Abstract
The PABP [poly(A)-binding protein] is able to interact with the 3′ poly(A) tail of eukaryotic mRNA, promoting its translation. Cleavage of PABP by viral proteases encoded by several picornaviruses and caliciviruses plays a role in the abrogation of cellular protein synthesis. We report that infection of MT-2 cells with HIV-1 leads to efficient proteolysis of PABP. Analysis of PABP integrity was carried out in BHK-21 (baby-hamster kidney) and COS-7 cells upon individual expression of the protease from several members of the Retroviridae family, e.g. MoMLV (Moloney murine leukaemia virus), MMTV (mouse mammary tumour virus), HTLV-I (human T-cell leukaemia virus type I), SIV (simian immunodeficiency virus), HIV-1 and HIV-2. Moreover, protease activity against PABP was tested in a HeLa-cell-free system. Only MMTV, HIV-1 and HIV-2 proteases were able to cleave PABP in the absence of other viral proteins. Purified HIV-1 and HIV-2 proteases cleave PABP1 directly at positions 237 and 477, separating the two first RNA-recognition motifs from the C-terminal domain of PABP. An additional cleavage site located at position 410 was detected for HIV-2 protease. These findings indicate that some retroviruses may share with picornaviruses and caliciviruses the capacity to proteolyse PABP.
Keywords: eukaryotic initiation factor (eIF), HIV, poly(A)-binding protein (PABP), protease, translation
Abbreviations: 2Apro, 2A protease; 3Cpro, 3C protease; BHK-21, baby-hamster kidney; CA, capsid protein; DMEM, Dulbecco's modified Eagle's Medium; dpi, days post-infection; eIF, eukaryotic initiation factor; eRF, eukaryotic release factor; GST, glutathione S-transferase; hpe, hours post-electroporation; hpt, hours post-transfection; HTLV-I, human T-cell leukaemia virus type I; IRES, internal ribosome entry site; MBP, maltose-binding protein; MMTV, mouse mammary tumour virus; MOI, multiplicity of infection; MoMLV, Moloney murine leukaemia virus; NSP3, non-structural protein 3; PABP, poly(A)-binding protein; Paip, PABP-interacting protein; pfu, plaque-forming units; RRM, RNA-recognition motif; SIV, simian immunodeficiency virus; SQ, saquinavir; SV, Sindbis virus; SV-2A, SV that expresses poliovirus 2Apro; SV-PR, SV that expresses the HIV-1 protease in the absence of other HIV-1 proteins; UTR, untranslated region
INTRODUCTION
The initiation of translation is a multistep process, being a major regulatory target for translational control in animal-virus-infected cells. In the early steps of translation, the 5′ cap structure of the mRNA is recognized by the eIF4F (eukaryotic initiation factor 4F) complex. eIF4F also binds to the small ribosome subunit by its interaction with eIF3 forming the 48 S complex. Then, the small ribosomal subunit migrates along the 5′-UTR (untranslated region) until an AUG initiation codon is encountered in a favourable context. The eIF4F complex is composed of three polypeptides: the cap-binding protein eIF4E, the ATP-dependent RNA helicase eIF4A and the scaffolding protein eIF4G [1]. Another motif recognized by the translation machinery in the mRNA is the poly(A) tail, which is achieved by means of the PABP [poly(A)-binding protein]. In addition, PABP interacts with the N-terminal domain of eIF4G, promoting the circularization of the mRNA [2,3]. The eIF4G–PABP interaction may induce several changes in the translation initiation complex that increase the affinity of eIF4E for the cap structure [4]. In this regard, simultaneous interactions between the 5′ cap structure and the 3′ poly(A) tail of the mRNA synergistically stimulate translation, both in vitro and in vivo [5,6]. In human cells, there are three types of PABPs, with several isoforms encoded by different genes and with different localizations and functions: cytoplasmic PABPs (PABPC group), nuclear PABPs (PABPN group) and X-linked PABPs. Cytoplasmic PABPs are highly conserved proteins that contain two domains linked by an unstructured region that is rich in proline and methionine residues: an N-terminal domain with four conserved RRMs (RNA-recognition motifs) and a C-terminal helical domain that is not required for RNA recognition, but is essential for PABP oligomerization and for the interaction with several regulatory proteins that are implicated in deadenylation of the poly(A) tail and the initiation and termination of translation [7,8]. PABP interacts with translation initiation factors such as eIF4G and eIF4B [5,9,10], with eRF3 (eukaryotic release factor 3) [11–13], and with two regulatory proteins termed Paip1 and Paip2 (PABP-interacting proteins 1 and 2), which are implicated in the regulation of translation [14,15].
Animal viruses have evolved mechanisms to manipulate the host's translational machinery in order to maximize efficient viral mRNA translation and facilitate the selective translation of viral mRNA. For example, in picornavirus-infected cells, the proteolytic cleavage of eIF4G by rhinovirus or poliovirus 2Apro (2A protease) or by the aphtovirus protease L inhibits translation of capped cellular mRNAs [16]. In contrast, translation of the uncapped picornavirus RNA that occurs by a cap-independent mechanism involving the IRES (internal ribosome entry site) is not affected by eIF4G hydrolysis. This mechanism is not exclusive to some picornaviruses. Retroviral genomic RNAs are capped at their 5′ ends and contain a 3′ poly(A) tail. However, retroviral RNAs have a relatively long 5′-UTR, and the presence of stable secondary structures between the cap and the initiation codon has been proposed to strongly interfere with the scanning mechanism that operates during the initiation of translation [17]. Interestingly, IRES elements have been found in several retroviruses, including HIV-1, and their effects promoting cap-independent translation of retroviral genomic RNAs have been demonstrated [18]. In addition, recent reports have shown that eIF4G is cleaved by the proteases of several members of the family Retroviridae, including MMTV (mouse mammary tumour virus), MoMLV (Moloney murine leukaemia virus), HTLV-I (human T-cell leukaemia virus type I), SIV (simian immunodeficiency virus), HIV-1 and HIV-2 [19,20]. The hydrolysis of eIF4G leads to the inhibition of cap-dependent translation without affecting IRES-driven protein synthesis [19,20].
PABP plays an important role in translation, and it is not surprising that certain viruses have developed mechanisms to target this protein to abrogate cellular translation. Rotavirus NSP3 (nonstructural protein 3) interacts with the N-terminal region of eIF4G, replacing PABP. This interaction leads to the shut-off of cellular protein synthesis, whereas viral mRNA translation remains unaffected by circularization via NSP3 interaction with the 3′-UTR of viral transcripts [21]. Several reports have shown that PABP is cleaved by 2Apro and 3Cpro (3C protease) during enterovirus infection [22–24]. Poliovirus 2Apro bisects PABP, while poliovirus 3Cpro cleaves this factor at three sites separating its C-terminal and N-terminal domains. It has been proposed that the removal of the C-terminal domain inhibits cellular translation without affecting binding of the N-terminal domain of PABP to the poly(A) tail and eIF4G [25]. A similar strategy has recently been described in caliciviruses, in which the 3C-like protease, like poliovirus 3Cpro, cleaves PABP during infection [26]. In the present paper, we provide evidence showing that retroviral proteases such as those from MMTV, HIV-1 and HIV-2 are able to hydrolyse PABP.
EXPERIMENTAL
Plasmids and in vitro transcription
The plasmids pT7SV-HIV-1PR and pT7SV-2Apro and pT7SVwt were described previously [27].
The pTM1-derived plasmids containing the protease-coding regions of several retroviruses were described in detail earlier [19,20]. The pTM1-Luc plasmid, which contains the luciferase gene, and the plasmid pTM1-2Apro have also been described previously [28,29].
