Abstract
Distinct domains within the SNARE (soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor) proteins, STX1A (syntaxin 1A) and SNAP-25 (synaptosome-associated protein-25 kDa), regulate hormone secretion by their actions on the cell's exocytotic machinery, as well as voltage-gated Ca2+ and K+ channels. We examined the action of distinct domains within SNAP-25 on Kv2.1 (voltage gated K+ 2.1) channel gating. Dialysis of N-terminal SNAP-25 domains, S197 (SNAP-251–197) and S180 (SNAP-251–180), but not S206 (full-length SNAP-251–206) increased the rate of Kv2.1 channel activation and slowed channel inactivation. Remarkably, these N-terminal SNAP-25 domains, acting on the Kv2.1 cytoplasmic N-terminus, potentiated the external TEA (tetraethylammonium)-mediated block of Kv2.1. To further examine whether these are effects of the channel pore domain, internal K+ was replaced with Na+ and external K+ was decreased from 4 to 1 mM, which decreased the IC50 of the TEA block from 6.8±0.9 mM to >100 mM. Under these conditions S180 completely restored TEA sensitivity (7.9±1.5 mM). SNAP-25 C-terminal domains, SNAP-25198–206 and SNAP-25181–197, had no effect on Kv2.1 gating kinetics. We conclude that different domains within SNAP-25 can form distinct complexes with Kv2.1 to execute a fine allosteric regulation of channel gating and the architecture of the outer pore structure in order to modulate cell excitability.
Keywords: Kv2.1 gating, K+ channel, soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor (SNARE), synaptosome-associated protein-25 kDa (SNAP-25), tetraethylammonium (TEA)
Abbreviations: BoNT, botulinum neurotoxin; EGFP, enhanced green fluorescent protein; GST, glutathione S-transferase; HEK, human embryonic kidney; [K+]o, external K+ concentration; Kv channel, voltage-gated K+ channel; S197, N-terminal SNAP-25 peptide containing amino acid residues 1–197; S180, N-terminal SNAP-25 peptide containing amino acid residues 1–180; S206, full-length SNAP-25; SNAP-25, synaptosome-associated protein-25 kDa; SNARE, soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor; STX1A, syntaxin 1A; T1 domain, tetramerization domain; TEA, tetraethylammonium
INTRODUCTION
The plasma membrane SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) proteins, STX1A (syntaxin 1A) and SNAP-25 (synaptosome-associated protein-25 kDa), are critical in co-ordinating the spatial and temporal actions of vesicular exocytosis [1]. Moreover, they have been shown to be physically associated with voltage-gated Ca2+ channels [2,3], tethering the Ca2+ channels to the immediate vicinity of exocytosis [4]. This results in formation of a multimolecular complex with the secretory granule bound to the SNARE protein, synaptotagmin, and has been referred to as the excitosome [5]. The excitosome complex serves to regulate Ca2+ influx precisely at the site of exocytosis [1–5]. We have subsequently shown that the membrane-repolarizing Kv2.1 (voltage-gated K+ 2.1) channel [6] can also be physically bound and regulated by STX1A and SNAP-25 [7–9] in order to further modulate membrane excitability and insulin secretion.
Much is known about the structure–function relationship of STX1A in modulating different steps in exocytosis [10,11] and the gating of voltage-gated Ca2+ [2,3,5,12] or Kv channels [2,9,13]. Specifically, the C-terminal H3 domain of STX1A [14] modulates the exocytic steps and voltage-gated Ca2+ or Kv channels [8,10–12], whereas the N-terminal HABC domain plays a negative regulatory role [14,15]. In fact, we have recently reported that there is interaction between these STX1A domains and the islet β-cell and cardiac KATP channels [16–18]. SNAP-25, the cognate partner of STX1A, has independent inhibitory actions on voltage-gated Ca2+ [19] or Kv2.1 channels [20]. SNAP-25 interacts with STX1A to form a complex, which profoundly modulates both voltage-gated Ca2+ [5] or Kv2.1 channels [20] in a manner quite distinct from either SNARE protein acting alone. It therefore appears that the STX1A–SNAP-25 complex assembly and disassembly that occurs during the secretory process could reveal distinct functional domains within each SNARE protein which function to modulate the different exocytotic steps and gating of the different membrane ion channels involved in secretion.
In contrast with STX-1A, relatively little is known about the functional domains within SNAP-25. BoNT (botulinum neurotoxin)/A and BoNT/E specifically cleave SNAP-25 and inhibit insulin secretion [21] and neurotransmitter release [22], thus acting as invaluable tools with which to examine SNAP-25 function. BoNT/A and BoNT/E cleave the 206 amino acid SNAP-25 protein at residues 197–198 and 180–181 respectively [22], and the resulting N-terminal and C-terminal proteolytic cleavage products of SNAP-25 have been shown to inhibit exocytosis [23–26]. We have shown that the S197 (SNAP-251–197) domain played a positive regulatory role, whereas the C-terminal SNAP-25198–206 domain exhibited inhibitory actions on β-cell voltage-gated Ca2+ channels [19]. Taking these results together, the proteolytic products of SNAP-25 generated by these neurotoxins have been revealed to be important functional domains with distinct actions on exocytosis and ion channel gating.
In the present study, we have postulated that the proteolytic domains of SNAP-25 may exhibit distinct actions on Kv2.1 channel gating. We therefore examined the SNAP-25 domains resulting from BoNT/A and BoNT/E proteolytic cleavage, including the N-terminal domain peptides S197 and S180 (SNAP-251–180), and the C-terminal peptides SNAP-25181–197 and SNAP-25198–206. We found that the N-terminal SNAP-25 domains modulated Kv2.1 channel gating through interaction with the channel's N-terminus. More surprisingly, these domains also induced profound allosteric changes to the Kv2.1 channel pore, which is located distal to the channel N-terminus.
MATERIALS AND METHODS
DNA constructs and generation of GST (glutathione S-transferase) fusion proteins
The rat Kv2.1 and Kv2.1ΔC416-pBluescript constructs were generously provided by Dr R. Joho (University of Texas, Southwestern Medical Center, Center for Basic Neuroscience, Dallas, TX, U.S.A.) and the rat Kv2.1ΔN139-pBluescript construct was kindly provided by Dr C. Deutsch (University of Pennsylvania, Department of Physiology, Philadelphia, PA, U.S.A.). Both constructs were subcloned into pcDNA3 (Invitrogen, Burlington ON, Canada). The coding sequences corresponding to the full-length rat SNAP-25, S197, S180, cytoplasmic N-terminus (amino acids 1–182) and C-terminus (amino acids 411–853), C1 (amino acids 412–633) and C2 (amino acids 634–853) of Kv2.1 were amplified by PCR and cloned into the pGEX-5X-1 expression vector (GE Healthcare, Baie d'Urfe QC, Canada) for the generation of GST fusion proteins as previously described [7,8]. GST fusion protein expression and purification were performed according to the manufacturer's instructions. The plasmid constructs pcDNA3-SNAP-25-WT, pcDNA3-S180 and pGEX-4T-1-S180 were generated as described previously [19]. SNAP-25198–206 and SNAP-25181–197 peptides were synthesized and purified (>95% purity by HPLC) at the Hospital for Sick Children of Toronto Biotechnology Center.
