless R. W., Yuen C.-T., Uhlin-Hansen L., Combarnous Y., Jankovic M., Maric S. C., Misulovin Z., Nussenzweig M. C., Feizi T. The cysteine-rich domain of the macrophage mannose receptor is a multispecific lectin that recognizes chondroitin sulfates A and B and sulfated oligosaccharides of blood group Lewisa and Lewisx types in addition to sulfated N-glycans of lutropin. J. Exp. Med. 2000;191:1117–1126. doi: 10.1084/jem.191.7.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang W., Swiggard W. J., Heufler C., Peng M., Mirza A., Steinman R. M., Nussenzweig M. C. The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature (London) 1995;375:151–155. doi: 10.1038/375151a0. [DOI] [PubMed] [Google Scholar]