The plasmid pGEX-2T-PABP1 containing the sequence encoding the human PABP1, lacking the first nine amino acids and fused to the GST (glutathione S-transferase) gene, was obtained as described previously [30] and was kindly provided by Dr Amelia Nieto, Centro Nacional de Biotecnología, CSIC, Madrid, Spain.
Capped genomic SV (Sindbis virus) mRNAs were synthesized in vitro using the T7 RNA polymerase kit (Promega). Plasmids used as DNA templates in these assays were linearized with XhoI.
Cell culture, virus infection and transfection
MT-2 cells were grown in RPMI 1640 containing 10% (v/v) foetal calf serum. MT-2 cells were infected with HIV-1 (NL3.4 strain) using an MOI (multiplicity of infection) of approx. 1 pfu (plaque-forming unit)/cell, and, at 3 dpi (days post-infection), cells were lysed in sample buffer as described previously [29]. HIV-1-infected cells were provided by Dr Balbino Alarcón, Centro de Biología Molecular “Severo Ochoa”, CSIC-UAM, Madrid, Spain.
BHK-21 (baby-hamster kidney) cells were electroporated with 30 μg of recombinant SV genomic RNAs in final volume of 50 μl as described in [27]. Electroporated cells were grown in DMEM (Dulbecco's modified Eagle's medium) containing 10% (v/v) foetal calf serum and non-essential amino acids, and, at 8 hpe (hours post-electroporation), cells were lysed in sample buffer.
COS-7 cells were grown in DMEM containing 10% (v/v) foetal calf serum and non-essential amino acids. Coupled infection/DNA transfection of COS-7 cells with recombinant vaccinia virus (vvT7) and pTM1-derived plasmids have been described in detail previously [28]. Transfection efficiencies were determined by immunofluorescence using a luciferase antiserum (Promega) after transfecting the cells with the plasmid pTM1-Luc as described previously [19].
Western blotting
Proteins were transferred on to a 0.45-μm-pore-size nitrocellulose membrane (Bio-Rad) for Western blot analysis. Western blots were developed with the following antibodies: mouse monoclonal anti-PABP antibody (Abcam) at a 1:250 dilution; eIF4GI antisera raised against peptides derived from the N- and C-terminal regions of the human eIF4GI [28] at a 1:1000 dilution; rabbit antisera raised against the C-terminal region of eIF4GII (a gift from Professor Nahum Sonenberg, McGill University, Montréal, Québec, Canada) at 1:500 dilution; hybridoma supernatant of a monoclonal antibody against eIF4A factor at a 1:50 dilution (a gift from Professor Dr Hans Trachsel, Institute for Biochemistry and Molecular Biology, University of Berne, Berne, Switzerland) and mouse ascites of a monoclonal antibody against HIV-1 p24 antigen (Centralised Facility for AIDS Reagents, NIBSC, Potters Bar, Herts., U.K.) was used at a 1:100 dilution. Goat anti-(rabbit IgG) antibody coupled to peroxidase and goat anti-(mouse IgG) antibody coupled to peroxidase (Pierce) were used at a 1:10000 dilution.
Protein purification
HIV-1 protease was purchased from the Centralised Facility for AIDS Reagents. Purified HIV-2 protease [31] was obtained through the NIH (National Institutes of Health) AIDS Research and Reference Reagent Program, Division of AIDS, NIAID (National Institute of Allergy and Infectious Diseases), NIH, Bethesda, MD, U.S.A., from Dr Bret Shirley and Mr Michael Cappola (Boehringer Ingelheim Pharmaceuticals). MoMLV protease was expressed and purified as described previously [32]. The chimaeric MBP (maltose-binding protein)–2Apro was purified by affinity chromatography, as described previously [33]. The pGEX-2T-PABP1 plasmid was used to purify the GST–PABP1 fusion protein by affinity chromatography, using a glutathione–agarose 4B resin (Amersham Biosciences) as described previously [30].
Protease cleavage assays
To detect PABP processing by HIV proteases in cell-free systems, crude HeLa S10 extracts were incubated with 2.5 ng/μl of recombinant HIV-1 protease, HIV-2 protease or MBP–2Apro, and, at the indicated times, reactions were stopped by adding sample buffer. Lysates were analysed by SDS/PAGE and Western blotting. Degradation kinetics of translation factors were determined by densitometric scanning of the protein band corresponding to each factor analysed using a GS-710 calibrated Imagin Densitometer (Bio-Rad). In order to map the cleavage sites of HIV-1 protease and HIV-2 protease on PABP, 50 μg of recombinant GST–PABP1 protein were incubated with 1 μg of recombinant HIV-1 protease or HIV-2 protease in a total volume of 200 μl for 3 h at 30 °C in a buffer containing 50 mM sodium phosphate, pH 6.0, 25 mM NaCl, 5 mM EDTA and 1 mM DTT. Cleavage products were separated by SDS/PAGE, transferred to an Immobilon PVDF membrane (Bio-Rad) and then subjected to automated Edman degradation with an Applied Biosystems Procise Sequencer in the Proteomics Service of Centro de Investigaciones Biológicas CSIC.
RESULTS
Cleavage of PABP in HIV-1-infected cells
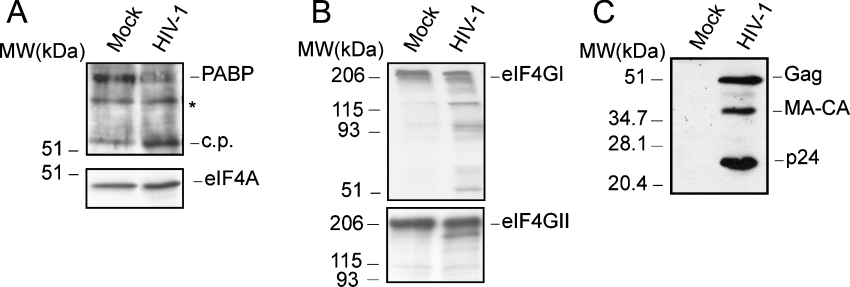
The hydrolysis of both PABP and eIF4G could contribute to the drastic shut-off of host translation in poliovirus-infected cells. In order to investigate the susceptibility of PABP to proteolysis after HIV-1 infection, MT-2 cells were infected with HIV-1 at a multiplicity of approx. 1 pfu/cell. The integrity of PABP was assessed by Western blotting at 3 dpi using PABP antiserum (Figure 1A, upper panel). Proteolytic cleavage of PABP was clearly apparent in MT-2 cells infected with HIV-1. Thus intact PABP decreased considerably, while a smaller immunoreactive protein band with a molecular mass of approx. 53 kDa appeared. To analyse the integrity of other initiation factors, these samples were blotted using antibodies against eIF4GI, eIF4GII and eIF4A (Figure 1). The amount of eIF4A remained constant after infection, indicating that this protein was not modified by HIV-1 and that similar amounts of proteins were loaded in all cases (Figure 1A, lower panel). Cleavage of eIF4GI was detected in MT-2 infected-cells, using antibodies that recognize epitopes at the N-terminal or C-terminal regions of the initiation factor. (Figure 1B). Hydrolysis of eIF4GI was estimated to be approx. 50–60%. In agreement with previous reports, eIF4GII was much less affected by HIV-1 infection [20]. Finally, the activity of HIV-1 protease in infected cells was also tested by Western blotting using p24 antiserum (Figure 1C). The Gag (p55) precursor was processed to CA-MA (capsid-matrix intermediate product) and to CA (capsid protein; p24) by HIV-1 protease.
Figure 1. PABP cleavage in HIV-1-infected cells.