Cell culture and transfections
HEK (human embryonic kidney)-293 cells were grown at 37 °C in 5% CO2 in DMEM (Dulbeco's modified Eagle's medium) containing 4.5 g/l glucose supplemented with 10% FBS (foetal bovine serum) and penicillin/streptomycin. For electrophysiological experiments, when HEK-293 cells were 50% confluent, they were transiently transfected with Kv2.1, Kv2.1ΔN139 or Kv2.1ΔC416 cDNA, and co-transfected with EGFP (enhanced green fluorescent protein) (in pcDNA3) using Lipofectamine™ 2000 (Invitrogen). For biochemical experiments, HEK-293 cells were transfected with pcDNA3-SNAP25-WT (wild-type), pcDNA3-S180, or pcDNA3-Kv2.1. The HEK cells reliably take up multiple plasmids with >90% efficiency. After transfection (1–2 days), cells were trypsinized and placed in 35 mm dishes for electrophysiological studies. Transfected cells were identified by visualization of the fluorescence of the co-expressed EGFP.
In vitro binding assay
After transfection (2 days), the HEK-293 cells were washed with 1×PBS (pH 7.4) and then harvested in binding buffer [25 mM Hepes (pH 7.4), 100 mM KCl, 1.5% Triton X-100, 2 μM pepstatin A, 1 μg/ml leupeptin and 10 μg/ml aprotinin]. The cells were lysed by sonication and insoluble materials were removed by centrifugation at 22500 g for 30 min at 4 °C. The detergent extract (0.3 ml, 1.5–2.1 μg/μl protein) of HEK-293 cell lysate was incubated with GST (as a negative control) or GST–Kv2.1-N, –C1 or –C2 fusion proteins (all bound to glutathione–agarose beads, 450 pmol of protein) at 4 °C for 2 h. The beads were then washed three times with binding buffer and the samples were separated on SDS/(12%)PAGE, transferred to a nitrocellulose membrane and identified using a mouse anti-SNAP-25 N-terminus antibody (1:750). In a reciprocal study, GST, GST–SNAP-25-WT or truncated fusion proteins (all bound to agarose beads, 550 pmol of protein) were used in a pulldown assay for overpressed Kv2.1 protein from a HEK-293 cell lysate extract (0.3 ml, 1.4–1.9 μg of protein/μl). The precipitated proteins were probed with an anti-Kv2.1 antibody (1:750; Upstate Biotech, Waltham, MA, U.S.A.).
Electrophysiology
HEK-293 cells were continually perfused with a bath solution containing (in mM): 140 NaCl, 4 KCl, 1 MgCl2, 2 CaCl2, and 10 Hepes (pH 7.4 with NaOH). Recording pipettes were generated by stretching 1.5 mm borosilicate glass capillary tubes (World Precision Instruments, Inc., Sarasota, FL, U.S.A.) using a pre-programmed pipette puller (Sutter Instrument, Novato, CA, U.S.A.). Pipettes were heat polished and the tip resistance ranged from 2–3 MΩ when filled with the intracellular solution containing (in mM): 140 KCl, 1 MgCl2, 1 EGTA, 10 Hepes and 5 MgATP (pH 7.25 with KOH). In some experiments symmetrical Na+ solutions were used. The internal solution contained (mM): 140 NaCl, 1 MgCl2, 1 EGTA, 10 Hepes and 5 MgATP (pH 7.25 with NaOH), and the external solution consisted of (in mM): 140 NaCl, 1 KCl, 1 MgCl2, 2 CaCl2, and 10 Hepes (pH 7.4 with NaOH). Recordings were performed using an EPC-9 amplifier and Pulse software (HEKA Electronik, Lambrecht, Germany). After whole-cell configuration was established, membrane potential was held at −70 mV and the outward current was triggered with a 200 ms depolarizing voltage pulse (−50 to 50 mV, with an increment of 10 mV). Currents were digitized at 10 kHz and filtered at 2 kHz. Stock solutions of GST or GST–SNAP-25 protein constructs were made in the pipette solution, and diluted to the desired concentration. They were dialysed directly into the cell via the patch pipette.
Statistical significance was determined using a Student's paired t test or ANOVA (analysis of variance) followed by a post-hoc Student-Neuman-Keuls test. P values of <0.05 were considered to be statistically significant. Data are shown as the means±S.E.M.
RESULTS
Effect of N-terminal SNAP-25 domains on Kv2.1 channel gating
To examine the precise action of the SNAP-25 domain peptides on the Kv2.1 channel, we expressed Kv2.1 in HEK-293 cells, which are devoid of Kv2.1 or SNAP-25 proteins. In the present study, we examined the effects of S197 and S180, which are the N-terminal fragments resulting from BoNT/A and BoNT/E cleavage respectively [22], on the Kv2.1 current. As in the case of full-length S206 (1 nM), which decreased Kv2.1 current by 37±5% compared with controls, 1 nM of either S180 or S197 similarly decreased the Kv2.1 current by 39±2% and 35±3% respectively. The C-terminal peptides SNAP-25198–206 and SNAP-25181–197, at up to 1 μM, had no effect on Kv2.1 current amplitude.
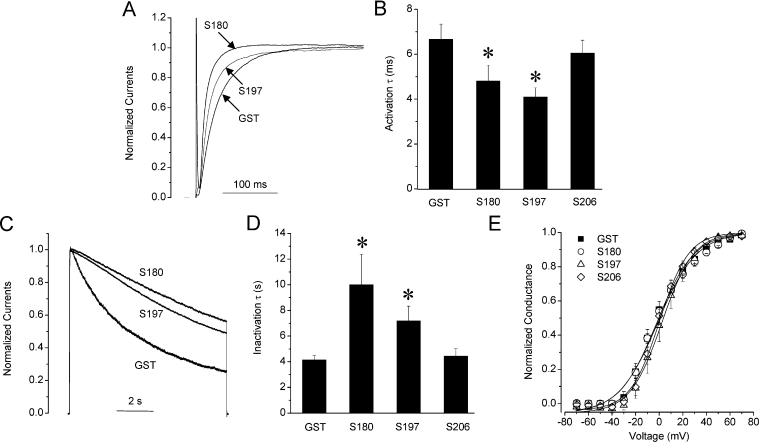
SNAP-25 N-terminal peptides also affected Kv2.1 channel gating. S197 (1 nM) increased the rate of channel activation as measured by fitting the currents at +50 mV to a monoexponential function. The time constants for channel activation decreased from 6.9±0.6 ms (GST control, n=27) to 4.1±0.4 ms (S197, n=15; P<0.05) (Figures 1A and 1B) measured after 10 min of dialysis. S180 (1 nM) similarly accelerated the rate of channel activation to 4.8±0.7 ms (n=19; P<0.05) compared with control. A more prominent change was the slowing of the rate of channel inactivation. Current decay was fit with a monoexponential function to derive the time constants of channel inactivation. The time constants increased from 4.2±0.3 s (n=20) in the GST control to 6.1±0.7 s (S197, n=12; P<0.05) and 10.0±2.4 s (S180, n=15; P<0.05) (Figures 1C and 1D). In stark contrast, full-length S206 (1 nM S206) did not affect either the rate of activation (6.0±1.2 ms; n=4) or current inactivation (4.4±0.6 s; n=4). Neither S180, S197 or S206 had any significant effects on the voltage-dependence of channel activation (Figure 1E) or inactivation (results not shown). Because S197 and S180 exhibit very similar effects on Kv2.1 channel gating, we conclude that the active domain must be the S180 fragment, and gating is not dependent on the SNAP-25181–197 region.
