Abstract
The Slr1991 adenylyl cyclase of the model prokaroyte Synechocystis PCC 6803 was stimulated 2-fold at 20 mM total Ci (inorganic carbon) at pH 7.5 through an increase in kcat. A dose response demonstrated an EC50 of 52.7 mM total Ci at pH 6.5. Slr1991 adenylyl cyclase was activated by CO2, but not by HCO3−. CO2 regulation of adenylyl cyclase was conserved in the CyaB1 adenylyl cyclase of Anabaena PCC 7120. These adenylyl cyclases represent the only identified signalling enzymes directly activated by CO2. The findings prompt an urgent reassessment of the activating carbon species for proposed HCO3−-activated adenylyl cyclases.
Keywords: adenylyl cyclase (adenyl cyclase, adenylate cyclase); bicarbonate (HCO3−); cAMP; carbon dioxide (CO2); Synechocystis
Abbreviations: (s)AC, (soluble) adenylyl cyclase; Ci, inorganic carbon
INTRODUCTION
Inorganic carbon (Ci) is fundamental to the physiology of all organisms. CO2 and HCO3− exist in a pH-dependent equilibrium and are the major biologically active forms of Ci. CO2 and HCO3− are vital to such diverse physiological processes as photosynthetic carbon fixation [1], pH homoeostasis [2] and carbon metabolism [3]. Study of Ci biology is essential to understand these vital physiological processes. Relatively little is known of the signalling mechanisms through which prokaryotic and eukaryotic cells directly detect CO2/HCO3− fluctuations [4]. The identification of Ci-activated signalling molecules and their role in physiology is fundamental to understanding the diverse roles of Ci in biology. Currently, no signalling enzymes directly activated by CO2 are known.
The mammalian sAC (soluble adenylyl cyclase) synthesizes the second messenger 3′,5′-cAMP and is stimulated by HCO3− [5,6]. It was observed that HCO3− regulation of AC (adenylyl cyclase) was conserved in a cyanobacterial AC, CyaC of Spirulina (Arthrospira) platensis, which had significant sequence similarity in the AC domain to sAC [6]. More recently, an active-site Asp→Thr polymorphism in the Class III AC family has been proposed as a marker for HCO3−-responsiveness [7]. On this basis, proposed HCO3−-responsive ACs are predicted to be widespread among the genomes of prokaryotes and eukaryotes [8]. To date, Ci regulation of AC has been confirmed in prokaryotes as diverse as Anabaena PCC 7120, Mycobacterium tuberculosis, Stigmatella aurantiaca and Chloroflexus aurantiacus [7,9]. An implicit assumption is made in the literature that the activating Ci ligand for AC is HCO3−, on the basis that the ionic form is more likely to bind in the active site than CO2. Identification of the activating carbon ligand for AC is essential to validate or question the relevance of significant recent literature in the field.
The photosynthetic cyanobacteria are an excellent model for investigating Ci signalling through AC, since hypothesized HCO3−-responsive ACs are widespread in these organisms and Ci has clearly defined roles in their physiology. Here we demonstrate that the single Class III AC, Slr1991 (Cya1), of the unicellular cyanobacterium Synechocystis PCC 6803, is activated by Ci. Furthermore, we demonstrate, surprisingly, that the activating ligand for this enzyme is CO2 and not HCO3−. A previously characterized proposed HCO3− regulated AC, CyaB1 of Anabaena PCC 7120, is also shown to respond to CO2 rather than HCO3−. The present work provides the first evidence for AC as a CO2-activated signalling molecule. This original finding prompts an immediate reassessment of the true activating carbon species in reported HCO3−-responsive ACs.
MATERIALS AND METHODS
Recombinant proteins
DNA corresponding to amino acids 120–337 of slr1991 was isolated by PCR from the genomic DNA of Synechocystis PCC 6803, subcloned into pQE30, and fitted with an N-terminal MRGSH6GS dodecapeptide affinity tag. Constructs were confirmed by double-stranded sequencing. Slr1991120–337 protein was expressed in Escherichia coli M15 [pREP4] cells at 25 °C, for 3 h with 300 μM isopropyl β-D-thiogalactoside. Pelleted cells were washed with 50 mM Tris/HCl (pH 8.5)/1 mM EDTA, resuspended in 50 mM Tris/HCl (pH 8.5)/250 mM NaCl/10 mM 1-thioglycerol, lysed by sonication (1×150 s) and protein was purified from the supernatant with Ni2+-nitrilotriacetic acid (Qiagen) as previously described [10]. CyaB1595–859 protein was generated as previously described [10]. Primer sequences are available on request from M. J. C.