(A) MT-2 cells were infected with HIV-1 (at an MOI of 1). At 3 dpi, the integrity of PABP was determined by Western blotting (upper panel; 10% gel). The corresponding Western blot against eIF4A is shown in the lower panel (10% gel). Mock, mock-infected cells; HIV-1, cells infected with HIV-1; c.p., cleavage product derived from PABP in infected cells; MW, molecular mass (sizes given in kDa). An unknown anti-PABP-reactive band is also denoted by an asterisk. (B) Western blots against eIF4GI (upper panel) and eIF4GII (lower panel) at 3 dpi (7.5% gel). (C) Western blot against the HIV-1 CA, p24 at 3 dpi (15% gel). The position of the intact initiation factors is also indicated in the respective panel.
HIV-1 protease cleaves PABP
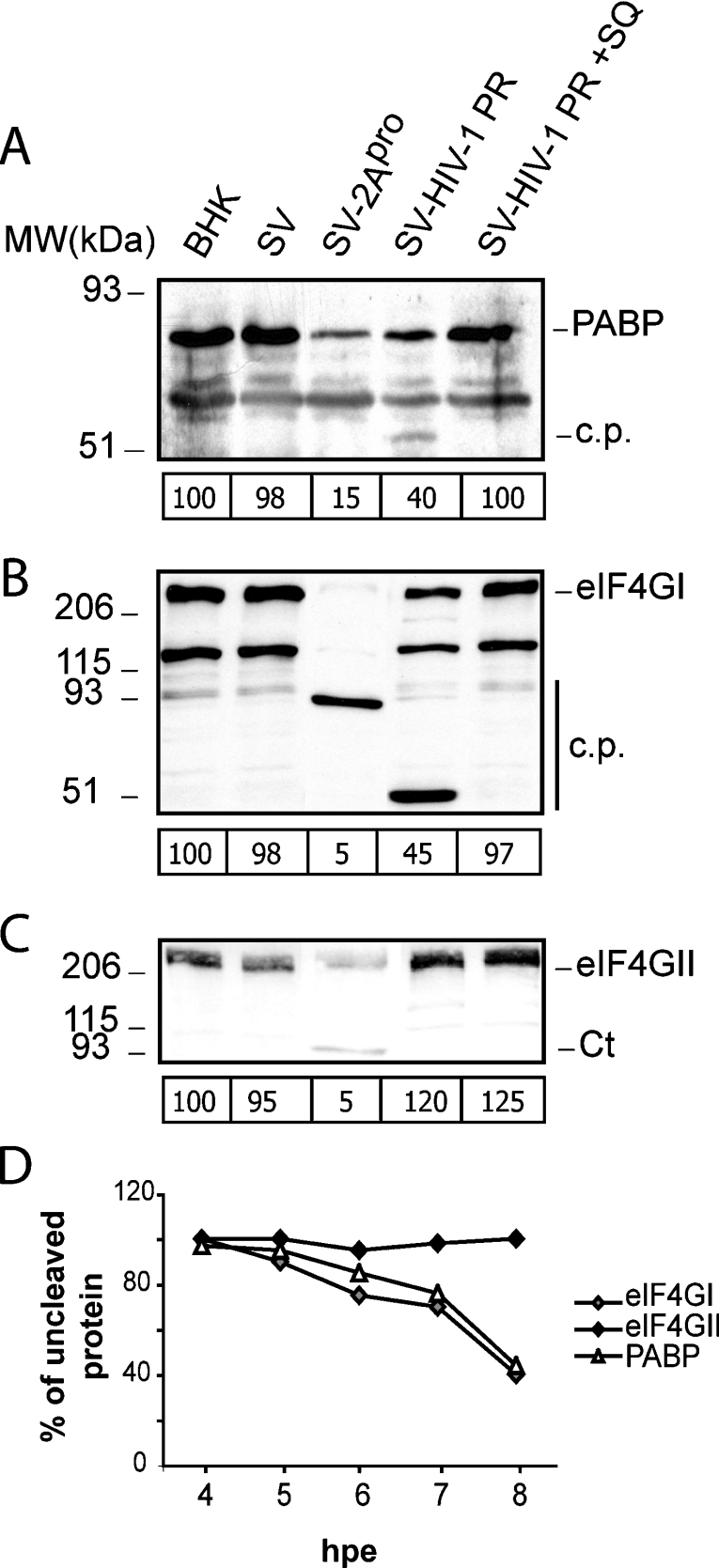
Next, the ability of HIV-1 protease to cleave PABP was tested when this protease was expressed alone in culture cells. For this purpose, the HIV-1 protease-coding region within the pol gene was placed under the control of a duplicated late promoter in a SV construct in order to generate a recombinant SV that expresses the HIV-1 protease in the absence of other HIV-1 proteins (SV-PR) [27]. In addition, the poliovirus 2A gene was cloned using a similar strategy to generate a recombinant SV that expresses poliovirus 2Apro (SV-2A) [27]. The transcribed RNAs corresponding to wild-type SV, SV-PR and SV-2A were electroporated in BHK-21 cells, and the integrity of PABP was examined at 8 hpe (Figure 2A). The expression of HIV-1 protease led to over 60% cleavage of PABP, a result similar to that found after expression of poliovirus 2Apro. A polypeptide of approx. 53 kDa, which could be a proteolytic product of PABP, was detected in cells electroporated with SV-PR RNA. This band has only been detected sporadically, and in low abundance, in overexposed immunoblots, but it is identical in size with those observed in HIV-1-infected cells. Moreover, cleavage products were not detected in cells expressing the poliovirus 2Apro, most probably due to the small amount of proteolytic products which are not well detected by the monoclonal antibody against PABP. In this regard, in a previous report, PABP cleavage products could not be detected using this antibody either in poliovirus-infected cells or in cell-free systems [22]. The decrease of PABP induced by expression of HIV-1 protease was abolished when 12 μM SQ (saquinavir), a specific inhibitor of the HIV protease, was present (Figure 2A). Furthermore, the 53 kDa cleavage product was not detectable in SV-PR-expressing cells when SQ was present (Figure 2A). Cell extracts were analysed further to determine the cleavage of eIF4GI and eIF4GII (Figures 2B and 2C). Previous findings revealed the presence of two forms of eIF4G of 220 and 150 kDa in BHK-21 cells [27,28]. These different eIF4G forms may be the result of post-translational modifications. The 150 kDa product may be a breakdown product of eIF4GI. Both polypeptides of 220 and 150 kDa are absent from cells that express 2Apro and HIV-1 protease. In agreement with our results reported previously, the expression of HIV-1 protease induces an effective cleavage of eIF4GI, while eIF4GII remained intact (Figures 2B and 2C) [19,20]. The kinetics of the hydrolysis of PABP, eIF4GI and eIF4GII by HIV-1 protease were analysed after electroporation of BHK-21 cells (Figure 2D). The cleavage of PABP by HIV-1 protease was similar to that observed with eIF4GI proteolysis. In addition, cleavage of eIF4GII by HIV-1 protease was very inefficient in this assay.
Figure 2. Cleavage of PABP in cells electroporated with recombinant SVs that express heterologous proteases.
(A) Cells were electroporated with transcription buffer (BHK), or wild-type SV (SV), SV-PR or SV-2A RNA and were incubated in the presence or absence of 12 μM SQ. At 8 hpe, cell extracts were analysed by Western blotting using specific antibodies against human PABP1 (10% gel). c.p., cleavage product derived from PABP in SV-PR-infected cells; MW, molecular mass (sizes given in kDa). The relative amount of intact PABP for each transfection experiment is indicated below each corresponding lane. (B) Detection of eIF4GI cleavage products by Western blotting using a mixture of antisera against its N- and C-terminal regions (7.5% gel). c.p., cleavage fragments. The amount of intact eIF4GI for each transfection experiment is indicated below each corresponding lane. (C) Detection of eIF4GII cleavage products by Western blotting using an antiserum against the C-terminal region (7.5% gel). Ct, C-terminal fragments of eIF4GII. The amount of intact eIF4GII for each transfection experiment is indicated below each lane. (D) Cleavage kinetics of PABP, eIF4GI and eIF4GII by HIV-1 protease. BHK-21 cells were electroporated as described in the Experimental section, and cell lysates were obtained at the indicated times. The values were obtained by densitometric scanning of the corresponding intact protein band at each time indicated.