Figure 1. Effect of N-terminal SNAP-25 domains on Kv2.1 channel gating.
(A) S180 and S197 accelerate the rate of current activation compared with control SNAP-25. Whole-cell normalized Kv2.1 current traces in HEK-293 cells in the absence and presence of GST (control), S180 or S197 (1 nM) are shown. Activation rates were measured from currents evoked by a 200 ms stepwise depolarization to +50 mV from a holding potential of −70 mV. Currents were fit with the monoexponential function I=A*exp−t/τ, where A is the amplitude, t is time and τ is the time constant. Summarized activation time constants (activation τ) of Kv2.1 using control, S180, S197 and S206 are shown in panel (B). (C) S180 and S197 but not S206 slow Kv2.1 current inactivation. Inactivation rates were determined from current evoked by a 10 s stepwise depolarization to +50 mV from a holding potential of −70 mV, and fit with a monoexponential function. (D) The summarized inactivation time constants (inactivation τ). (E) The voltage dependence of channel activation. Tail currents were measured at −70 mV following the 200 ms stepwise depolarizations from −70 to +70 mV. Data were fit with the Boltzmann function I/Imax=1/{1+exp[(V−V1/2)/k]}, where V1/2 is the half-maximal inactivation potential and k is the slope factor. *, P<0.05.
Binding of SNAP-25 domains to Kv2.1 channels
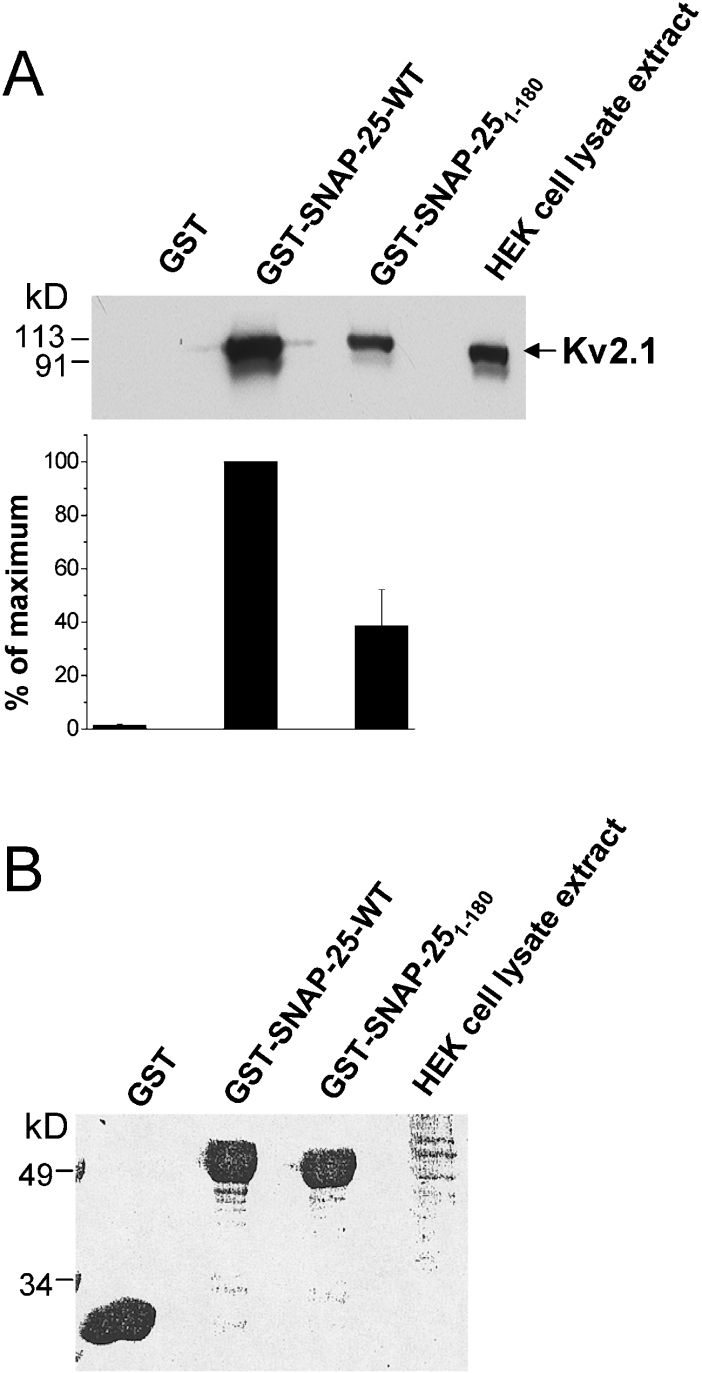
We determined that the effects of the N-terminal S180 domain are mediated through a direct interaction with the Kv2.1 channel, by GST pulldown assay. First, we examined whether S180 can bind to the full-length Kv2.1 channel. Figure 2(A) demonstrates that both full-length SNAP-25 (SNAP-25-WT) and S180 can physically interact with Kv2.1 and can pull down the channel from a HEK-293 cell lysate. An unexpected outcome was that S180 pulled down less Kv2.1 protein (38.6±13.6%) compared with WT SNAP-25.
Figure 2. Kv2.1 binds SNAP-25 and S180.
GST, GST–SNAP-25-WT or GST–S180 fusion proteins (all bound to glutathione–agarose beads, 550 pmol of protein each) were used to pull-down overexpressed Kv2.1 from an HEK-293 cell lysate extract. (A) Top panel shows a representative blot from three separate experiments. Cell lysate extracts from HEK-293 cells were used as a positive control. Bottom panel, a summary of three experiments is shown, in which the values (means±S.E.M.) are expressed as a percentage of the maximal binding (GST–SNAP-25-WT). (B) Ponceau S staining of the blot in order to demonstrate that the same amount of GST proteins was loaded.
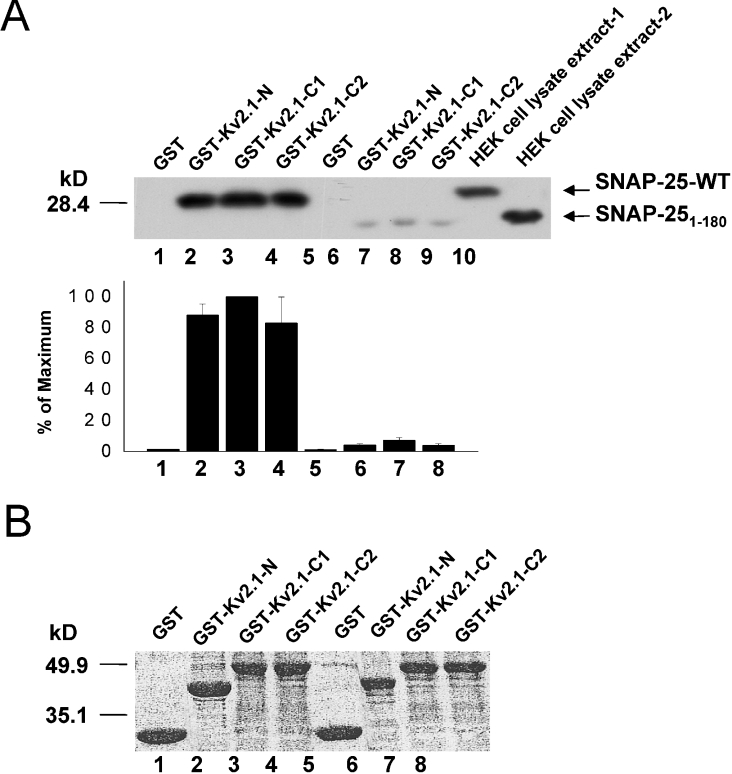
We performed the converse experiment whereby we determined the presence of binding of both WT SNAP-25 and S180 with GST fusion proteins containing the cytoplasmic N- and C-termini (C1, amino acids 412–633, C2, amino acids 634–853) of Kv2.1 (Figure 3A). As previously shown [9], SNAP-25 interacts with both the N and C-terminal domains of Kv2.1 (Figure 3A). S180 also bound to both the cytoplasmic N- and C-termini; however, we again observed significantly less binding compared with full-length SNAP-25.
Figure 3. SNAP-25 and S180 bind Kv2.1 cytoplasmic domain proteins.
Binding assays were performed using GST, GST–Kv2.1-N, –C1, or –C2 fusion proteins (all bound to glutathione–agarose beads, 450 pmol of protein) to pull-down overexpressed SNAP-25-WT or S180 from an HEK-293 cell lysate extract. (A) Top panel shows a representative Western blot of the precipitated SNAP-25 (lanes 1–4) and S180 proteins (lanes 5–8). The HEK-293 cell lysate extract-1 contained overexpressed SNAP-25-WT protein (lane 9), and -2 contained overexpressed S180 protein (lane 10). Bottom panel, a summary of the quantitative densitometric scanning of blots from three separate experiments. The values (means±S.E.M.) are expressed as a percentage of the maximum. (B) Representative Ponceau S staining of the blot shows that equal amounts of GST proteins were loaded.
We were surprised by the outcomes of the experiments shown in Figures 2(A) and 3(A), as the N-terminal SNAP-25 fragments were effective at modulating Kv2.1 channel gating, whereas S206 had no effect. It is possible that the C-terminal region of SNAP-25 is an important contributor to SNAP-25 binding to the channel. An alternative explanation is that simultaneous binding of S180 to both Kv2.1 N- and C-termini in vivo may occur to a much greater extent than S180 interactions with the individual cytoplasmic domains of the channel in vitro. Similar results were recently reported by Tsuk et al. [27] who demonstrated a greater extent of in vitro physical interaction of full-length SNAP-25 with both the N- and C-termini of Kv2.1 compared with when either Kv2.1 cytoplasmic domain was present alone.
SNAP-25 N-terminal domains potentiate TEA blockade
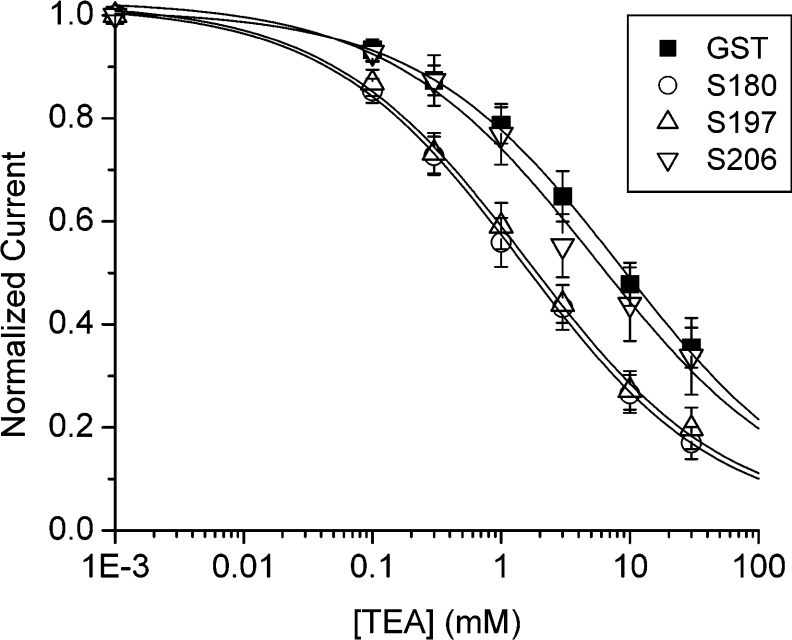
The observed effects of S180 and S197 on Kv2.1 current decay described above are reminiscent of a slowing in C-type inactivation [28,29]. A unique feature in the development of C-type inactivation is the sensitivity to external TEA or K+, resulting from their interference with the structural alterations in the outer pore vestibule of the Kv channel protein [30–32]. Specifically, an increase in extracellular K+ retards C-type inactivation [31,33] and enhances the external TEA block of the Kv channel pore [34]. This suggested the possibility that the N-terminal SNAP-25 fragments might conceivably alter the Kv2.1 channel pore region in a manner that would influence the TEA channel block. We dialysed a minimally active concentration of N-terminal GST–SNAP-25 domain proteins (1 pM for 5 min) or GST (control) into Kv2.1 channel-expressing HEK-293 cells, and then externally perfused the cell with increasing concentrations of TEA (Figure 4). Remarkably, both S180 and S197 produced a significant enhancement of external TEA block compared with WT SNAP-25. Specifically, the dose response for the TEA block showed a significant leftward shift in the IC50 from 4.9±0.8 mM (GST control, n=10) to 1.6±0.6 mM (S180, n=7) and 1.5±0.8 mM (S197, n=8) (P<0.05) (Figure 4).