Other retroviral proteases cleave PABP in transfected cells
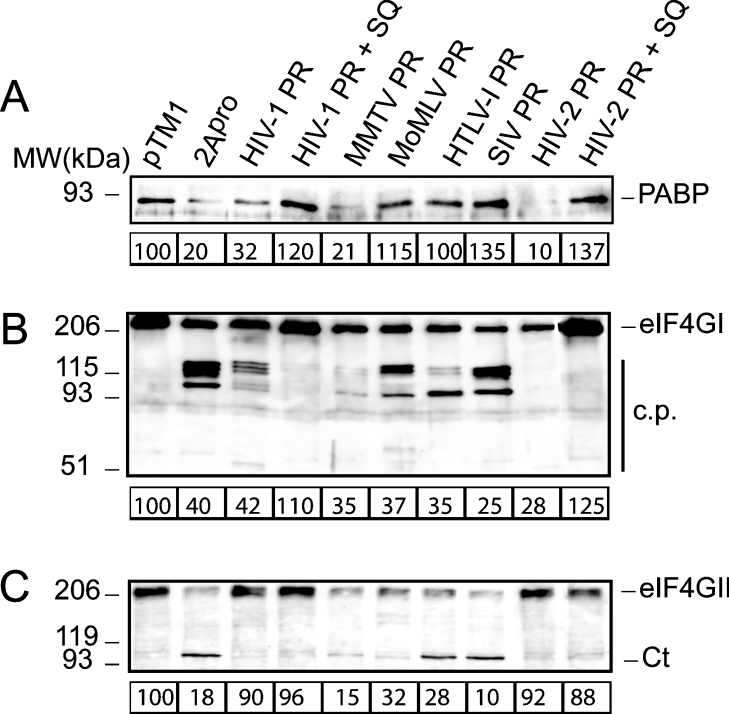
The susceptibility of PABP to proteolysis mediated by different retroviral proteases encoded by MoMLV, MMTV, HTLV-I, SIV, HIV-1 and HIV-2 was tested in transfected cells. For this purpose, the pTM1-derived plasmids containing the corresponding retroviral protease-coding sequences were transfected in COS-7 cells with the vaccinia virus vvT7 system. Poliovirus 2Apro was expressed in parallel to serve as a control of PABP cleavage. The integrity of PABP was assessed by Western blot at 16 hpt (hours post-transfection) (Figure 3A). Expression of HIV-1, HIV-2 and MMTV protease induced a substantial decrease in intact PABP comparable with that obtained after transfection of poliovirus 2Apro. Cleavage products of PABP were not detected by Western blot analysis in these cells, in a similar fashion to that observed in cells electroporated with SV-2A mRNA. The addition of 2 μM SQ prevented the loss of PABP caused by HIV-1 and HIV-2 proteases, suggesting that the lower level of intact PABP in HIV protease-transfected cells was due to their proteolytic activity. On the other hand, a decrease in PABP was not detect-able in cells transfected with plasmids containing the retroviral protease-coding regions of MoMLV, HTLV-I and SIV. The integrity of eIF4GI and eIF4GII was also analysed in protease-transfected cells (Figures 3B and 3C). In agreement with previous data, all retroviral proteases were able to cleave eIF4GI (Figure 3B), while the proteases of MMTV, MoMLV, HTLV-I and SIV cleaved eIF4GII in transfected cells (Figure 3C). The efficiency of transfection in these experiments was approx. 70%, as determined by immunofluorescence of cells transfected with the pTM1-Luc plasmid, using a luciferase antiserum (results not shown).
Figure 3. Cleavage of PABP, eIF4GI and eIF4GII in transfected cells.
(A) COS-7 cells were transfected with the empty pTM1 vector or with pTM1 carrying inserts containing the protease gene of several retroviruses or poliovirus 2Apro. At 16 hpt, equal amounts of protein extract were loaded in a polyacrylamide gel and analysed by Western blotting using a monoclonal antibody against PABP (10% gel). Data are referred to control experiments carried out with the empty pTM1 vector, and the values were obtained by densitometric scanning of the protein band of approx. 70 kDa corresponding to PABP. (B) Western blot against eIF4GI (7.5% gel). (C) Western blot against eIF4GII (7.5% gel). The estimated amount of intact protein in cell extracts obtained from transfections with different constructs is indicated below each panel. MW, molecular mass (sizes are given in kDa); c.p., cleavage fragments; Ct, C-terminal fragments of eIF4GII.
In vitro cleavage of PABP by HIV-1 and HIV-2 proteases
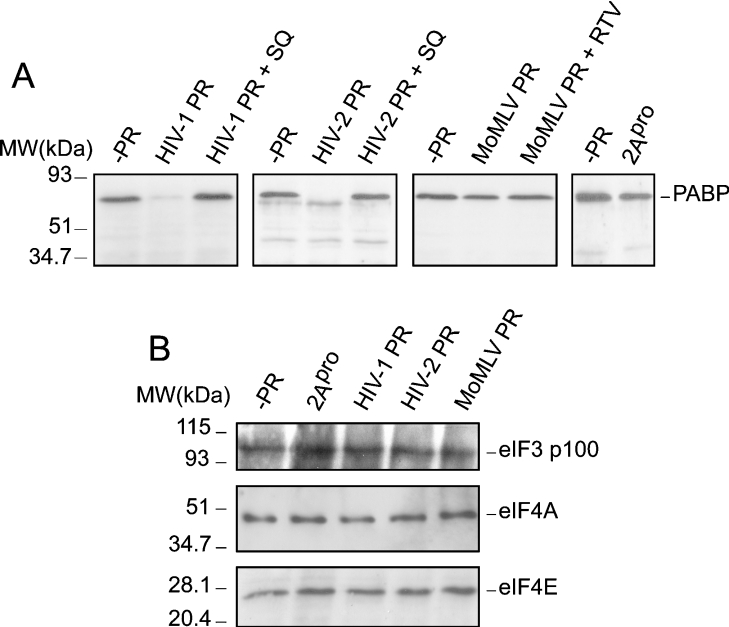
The proteolysis of PABP was studied further in cell-free systems using purified recombinant proteases from HIV-1, HIV-2 and MoMLV. The recombinant MBP–2Apro was included as a control. HeLa cell extracts were incubated for 90 min at 30 °C with 3 ng/μl of each purified protease. Under the experimental conditions used, complete proteolytic processing of intact PABP by HIV-1 and HIV-2 proteases occurs, while some intact PABP still remains upon incubation with MBP–2Apro (Figure 4A). This result indicates that the purified HIV-1 and HIV-2 proteases hydrolyse PABP more efficiently than MBP–2Apro. On the other hand, the MoMLV protease was unable to cleave PABP in this assay. The specificity of these cleavages was evidenced by using an HIV-1 protease inhibitor such as SQ, which blocked cleavage of PABP1 by both HIV-1 and HIV-2 proteases (Figure 4A). Consistent with the above results, the purified HIV-1 and HIV-2 proteases proteolysed eIF4GI in vitro, while eIF4GII was very inefficiently degraded by both proteases (results not shown). In addition, the integrity of other initiation factors, such as eIF4E, eIF4A and the 100 kDa subunit of eIF3, was analysed (Figure 4B, bottom, middle and top panels respectively). The amount of these translation factors remained constant after incubation with recombinant HIV-1 and HIV-2 proteases.