Figure 4. N-terminal domains of SNAP-25 sensitize Kv2.1 channels to TEA inhibition.
HEK-293 cells transiently transfected with Kv2.1 were perfused externally with TEA from 0.01 to 30 mM in the presence of internally dialysed GST (1 pM, n=6), S180 (1 pM, n=5), S197 or S206 (1 pM, n=5). Currents evoked are as described in Figure 1 and were measured at +50 mV. The current in the presence of TEA was normalized to pre-drug values, and expressed as the fraction of the current that was blocked. Data were fit with the Hill equation I/Io=1/{1+(IC50h/[TEA]h)}, where I/Io is the fraction of current blocked, IC50 is the concentration at which 50% of the current is blocked by TEA, and h is the Hill coefficient.
We performed additional experiments on the cytoplasmic N- and C-terminal truncation mutants, Kv2.1ΔN139 and Kv2.1ΔC416, which lack the first 139 residues (1–139) and distal 416 amino acids (438–853) respectively. Dialysis of S180 (1 nM) into HEK-293 cells expressing Kv2.1ΔN139 had no effect on the TEA sensitivity of either Kv2.1ΔN139 (IC50 1.2±0.5 mM for GST control versus 1.2±0.9 mM for S180; n=5) or Kv2.1ΔC416 channels (IC50 2.1±1.2 mM for GST control versus 2.6±1.1 mM for S180; n=6–7). As observed in the binding results above, these data suggest the alteration in the external TEA block of Kv2.1 by the N-terminal SNAP-25 domains is dependent on functional association with both the N- and C-termini of Kv2.1.
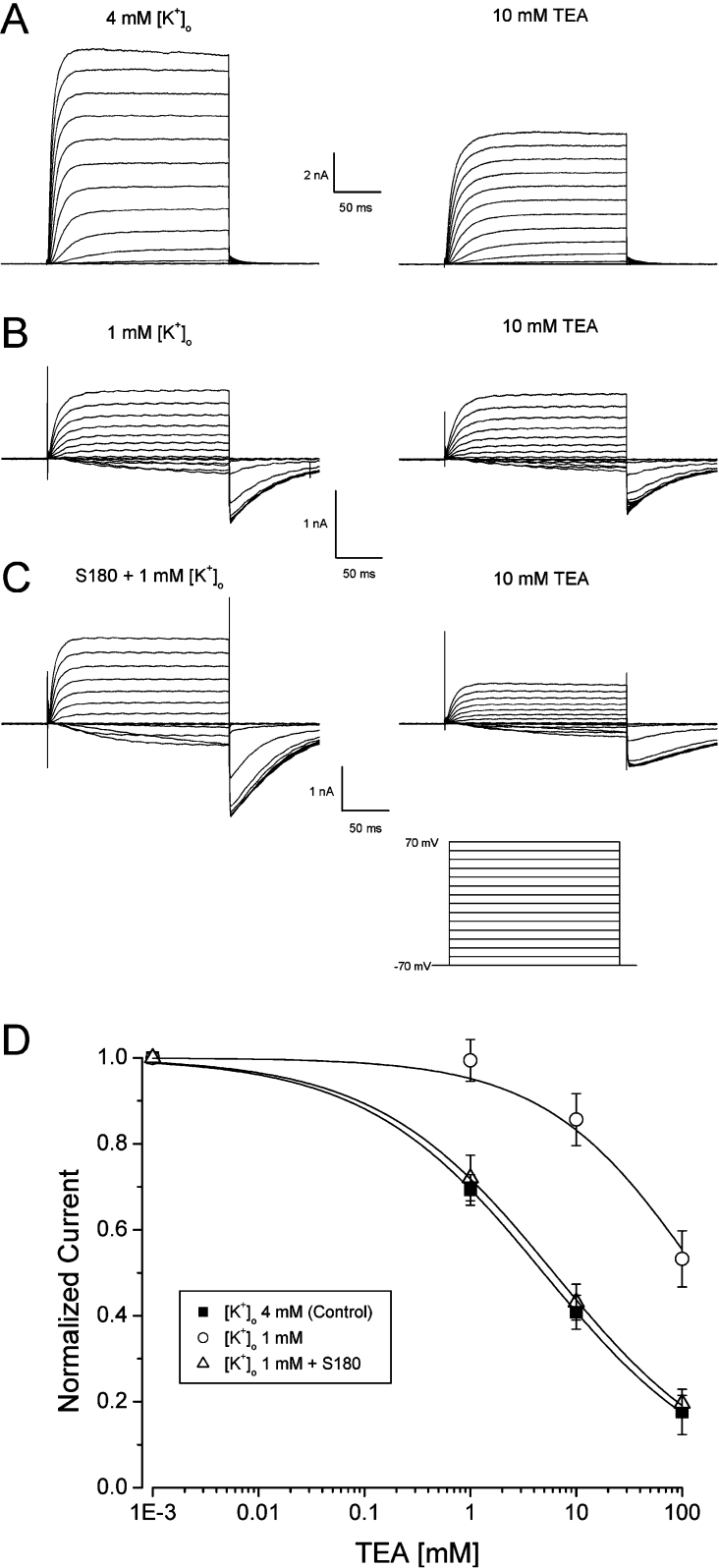
K+ dependency of the external TEA blockade of Kv2.1 is due to conformational rearrangements in the outer vestibule produced by changes in K+ ion occupancy in the selectivity filter [34,35]. Specifically, in the absence of external and internal K+ (Na+ substitution), Kv2.1 channels become insensitive to TEA blockade even up to 100 mM, but are still capable of allowing Na+ permeation [34,35] (as shown in Figure 5B). Since S180 was able to increase the sensitivity to the TEA block (Figure 4A), we examined whether S180 could increase and even possibly restore the TEA block of the Kv2.1 channel under conditions of absent or low external and internal K+ concentration. The IC50 of the TEA block of Kv2.1 channels recorded in the presence of a normal 4 mM [K+]o (external K+ concentration) was 6.8±0.9 mM (n=5) (Figure 5D). TEA (100 mM) did not inhibit outward Kv2.1 currents in HEK-293 cells in the absence of external K+ (equimolar 140 mM Na+ replacement; results not shown). When the [K+]o was decreased to 1 mM, 10 mM TEA elicited only a small decrease in Kv2.1 currents (Figure 5B) as expected. The IC50 for the TEA block under these recording conditions was >100 mM (Figures 5B and 5D). However, in the presence of 1 mM [K+]o and S180 (1 pM) inside the cell, the IC50 for the TEA block was shifted to the left to values that were indistinguishable from those in the control K+ and Na+ concentrations (7.9±1.5 mM, n=6) (Figures 5C and 5D). Thus at a low [K+]o (1 mM), S180 was able to sensitize Kv2.1 channels to TEA blockade to similar extent as in control [K+]o (4 mM). These results suggest that S180 may induce a long-range allosteric rearrangement of the outer pore regions leading to enhanced sensitivity to an external TEA channel block.