Figure 4. Cleavage of PABP in vitro by purified recombinant HIV-1, HIV-2 and MoMLV proteases.
Crude HeLa S10 extracts (50 μg) were incubated with 3 ng/μl of each recombinant protease in a total volume of 20 μl for 90 min and subjected to Western blotting analysis by using: (A) a monoclonal antibody against PABP (10% gel); (B) a polyclonal antiserum against the 100 kDa subunit of eIF3 (top panel; 10% gel), an antibody against eIF4A (middle panel; 15% gel) or a monoclonal antibody raised against eIF4E (bottom panel; 15% gel). MW, molecular mass (sizes given in kDa).
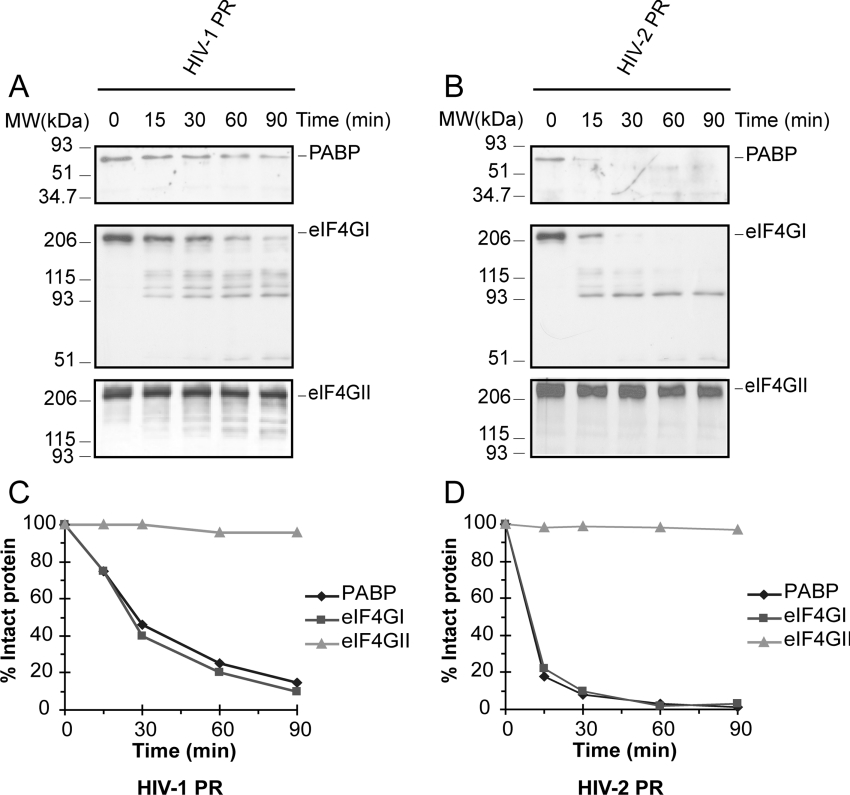
To analyse the kinetics of proteolysis of PABP, eIF4GI and eIF4GII, HeLa cell extracts were incubated with recombinant HIV-1 protease and HIV-2 protease, and, at the indicated times, the reactions were stopped (Figures 5A and 5B). As observed with SV-PR in culture cells, the kinetics of cleavage of PABP by HIV-1 and HIV-2 proteases were very similar to those observed for eIF4GI (Figures 5C and 5D). Consistent with previous observations using HIV-1 and HIV-2 proteases [19,20], hydrolysis of eIF4GII does not occur with the retroviral proteases throughout the assay.
Figure 5. Cleavage kinetics of PABP, eIF4GI and eIF4GII in vitro by purified recombinant HIV-1 and HIV-2 proteases.
Crude HeLa S10 extracts (50 μg) were incubated with 3 ng/μl HIV-1 protease (A) or HIV-2 protease (B) in a total volume of 20 μl during the indicated times and subjected to Western blot analysis by using a monoclonal antibody against PABP (top panel; 10% gel), a mixture of antisera against N-terminal and C-terminal regions of eIF4GI (middle panel; 7.5% gel) or an antiserum against the C-terminal region of eIF4GII (bottom panel; 7.5% gel). Degradation kinetics of PABP, eIF4GI and eIF4GII in HeLa cell extracts incubated with HIV-1 protease (C) and HIV-2 protease (D). Determinations were obtained by densitometric scanning of the corresponding intact protein band at the indicated times. MW, molecular mass (sizes given in kDa).
Identification of the cleavage sites of HIV-1 and HIV-2 proteases on PABP1
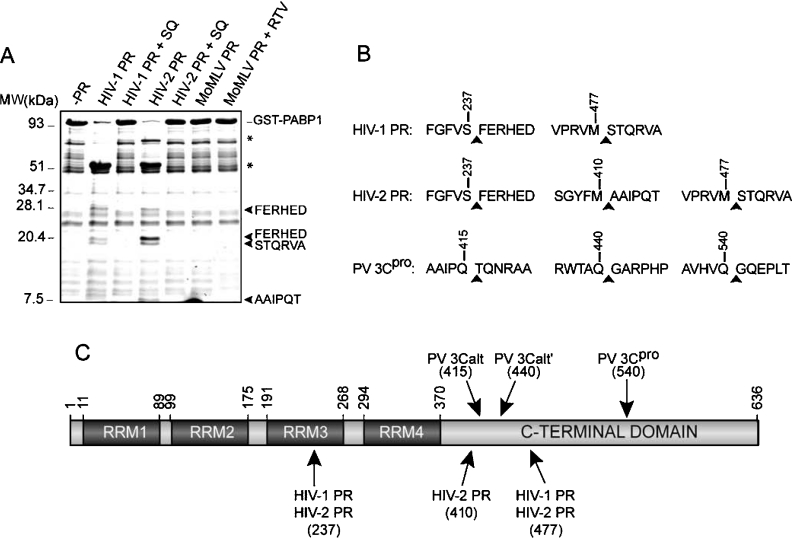
To determine whether the cleavage of HIV-1 and HIV-2 proteases on PABP was the result of a direct or indirect activation of cellular proteases, the fusion protein GST–PABP1-(9–636) was incubated with the recombinant HIV-1, HIV-2 and MoMLV proteases. In some assays, 2 μM SQ (in the case of HIV-1 protease or HIV-2 protease) and 20 μM ritonavir (in the case of MoMLV protease) were added. The staining of SDS/polyacrylamide gels with Coomassie Brilliant Blue revealed the generation of several cleavage products (Figure 6A). Incubation of GST–PABP1-(9–636) with both HIV-1 protease and HIV-2 protease revealed a similar degradation pattern, but with some slight differences (Figure 6A). Cleavage of GST–PABP1 by HIV-1 and HIV-2 proteases was inhibited by addition of 2 μM SQ. In addition, the purified MoMLV protease was unable to cleave the recombinant GST–PABP1 under the conditions used in this assay (Figure 6A).
Figure 6. Identification of HIV-1 protease and HIV-2 protease cleavage sites on PABP1.
(A) Recombinant GST–PABP1 protein (5 μg) was incubated with 100 ng of recombinant HIV-1 protease, HIV-2 protease or MoMLV protease in a total volume of 20 μl as described in the Experimental section. Cleavage products were separated by SDS/17% PAGE, and the hydrolysis fragments were stained with Coomassie Brilliant Blue. The sequenced polypeptides are indicated with an arrowhead. Bands corresponding to GST N-terminal polypeptides are denoted by asterisks. MW, molecular mass (sizes given in kDa). (B) Amino acid sequence of the PABP1 cleavage sites identified using HIV-1 and HIV-2 proteases. (C) Diagram showing the functional domains found in PABP1 based on the available data, including the position of mapped cleavage sites for HIV-1 and HIV-2 proteases. The RRMs and the regions involved in the binding to other translation factors are shown. Sequence numbering refers to the human PABP1 isoform. RTV, ritonavir.