Figure 5. S180 enhances the TEA block under low [K+]o.
(A) Representative Kv2.1 currents recorded under normal (4 mM) or decreased (1 mM) [K+]o, in the absence (left panel) or presence of 10 mM TEA (right panel) with either GST or S180 in the pipette. Currents were elicited as described in Figure 1(A) with external 140 mM NaCl and 4 mM KCl (140 mM KCl in the pipette), or (B) and (C) under symmetrical 140 mM NaCl with decreased [K+]o (1 mM). (D) Dose-dependent blockade of Kv2.1 current measured under normal [K+]o (4 mM) (IC50 6.8±0.9 mM), decreased [K+]o (1 mM) (IC50>100 mM), or when internally dialysed with 1 pM S180 in the presence of 1 mM [K+]o (IC50 7.9±1.5 mM). n=5 or 6 cells.
DISCUSSION
In the present study, we have demonstrated novel functions for the N-terminal SNAP-25 domains on Kv2.1 channels. First, the N-terminal cleavage products, S180 and S197, bound to both the cytoplasmic N- and C-termini of Kv2.1 to modulate channel activation and C-type inactivation. Second, S180 and S197 increased the sensitivity of the Kv2.1 channel pore to external TEA blockade. We postulate that the N-terminal SNAP-25 domains modulate channel activation and C-type inactivation via long-range allosteric alterations of the outer pore vestibule in Kv2.1 channels through their interaction with the cytoplasmic Kv2.1 N- and C-terminal regions. Neither the full-length SNAP-25 nor the C-terminal SNAP-25 peptides alone had any effect on Kv2.1 gating or on TEA sensitivity.
We observed accelerated kinetics in channel opening in the absence of any change in the voltage dependence of channel activation using both S180 and S197, but not S206. SNAP-25 N-terminal domains may be modulating the inner Kv2.1 channel pore that is involved in channel opening. The lack of an effect on voltage-dependent activation suggests that there is no influence from S180 or S197 on the voltage sensitivity of S4 domain movement or stability of the closed and open states. Ju et al. [36] have demonstrated similar results when comparing rat with human Kv2.1 isoforms. The rat isoform was found to be activated much faster than human Kv2.1, but no difference was observed in the voltage dependence of channel activation between the two Kv2.1 channels. The authors further revealed that both the cytoplasmic N- and C-termini physically interact to regulate the kinetics of channel activation. Interestingly, we observed that the SNAP-25 N-terminal domains bind to both the cytoplasmic N- and C-terminal regions of Kv2.1, which we suggest induces conformational changes in these coupled channel domains that further influence channel gating.
C-type inactivation results from structural rearrangements of the outer pore region of Kv channels, which is influenced by pore residues [31,32,37], as well as in the S6 transmembrane segment [29] and the cytoplasmic C-terminus [28]. Increased K+ occupancy within the pore region slows the development of C-type inactivation [31]. In Kv2.1 channels, an increase in K+ occupancy of the external pore accelerates channel activation [38], slows inactivation [39] and increases TEA sensitivity [35], which is remarkably similar to the effects of the N-terminal SNAP-25 domains that we observed. How does the binding of these SNAP-25 domains elicit changes in C-type inactivation through conformational alterations in the structure of the Kv2.1 pore? The N-terminal SNAP-25 domains bind to both the N- and C-termini of Kv2.1. Both the cytoplasmic N- and C-termini of Kv2.1 are important for determining channel activation and inactivation kinetics [28,40]. Kv2.1ΔN139 displays slower activation kinetics and a small amount of C-type inactivation, whereas truncation of C-terminal residues hastens channel activation and retards inactivation compared with WT Kv2.1 [28]. The T1 (tetramerization) domain within this Kv2.1 N-terminus also interacts with the distal portion of the cytoplasmic C-terminus of Kv2.1 leading to faster activation kinetics [40]. The T1 domain has been suggested to lie under the inner conduction pathway and to interact directly with the C-terminal cytoplasmic domain [36,40]. Binding of the N-terminal SNAP-25 domain proteins to the cytoplasmic N-terminus of Kv2.1 may result in partial occlusion of the inner conduction pathway leading to a decrease in outward currents. The SNAP-25 N-terminal domains may also provide stronger coupling between the N- and C-termini. Such conformational changes have been observed in the C-terminus of the Shaker K+ channels upon binding of the Kvβ subunit to the N-terminal T1 domain [41]. Further studies will be required to dissect out these possibilities.
We have recently shown that SNAP-25 decreased Kv2.1 current in rat β-cells by acting on the Kv2.1 cytoplasmic N-terminal domain [9]. In the present study, we demonstrated that the S180 domain of SNAP-25 contains a putative active site for inhibiting Kv2.1 current, and have further showed its action in the fine modulation of channel activation and inactivation gating kinetics. The C-terminal SNAP-25 domains did not have any primary effects on Kv2.1, but this may explain the inability of the full-length SNAP-25 protein to influence the gating kinetics of the Kv2.1 channel, by negating the effects of the SNAP-25 N-terminus in modulating channel gating. These results demonstrate that the SNAP-25 protein contains both positive and negative regulatory domains, which may be revealed following proteolytic cleavage of SNAP-25 by BoNT/A and BoNT/E. Taken together with the results of previous work, our present study supports the idea that different domains within SNAP-25 (and STX1A) exert distinct actions on both voltage-gated Ca2+ and Kv2.1 channels.
Acknowledgments
The present study was supported by grants from the Heart and Stroke Foundation of Ontario (T5343 and T5537) and Canadian Institutes for Health Research (MOP 69083) to R.G.T. and H.Y.G. Y.H. is supported by a Postdoctoral Award from the Janssen-Ortho/Canadian Association of Gastroenterology, and Y.-M.L. is supported by a Canadian Diabetes Association Postdoctoral Fellowship Award. R.G.T. was supported by a New Investigator Award from the Heart and Stroke Foundation of Canada.