In order to identify precisely the proteolysis sites, the cleavage products were sequenced by the Edman degradation method. N-terminal sequencing of the polypeptides of approx. 27 and 20 kDa rendered FERHED, which corresponds to a cleavage site located between positions 237 and 238 of PABP1 (Figure 6B). Sequencing analysis of the 18 kDa polypeptide rendered a serine, threonine, glutamine, arginine, valine and alanine in sequencing cycles 1–6, which correspond to the cleavage site located at positions 477/478 of PABP1. In addition, AAIPQT was identified as the N-terminal sequence of the approx. 8 kDa fragment detected after incubation of GST–PABP1 with HIV-2 protease, which reveals a processing site located between positions 410 and 411 of PABP1 (Figure 6B). Edman degradation of polypeptides of approx. 72 and 52 kDa rendered a sequence that correspond with the six first amino acids of GST (Figure 6A, denoted by asterisks). These findings reflect that both HIV-1 and HIV-2 proteases cleave PABP1 around the same sites (Figure 6C). The presence of additional cleavage sites on PABP1, such as the cleavage site located at position 410/411, could also explain in part the variability observed among the degradation products rendered by HIV-1 and HIV-2 proteases. The relative conservation of the PABP1 cleavage sites of viral proteases supports the view that this protein is a genuine substrate for the HIV-1 and HIV-2 proteases.
DISCUSSION
A variety of viral products are responsible for cell damage that occurs during virus infection. Animal viruses encode proteases that cleave polyprotein precursors to render the mature viral proteins. Apart from those substrates, a number of host proteins are also cleaved by viral proteases. The hydrolysis of the host proteins alters different cellular functions. Thus proteases of several viruses cleave protein factors of the cellular translational machinery, causing a down-regulation of cellular mRNA translation, while increasing the synthesis of viral proteins. Thus cleavage of the initiation factor eIF4G by proteases of several picornaviruses leads to the inhibition of translation directed by cellular capped mRNAs [16]. Moreover, bisection of eIF4G by picornavirus proteases enhances the translation of the virus mRNA genome that contains an IRES motif at its 5′ end. Previous reports have shown that eIF4G is also a target of retroviral proteases [19,20]. In some retroviruses, including HIV-1, viral-protease-mediated cleavage of eIF4G leads to the inhibition of cap-dependent translation, without affecting the IRES-driven protein synthesis directed by retroviral mRNAs [19,20,34].
Previous reports have shown that PABP is also cleaved by the proteases of several picornaviruses and caliciviruses during infection [22–24,26]. Its degradation, together with the cleavage of eIF4G, contribute to the observed inhibition of cellular translation that occurs during viral infection. The cleavage of PABP by the poliovirus 3Cpro occurs at the C-terminal domain of the protein. As a result, the C-terminal portion, which is implicated in interaction with other proteins, is separated from the RNA-interacting N-terminal domain [24]. This cleavage may selectively inhibit poly(A)-dependent translation [24,25]. The C-terminal domain of PABP interacts with several proteins that are implicated in mRNA translation (i.e. eIF4B and eRF3) [5,9–13]. The cleavage of PABP by 3Cpro disrupts its interaction with eIF4B and eRF3, probably affecting late events in translation, such as ribosome recycling. Our results show that HIV proteases also cleaved PABP within its C-terminal domain. The identified cleavage sites (at positions 410–411 and 477–478) were relatively close to those recognized by poliovirus 3Cpro. In addition, HIV proteases also hydrolyse PABP between residues 237 and 238. This cleavage leads to the removal of the first two RRMs (RRM1 and RRM2) of PABP, which may retain some of the functions of the protein.
All of the RRMs of PABP possess RNA-binding ability [35], although the first two domains together (RRM1–RRM2) are the ones with the highest affinity for the poly(A) tail [36]. RRM2 is involved in direct interaction with eIF4G [9,37]. It has been shown that translation can be rescued in PABP-depleted Krebs-2 cell extracts by the addition of purified recombinant PABP fragments such as RRM1–RRM2 or RRM1–RRM4, although less efficiently than with intact PABP [38]. These observations suggested that the interaction of PABP and eIF4G is essential for efficient translation, although additional interactions of the C-terminal domain of PABP with other factors would be necessary for a complete stimulation of translation [38]. In agreement with that proposal, cleavage of PABP within RRM3 by HIV protease may abolish in part, but not completely, the function of its N-terminal domain, since the RRM1–RRM2 region still retains the eIF4G-binding site and the ability to bind RNA.
Infection of C8166 cells by HIV-1 led to a drastic decline in host translation coincident with the decrease of intact eIF4GI [20]. However, total proteolysis of eIF4GI is not sufficient to block translation of cellular mRNAs engaged in the translational machinery [39,40]. The shut-off of host protein synthesis is coincident with the hydrolysis of eIF4GII in poliovirus- and rhinovirus-infected cells, as well as in apoptotic cells [41,42]. PABP hydrolysis by poliovirus 3Cpro inhibits completely the translation of endogenous mRNAs in HeLa cell extracts [25]. Our present findings indicate that cleavage of both eIF4GI and PABP occur with similar kinetics in cell culture and in a cell-free system, suggesting that PABP proteolysis may also contribute to host cell translation inhibition and could be responsible for the shut-off of host protein synthesis, observed in some cell lines upon HIV-1 infection [20]. In HIV-1-infected cells, the correlation observed between eIF4GI hydrolysis and the inhibition of cellular protein synthesis could be attributed to the viral-protease cleavage of both eIF4GI and PABP.
Interestingly, retroviral proteases such as those from MoMLV, HTLV-I or SIV-1 are able to hydrolyse eIF4GI and eIF4GII, but not PABP, while inhibiting protein synthesis in transfected COS-7 cells [19]. Although both HIV-1 and HIV-2 proteases are able to proteolyse eIF4GI and PABP very efficiently, eIF4GII remains a poor substrate of both enzymes [19,20]. Taken together, available data suggest that, at least two of those factors must be inactivated to efficiently inhibit cap- and poly(A)-dependent translation of cellular mRNAs.
Apart from factors implicated in translation, other host proteins have been identified as substrates of HIV proteases and the potential role of cleavage of cellular proteins in the cytotoxic effect inflicted by HIV has been pointed out by several laboratory groups [43,44]. Examples of cell proteins cleaved by HIV proteases are microtubule-associated proteins, cytoskeletal proteins and nuclear factor-κB, among others [45,46]. Nevertheless, further investigations are required to define the precise role of each of those proteolytic cleavages in the cytopathogenicity provoked by HIV infection.
It is widely accepted that most retroviruses do not affect cellular protein synthesis after infection. This is not the case for HIV, which blocks cellular protein synthesis more or less efficiently depending on the viral strain and cell line analysed [47,48]. In HIV-1, the Vpr protein blocks proliferation of CD4+ T-cells at the G2 cell-cycle checkpoint [18,49]. At this stage, the initiation of cap-dependent translation is suppressed, probably due to the cleavage of both eIF4GI and PABP, while the initiation of IRES-containing mRNAs ensures Gag and Gag-Pol synthesis. In this scenario, cleavage of both PABP and eIF4GI could facilitate viral gene expression, while contributing to the inhibition of host protein synthesis. The finding that many retroviral and picornaviral proteases target eIF4G, and, in some cases, PABP, indicates that those viruses may share a common mechanism of translational control.
Acknowledgments
Financial support from Grant BMC2003-00494 from the Dirección General de Investigación Científica y Técnica and an Institutional Grant to the Centro de Biología Molecular “Severo Ochoa” from the Fundación Ramón Areces, are acknowledged. E.A. and A.C. hold fellowships from the Ministerio de Educación y Ciencia.
References
- 1.Hentze M. W. eIF4G: a multipurpose ribosome adapter? Science. 1997;275:500–501. doi: 10.1126/science.275.5299.500. [DOI] [PubMed] [Google Scholar]
- 2.Jacobson A., Favreau M. Possible involvement of poly(A) in protein synthesis. Nucleic Acids Res. 1983;11:6353–6368. doi: 10.1093/nar/11.18.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacobson A. Poly(A) metabolism and translation: the closed loop model. In: Hershey J. W. B., Mathews M. B., Sonenberg N., editors. Translational Control. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1996. pp. 451–480. [Google Scholar]
- 4.Borman A. M., Michel Y. M., Kean K. M. Biochemical characterisation of cap-poly(A) synergy in rabbit reticulocyte lysates: the eIF4G–PABP interaction increases the functional affinity of eIF4E for the capped mRNA 5′-end. Nucleic Acids Res. 2000;28:4068–4075. doi: 10.1093/nar/28.21.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tarun S. Z., Jr, Wells S. E., Deardorff J. A., Sachs A. B. Translation initiation factor eIF4G mediates in vitro poly(A) tail-dependent translation. Proc. Natl. Acad. Sci. U.S.A. 1997;94:9046–9051. doi: 10.1073/pnas.94.17.9046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallie D. R. The cap and poly(A) tail function synergistically to regulate mRNA translational efficiency. Genes Dev. 1991;5:2108–2116. doi: 10.1101/gad.5.11.2108. [DOI] [PubMed] [Google Scholar]
- 7.Adam S. A., Nakagawa T., Swanson M. S., Woodruff T. K., Dreyfuss G. mRNA polyadenylate-binding protein: gene isolation and sequencing and identification of a ribonucleoprotein consensus sequence. Mol. Cell. Biol. 1986;6:2932–2943. doi: 10.1128/mcb.6.8.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sachs A. B., Bond M. W., Kornberg R. D. A single gene from yeast for both nuclear and cytoplasmic polyadenylate-binding proteins: domain structure and expression. Cell. 1986;45:827–835. doi: 10.1016/0092-8674(86)90557-x. [DOI] [PubMed] [Google Scholar]
- 9.Imataka H., Gradi A., Sonenberg N. A newly identified N-terminal amino acid sequence of human eIF4G binds poly(A)-binding protein and functions in poly(A)-dependent translation. EMBO J. 1998;17:7480–7489. doi: 10.1093/emboj/17.24.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le H., Tanguay R. L., Balasta M. L., Wei C. C., Browning K. S., Metz A. M., Goss D. J., Gallie D. R. Translation initiation factors eIF-iso4G and eIF-4B interact with the poly(A)-binding protein and increase its RNA binding activity. J. Biol. Chem. 1997;272:16247–16255. doi: 10.1074/jbc.272.26.16247. [DOI] [PubMed] [Google Scholar]
- 11.Hoshino S., Imai M., Kobayashi T., Uchida N., Katada T. The eukaryotic polypeptide chain releasing factor (eRF3/GSPT) carrying the translation termination signal to the 3′-poly(A) tail of mRNA: direct association of erf3/GSPT with polyadenylate-binding protein. J. Biol. Chem. 1999;274:16677–16680. doi: 10.1074/jbc.274.24.16677. [DOI] [PubMed] [Google Scholar]
- 12.Kozlov G., Trempe J. F., Khaleghpour K., Kahvejian A., Ekiel I., Gehring K. Structure and function of the C-terminal PABC domain of human poly(A)-binding protein. Proc. Natl. Acad. Sci. U.S.A. 2001;98:4409–4413. doi: 10.1073/pnas.071024998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cosson B., Couturier A., Chabelskaya S., Kiktev D., Inge-Vechtomov S., Philippe M., Zhouravleva G. Poly(A)-binding protein acts in translation termination via eukaryotic release factor 3 interaction and does not influence [PSI+] propagation. Mol. Cell. Biol. 2002;22:3301–3315. doi: 10.1128/MCB.22.10.3301-3315.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Craig A. W., Haghighat A., Yu A. T., Sonenberg N. Interaction of polyadenylate-binding protein with the eIF4G homologue PAIP enhances translation. Nature (London) 1998;392:520–523. doi: 10.1038/33198. [DOI] [PubMed] [Google Scholar]
- 15.Khaleghpour K., Svitkin Y. V., Craig A. W., DeMaria C. T., Deo R. C., Burley S. K., Sonenberg N. Translational repression by a novel partner of human poly(A) binding protein, Paip2. Mol. Cell. 2001;7:205–216. doi: 10.1016/s1097-2765(01)00168-x. [DOI] [PubMed] [Google Scholar]
- 16.Prevot D., Darlix J. L., Ohlmann T. Conducting the initiation of protein synthesis: the role of eIF4G. Biol. Cell. 2003;95:141–156. doi: 10.1016/s0248-4900(03)00031-5. [DOI] [PubMed] [Google Scholar]
- 17.Baudin F., Marquet R., Isel C., Darlix J. L., Ehresmann B., Ehresmann C. Functional sites in the 5′ region of human immunodeficiency virus type 1 RNA form defined structural domains. J. Mol. Biol. 1993;229:382–397. doi: 10.1006/jmbi.1993.1041. [DOI] [PubMed] [Google Scholar]
- 18.Brasey A., Lopez-Lastra M., Ohlmann T., Beerens N., Berkhout B., Darlix J. L., Sonenberg N. The leader of human immunodeficiency virus type 1 genomic RNA harbors an internal ribosome entry segment that is active during the G2/M phase of the cell cycle. J. Virol. 2003;77:3939–3949. doi: 10.1128/JVI.77.7.3939-3949.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alvarez E., Menendez-Arias L., Carrasco L. The eukaryotic translation initiation factor 4GI is cleaved by different retroviral proteases. J. Virol. 2003;77:12392–12400. doi: 10.1128/JVI.77.23.12392-12400.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ventoso I., Blanco R., Perales C., Carrasco L. HIV-1 protease cleaves eukaryotic initiation factor 4G and inhibits cap-dependent translation. Proc. Natl. Acad. Sci. U.S.A. 2001;98:12966–12971. doi: 10.1073/pnas.231343498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piron M., Vende P., Cohen J., Poncet D. Rotavirus RNA-binding protein NSP3 interacts with eIF4GI and evicts the poly(A) binding protein from eIF4F. EMBO J. 1998;17:5811–5821. doi: 10.1093/emboj/17.19.5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joachims M., Van Breugel P. C., Lloyd R. E. Cleavage of poly(A)-binding protein by enterovirus proteases concurrent with inhibition of translation in vitro. J. Virol. 1999;73:718–727. doi: 10.1128/jvi.73.1.718-727.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kerekatte V., Keiper B. D., Badorff C., Cai A., Knowlton K. U., Rhoads R. E. Cleavage of Poly(A)-binding protein by coxsackievirus 2A protease in vitro and in vivo: another mechanism for host protein synthesis shutoff? J. Virol. 1999;73:709–717. doi: 10.1128/jvi.73.1.709-717.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuyumcu-Martinez N. M., Joachims M., Lloyd R. E. Efficient cleavage of ribosome-associated poly(A)-binding protein by enterovirus 3C protease. J. Virol. 2002;76:2062–2074. doi: 10.1128/jvi.76.5.2062-2074.