References
- 1.Lin R. C., Scheller R. H. Mechanisms of synaptic vesicle exocytosis. Annu. Rev. Cell Dev. Biol. 2000;16:19–49. doi: 10.1146/annurev.cellbio.16.1.19. [DOI] [PubMed] [Google Scholar]
- 2.Wiser O., Trus M., Hernandez A., Renström E., Barg S., Rorsman P., Atlas D. The voltage sensitive Lc-type Ca2+ channel is functionally coupled to the exocytotic machinery. Proc. Natl. Acad. Sci. U.S.A. 1999;96:248–253. doi: 10.1073/pnas.96.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang S.-N., Larsson O., Bränström R., Bertorello A. M., Leibiger B., Leibiger I. B., Moede T., Köhler T., Meister B., Berggren P.-O. Syntaxin interacts with the LD subtype of voltage-gated Ca2+ channels in pancreatic β cells. Proc. Natl. Acad. Sci. U.S.A. 1999;96:10164–10169. doi: 10.1073/pnas.96.18.10164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barg S., Ma X., Eliasson L., Galvanovskis J., Göpel S. O., Obermüller S., Platzer J., Renström E., Trus M., Atlas D., et al. Fast exocytosis with few Ca2+ channels in insulin-secreting mouse pancreatic β-cells. Biophys. J. 2001;81:3308–3323. doi: 10.1016/S0006-3495(01)75964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atlas D. Functional and physical coupling of voltage-sensitive calcium channels with exocytotic proteins: ramifications for the secretion mechanism. J. Neurochem. 2001;77:972–985. doi: 10.1046/j.1471-4159.2001.00347.x. [DOI] [PubMed] [Google Scholar]
- 6.MacDonald P. E., Ha X. F., Wang J., Smukler S. R., Sun A. M., Gaisano H. Y., Salapatek A. M. F., Backx P. H., Wheeler M. B. Members of the Kv1 and Kv2 voltage-dependent K+ channel families regulate insulin secretion. Mol. Endocrinol. 2001;15:1423–1435. doi: 10.1210/mend.15.8.0685. [DOI] [PubMed] [Google Scholar]
- 7.Leung Y. M., Kang Y. H., Gao X., Xia F., Xie H., Sheu L., Tsuk S., Lotan I., Tsushima R. G., Gaisano H. Y. Syntaxin 1A binds to the cytoplasmic C terminus of Kv2.1 to regulate channel gating and trafficking. J. Biol. Chem. 2003;278:17532–17538. doi: 10.1074/jbc.M213088200. [DOI] [PubMed] [Google Scholar]
- 8.Leung Y. M., Kang Y., Xia F., Sheu L., Gao X., Xie H., Tsushima R. G., Gaisano H. Y. Open form syntaxin-1A is more potent than the wild type form in inhibiting Kv2.1 channel: implications for membrane potential regulation in exocytosis. Biochem. J. 2005;387:195–202. doi: 10.1042/BJ20041625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacDonald P. E., Wang G., Tsuk S., Dodo C., Kang Y. H., Tang L., Wheeler M. B., Cattral M. S., Lakey J. R., Salapatek A. M. F., Gaisano H. Y. Synaptosome-associated protein of 25 kilodaltons modulates Kv2.1 voltage-dependent K+ channels in neuroendocrine islet β-cells through an interaction with the channel N terminus. Mol. Endocrinol. 2002;16:2452–2461. doi: 10.1210/me.2002-0058. [DOI] [PubMed] [Google Scholar]
- 10.Ohara-Imaizumi M., Nakamichi Y., Nishiwaki C., Nagamatsu S. Transduction of MIN6 β cells with TAT-syntaxin SNARE motif inhibits insulin exocytosis in biphasic insulin release in a distinct mechanism analyzed by evanescent wave microscopy. J. Biol. Chem. 2002;277:50805–50811. doi: 10.1074/jbc.M207988200. [DOI] [PubMed] [Google Scholar]
- 11.Dulubova I., Sugita S., Hill S., Hosaka M., Fernandez I., Südhof T. C., Rizo J. A conformational switch in syntaxin during exocytosis: role of munc18. EMBO J. 1999;18:4372–4382. doi: 10.1093/emboj/18.16.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jarvis S. E., Zamponi G. W. Distinct molecular determinants govern syntaxin 1A-mediated inactivation and G-protein inhibition of N-type calcium channels. J. Neurosci. 2001;21:2939–2948. doi: 10.1523/JNEUROSCI.21-09-02939.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fili O., Michaelevski I., Bledi Y., Chikvashvili D., Singer-Lahat D., Boshwitz H., Linial M., Lotan I. Direct interaction of a brain voltage-gated K+ channel with syntaxin 1A: functional impact on channel gating. J. Neurosci. 2001;21:1964–1974. doi: 10.1523/JNEUROSCI.21-06-01964.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu M. N., Fergestad T., Lloyd T. E, He Y., Broadie K., Bellen H. J. Syntaxin 1A interacts with multiple exocytic proteins to regulate neurotransmitter release in vivo. Neuron. 1999;23:593–605. doi: 10.1016/s0896-6273(00)80811-9. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez I., Ubach J., Dulubova I., Zhang X., Südhof T. C., Rizo J. Three-dimensional structure of an evolutionarily conserved N-terminal domain of syntaxin 1A. Cell. 1998;94:841–849. doi: 10.1016/s0092-8674(00)81742-0. [DOI] [PubMed] [Google Scholar]
- 16.Pasyk E. A., Kang Y., Huang X., Cui N., Sheu L., Gaisano H. Y. Syntaxin-1A binds the nucleotide-binding folds of sulphonylurea receptor 1 to regulate the KATP channel. J. Biol. Chem. 2004;279:4234–4240. doi: 10.1074/jbc.M309667200. [DOI] [PubMed] [Google Scholar]
- 17.Kang Y., Leung Y. M., Manning-Fox J. E., Xia F., Xie H., Sheu L., Tsushima R. G., Gaisano H. Y. Syntaxin-1A inhibits cardiac KATP channels by its actions on nucleotide binding folds 1 and 2 of sulfonylurea receptor 2A. J. Biol. Chem. 2004;279:47125–47131. doi: 10.1074/jbc.M404954200. [DOI] [PubMed] [Google Scholar]
- 18.Cui N., Kang Y., He Y., Leung Y. M., Pasyk E., Xie H., Gao X., Sheu L., Hansen J. B., Wahl P., Tsushima R. G., Gaisano H. Y. H3 domain of syntaxin 1A inhibits KATP channels by its actions on the sulfonylurea receptor 1 nucleotide-binding folds-1 and -2. J. Biol. Chem. 2004;279:53259–53265. doi: 10.1074/jbc.M410171200. [DOI] [PubMed] [Google Scholar]
- 19.Ji J., Yang S. N., Huang X., Li X., Sheu L., Diamant N., Berggren P.-O., Gaisano H. Y. Modulation of L-type Ca2+ channels by distinct domains within SNAP-25. Diabetes. 2002;51:1425–1436. doi: 10.2337/diabetes.51.5.1425. [DOI] [PubMed] [Google Scholar]