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuyumcu-Martinez N. M., Van Eden M. E., Younan P., Lloyd R. E. Cleavage of poly(A)-binding protein by poliovirus 3C protease inhibits host cell translation: a novel mechanism for host translation shutoff. Mol. Cell. Biol. 2004;24:1779–1790. doi: 10.1128/MCB.24.4.1779-1790.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuyumcu-Martinez M., Belliot G., Sosnovtsev S. V., Chang K. O., Green K. Y., Lloyd R. E. Calicivirus 3C-like proteinase inhibits cellular translation by cleavage of poly(A)-binding protein. J. Virol. 2004;78:8172–8182. doi: 10.1128/JVI.78.15.8172-8182.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castelló A., Sanz M. A., Molina S., Carrasco L. Translation of sindbis virus 26S mRNA does not require intact eukariotic initiation factor 4G. J. Mol. Biol. 2006;355:942–956. doi: 10.1016/j.jmb.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 28.Aldabe R., Feduchi E., Novoa I., Carrasco L. Efficient cleavage of p220 by poliovirus 2Apro expression in mammalian cells: effects on vaccinia virus. Biochem. Biophys. Res. Commun. 1995;215:928–936. doi: 10.1006/bbrc.1995.2553. [DOI] [PubMed] [Google Scholar]
- 29.Ventoso I., Barco A., Carrasco L. Mutational analysis of poliovirus 2Apro: distinct inhibitory functions of 2Apro on translation and transcription. J. Biol. Chem. 1998;273:27960–27967. doi: 10.1074/jbc.273.43.27960. [DOI] [PubMed] [Google Scholar]
- 30.Burgui I., Aragon T., Ortin J., Nieto A. PABP1 and eIF4GI associate with influenza virus NS1 protein in viral mRNA translation initiation complexes. J. Gen. Virol. 2003;84:3263–3274. doi: 10.1099/vir.0.19487-0. [DOI] [PubMed] [Google Scholar]
- 31.Rittenhouse J., Turon M. C., Helfrich R. J., Albrecht K. S., Weigl D., Simmer R. L., Mordini F., Erickson J., Kohlbrenner W. E. Affinity purification of HIV-1 and HIV-2 proteases from recombinant. E. coli strains using pepstatin–agarose. Biochem. Biophys. Res. Commun. 1990;171:60–66. doi: 10.1016/0006-291x(90)91356-w. [DOI] [PubMed] [Google Scholar]
- 32.Menendez-Arias L., Gotte D., Oroszlan S. Moloney murine leukemia virus protease: bacterial expression and characterization of the purified enzyme. Virology. 1993;196:557–563. doi: 10.1006/viro.1993.1511. [DOI] [PubMed] [Google Scholar]
- 33.Ventoso I., Barco A., Carrasco L. Genetic selection of poliovirus 2Apro-binding peptides. J. Virol. 1999;73:814–818. doi: 10.1128/jvi.73.1.814-818.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perales C., Carrasco L., Ventoso I. Cleavage of eIF4G by HIV-1 protease: effects on translation. FEBS Lett. 2003;533:89–94. doi: 10.1016/s0014-5793(02)03764-x. [DOI] [PubMed] [Google Scholar]
- 35.Deo R. C., Bonanno J. B., Sonenberg N., Burley S. K. Recognition of polyadenylate RNA by the poly(A)-binding protein. Cell. 1999;98:835–845. doi: 10.1016/s0092-8674(00)81517-2. [DOI] [PubMed] [Google Scholar]
- 36.Burd C. G., Matunis E. L., Dreyfuss G. The multiple RNA-binding domains of the mRNA poly(A)-binding protein have different RNA-binding activities. Mol. Cell. Biol. 1991;11:3419–3424. doi: 10.1128/mcb.11.7.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kessler S. H., Sachs A. B. RNA recognition motif 2 of yeast Pab1p is required for its functional interaction with eukaryotic translation initiation factor 4G. Mol. Cell. Biol. 1998;18:51–57. doi: 10.1128/mcb.18.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kahvejian A., Svitkin Y. V., Sukarieh R., M'Boutchou M. N., Sonenberg N. Mammalian poly(A)-binding protein is a eukaryotic translation initiation factor, which acts via multiple mechanisms. Genes Dev. 2005;19:104–113. doi: 10.1101/gad.1262905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perez L., Carrasco L. Lack of direct correlation between p220 cleavage and the shut-off of host translation after poliovirus infection. Virology. 1992;189:178–186. doi: 10.1016/0042-6822(92)90693-j. [DOI] [PubMed] [Google Scholar]
- 40.Irurzun A., Sanchez-Palomino S., Novoa I., Carrasco L. Monensin and nigericin prevent the inhibition of host translation by poliovirus, without affecting p220 cleavage. J. Virol. 1995;69:7453–7460. doi: 10.1128/jvi.69.12.7453-7460.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gradi A., Svitkin Y. V., Imataka H., Sonenberg N. Proteolysis of human eukaryotic translation initiation factor eIF4GII, but not eIF4GI, coincides with the shutoff of host protein synthesis after poliovirus infection. Proc. Natl. Acad. Sci. U.S.A. 1998;95:11089–11094. doi: 10.1073/pnas.95.19.11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Svitkin Y. V., Gradi A., Imataka H., Morino S., Sonenberg N. Eukaryotic initiation factor 4GII (eIF4GII), but not eIF4GI, cleavage correlates with inhibition of host cell protein synthesis after human rhinovirus infection. J. Virol. 1999;73:3467–3472. doi: 10.1128/jvi.73.4.3467-3472.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shoeman R. L., Honer B., Stoller T. J., Kesselmeier C., Miedel M. C., Traub P., Graves M. C. Human immunodeficiency virus type 1 protease cleaves the intermediate filament proteins vimentin, desmin, and glial fibrillary acidic protein. Proc. Natl. Acad. Sci. U.S.A. 1990;87:6336–6340. doi: 10.1073/pnas.87.16.6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tomasselli A. G., Hui J. O., Adams L., Chosay J., Lowery D., Greenberg B., Yem A., Deibel M. R., Zurcher-Neely H., Heinrikson R. L. Actin, troponin C, Alzheimer amyloid precursor protein and pro-interleukin 1β as substrates of the protease from human immunodeficiency virus. J. Biol. Chem. 1991;266:14548–14553. [PubMed] [Google Scholar]
- 45.Dunn B. M. Human immunodeficiency virus 1 retropepsin. In: Barret A. J., Rawlings N. D., Woessner J. F., editors. Handbook of Proteolytic Enzymes. London: Academic Press; 1998. pp. 919–928. [Google Scholar]
- 46.Wlodawer A., Gustchina A. Structural and biochemical studies of retroviral proteases. Biochim. Biophys. Acta. 2000;1477:16–34. doi: 10.1016/s0167-4838(99)00267-8. [DOI] [PubMed] [Google Scholar]
- 47.Agy M. B., Wambach M., Foy K., Katze M. G. Expression of cellular genes in CD4 positive lymphoid cells infected by the human immunodeficiency virus, HIV-1: evidence for a host protein synthesis shut-off induced by cellular mRNA degradation. Virology. 1990;177:251–258. doi: 10.1016/0042-6822(90)90478-a. [DOI] [PubMed] [Google Scholar]
- 48.Somasundaran M., Robinson H. L. Unexpectedly high levels of HIV-1 RNA and protein synthesis in a cytocidal infection. Science. 1988;242:1554–1557. doi: 10.1126/science.3201245. [DOI] [PubMed] [Google Scholar]
- 49.Goh W. C., Rogel M. E., Kinsey C. M., Michael S. F., Fultz P. N., Nowak M. A., Hahn B. H., Emerman M. HIV-1 Vpr increases viral expression by manipulation of the cell cycle: a mechanism for selection of Vpr in vivo. Nat. Med. 1998;4:65–71. doi: 10.1038/nm0198-065. [DOI] [PubMed] [Google Scholar]