- 20.Michaelevski I., Chikvashvili D., Tsuk S., Singer-Lahat D., Kang Y., Linial M., Gaisano H. Y., Fili O., Lotan I. Direct interaction of target SNAREs with the Kv2.1 channel. Modal regulation of channel activation and inactivation gating. J. Biol. Chem. 2002;278:34320–34330. doi: 10.1074/jbc.M304943200. [DOI] [PubMed] [Google Scholar]
- 21.Sadoul K., Lang J., Montecucco C., Weller U., Regazzi R., Catsicas S., Wollheim C. B., Halban P. A. SNAP-25 is expressed in islets of Langerhans and is involved in insulin release. J. Cell Biol. 1995;128:1019–1028. doi: 10.1083/jcb.128.6.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niemann H., Blasi J., Jahn R. Clostridial neurotoxins: new tools for dissecting exocytosis. Trends Cell Biol. 1994;4:179–185. doi: 10.1016/0962-8924(94)90203-8. [DOI] [PubMed] [Google Scholar]
- 23.Gutierrez L. M., Viniegra S., Rueda J., Ferrer-Montiel A. V., Canaves J. M., Montal M. A peptide that mimics the C-terminal sequence of SNAP-25 inhibits secretory vesicle docking in chromaffin cells. J. Biol. Chem. 1997;272:2634–2639. doi: 10.1074/jbc.272.5.2634. [DOI] [PubMed] [Google Scholar]
- 24.Ferrer-Montiel A. V., Gutierrez L. M., Apland J. P., Canaves J. M., Gil A., Viniegra S., Biser J. A., Adler M., Montal M. The 26-mer peptide released by SNAP-25 cleavage by botulinum neurotoxin E inhibits vesicle docking. FEBS Lett. 1998;435:84–88. doi: 10.1016/s0014-5793(98)01012-6. [DOI] [PubMed] [Google Scholar]
- 25.Huang X., Wheeler M. B., Kang Y. H., Sheu L., Lukacs G. L., Trimble W. S., Gaisano H. Y. Truncated SNAP-25 (1–197), like botulinum neurotoxin A inhibits insulin secretion from HIT-T15 insulinoma cells. Mol. Endocrinol. 1998;12:1060–1070. doi: 10.1210/mend.12.7.0130. [DOI] [PubMed] [Google Scholar]
- 26.Lawrence G. W., Foran P., Mohammed N., DasGupta B. R., Dolly J. O. Importance of two adjacent C-terminal sequences of SNAP-25 in exocytosis from intact and permeabilized chromaffin cells revealed by inhibition with botulinum neurotoxins A and E. Biochemistry. 1997;36:3061–3067. doi: 10.1021/bi9622478. [DOI] [PubMed] [Google Scholar]
- 27.Tsuk S., Michaelevski I., Bentley G. N., Joho R. H., Chikvashvili D., Lotan I. Kv2.1 channel activation and inactivation is influenced by physical interactions of both syntaxin 1A and the syntaxin1A/soluble N-ethylmaleimide-sensitive factor-25 (t-SNARE) complex with the C terminus of the channel. Mol. Pharmacol. 2005;67:480–488. doi: 10.1124/mol.104.005314. [DOI] [PubMed] [Google Scholar]
- 28.VanDongen A. M., Frech G. C., Drewe J. A., Joho R. H., Brown A. M. Alteration and restoration of K+ channel function by deletions at the N- and C-termini. Neuron. 1990;5:433–443. doi: 10.1016/0896-6273(90)90082-q. [DOI] [PubMed] [Google Scholar]
- 29.Hoshi T., Zagotta N., Aldrich R. W. Two types of inactivation in Shaker K+ channels: effects of alterations in the carboxy-terminal region. Neuron. 1991;7:547–556. doi: 10.1016/0896-6273(91)90367-9. [DOI] [PubMed] [Google Scholar]
- 30.Choi K. L., Aldrich R. W., Yellen G. Tetraethylammonium blockade distinguishes two inactivation mechanisms in voltage-activated K+ channels. Proc. Natl. Acad. Sci. U.S.A. 1991;88:5092–5095. doi: 10.1073/pnas.88.12.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baukrowitz T., Yellen G. Modulation of K+ current by frequency and external [K+]: a tale of two inactivation mechanisms. Neuron. 1995;15:951–960. doi: 10.1016/0896-6273(95)90185-x. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y., Jurman M. E., Yellen G. Dynamic rearrangement of the outer mouth of a K+ channel during gating. Neuron. 1996;16:859–867. doi: 10.1016/s0896-6273(00)80106-3. [DOI] [PubMed] [Google Scholar]
- 33.López-Barneo J., Hoshi T., Heinemann S. H., Aldrich R. W. Effects of external cations and mutations in the pore region on C-type inactivation of Shaker potassium channels. Receptors Channels. 1993;1:61–71. [PubMed] [Google Scholar]
- 34.Ikeda S. R., Korn S. J. Influence of permeating ions on potassium channel block by external tetraethylammonium. J. Physiol. 1995;486:267–272. doi: 10.1113/jphysiol.1995.sp020809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Immke D., Wood M., Kiss L., Korn S. J. Potassium-dependent changes in the conformation of the Kv2.1 potassium channel pore. J. Gen. Physiol. 1999;113:819–836. doi: 10.1085/jgp.113.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ju M., Stevens L., Leadbitter E., Wray D. The roles of N- and C-terminal determinants in the activation of the Kv2.1 potassium channel. J. Biol. Chem. 2003;278:12769–12778. doi: 10.1074/jbc.M212973200. [DOI] [PubMed] [Google Scholar]
- 37.Yellen G., Sodickson D., Chen T. Y., Jurman M. E. An engineered cysteine in the external mouth of a K+ channel allows inactivation to be modulated by metal binding. Biophys. J. 1994;66:1068–1975. doi: 10.1016/S0006-3495(94)80888-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Consiglio J. F., Korn S. J. Influence of permeant ions on voltage sensor function in the Kv2.1 potassium channel. J. Gen. Physiol. 2004;123:387–400. doi: 10.1085/jgp.200308976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kiss L., Korn S. J. Modulation of C-type inactivation by K+ at the potassium channel selectivity filter. Biophys. J. 1998;74:1840–1849. doi: 10.1016/S0006-3495(98)77894-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Minor D. L., Lin Y. F., Mobley B. C., Avelar A., Jan Y. N., Jan L. Y., Berger J. M. The polar T1 interface is linked to conformational changes that open the voltage-gated potassium channel. Cell. 2000;102:657–670. doi: 10.1016/s0092-8674(00)00088-x. [DOI] [PubMed] [Google Scholar]
- 41.Sokolova O., Accardi A., Gutierrez D., Lau A., Rigney M., Grigorieff N. Conformational changes in the C terminus of Shaker K+ channel bound to the rat Kvβ2-subunit. Proc. Natl. Acad. Sci. U.S.A. 2003;100:12607–12612. doi: 10.1073/pnas.2235650100. [DOI] [PMC free article] [PubMed] [Google Scholar]