AC assays
AC assays were performed at 40 °C in a final volume of 100 μl and typically contained 50 mM buffer, 2 mM MnCl2, 2 mM [2,8-3H]cAMP (150 Bq) and [α-32P]ATP (25 kBq) as substrate, if not stated otherwise [11]. Protein concentrations were adjusted to maintain substrate utilization at less than 10%. Kinetic constants were determined over a concentration range of substrate (Mn2+-ATP) of 1–100 μM. The following buffers were used: pH 6.5, Mes; pH 7.0–7.5, Mops; pH 8.0–8.5, Tris/HCl; and pH 9.0, Ches [2-(N-cyclohexylamino)ethanesulphonic acid]. Enzyme, buffer and substrate were all prepared at the appropriate pH for the required assay. CO2 was quantified by titration against NaOH. The assay pH was stable over a period of at least 40 min. All errors correspond to the S.E.M. If absent, errors are smaller than the symbol used to depict the data point.
RESULTS AND DISCUSSION
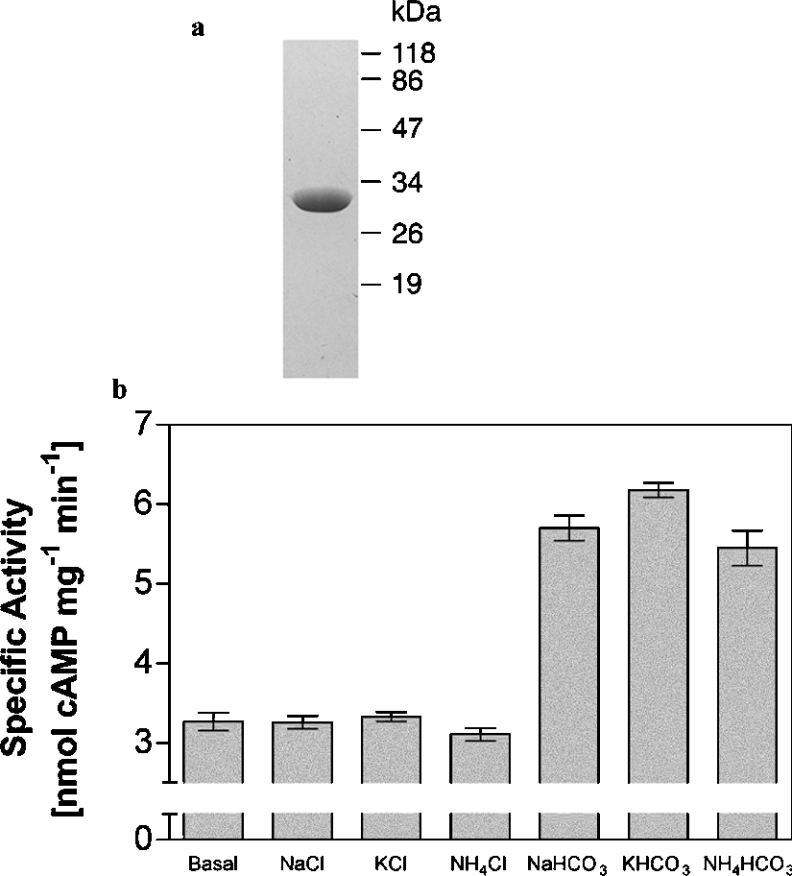
The cya1 (slr1991; http://www.kazusa.or.jp/cyano/Synechocystis) gene of the unicellular cyanobacterium Synechocystis PCC 6803 encodes an enzyme consisting of a single FHA (forkhead associated) domain and a Class III AC domain that contains an Asp→Thr polymorphism associated with a putative HCO3− responsiveness [7,12]. We expressed the AC domain of Slr1991 as a purified recombinant protein (Figure 1a). The purified wild-type protein had a significant AC specific activity in the presence of both Mg2+-ATP (154±2.0 pmol of cAMP·min−1·mg−1, n=8) and Mn2+-ATP (5816±87 pmol of cAMP·mg−1·min−1, n=8) under optimal conditions (pH 9.5, 40 °C, 0.3 mM ATP and 8 μM protein).
Figure 1. AC activity of purified recombinant Slr1991120–337.
(a) Purification of recombinant Slr1991120–337 (SDS/PAGE analysis and Coomassie Blue staining). A 1.5 μg portion of protein was applied and molecular-mass standards (in kDa) are indicated. (b) Slr1991120–337 specific activity (n=8) in the presence of 20 mM total Ci/salt (0.6 μM protein and 20 μM Mn2+-ATP, pH 7.5).
The Slr1991120–337 protein had a pH optimum of 9.5 and a temperature optimum of 40 °C. The enthalpy of activation (EA) derived from the linear arm of an Arrhenius plot using Mn2+-ATP was 33.5±1.4 kJ·mol−1 (n=6). We investigated whether Slr1991120–337 was regulated by Ci with a view to determining the identity of the activating species, CO2 or HCO3−. Slr1991120–337 specific activity was stimulated 2-fold by 20 mM total Ci (1.2 mM CO2/18.8 mM HCO3−) at pH 7.5 compared with Cl−. Stimulation was independent of cation and robust to 95% confidence intervals (Figure 1b). A previous report by Masuda and Ono [13] had not observed stimulation of Slr1991 by Ci at pH 7.5. We noted that an extended assay period (40 min) was required to observe robust Ci activation of Slr1991 at pH 7.5. Although Masuda and Ono [13] did not report the assay time, this is the most likely cause of the discrepancy.
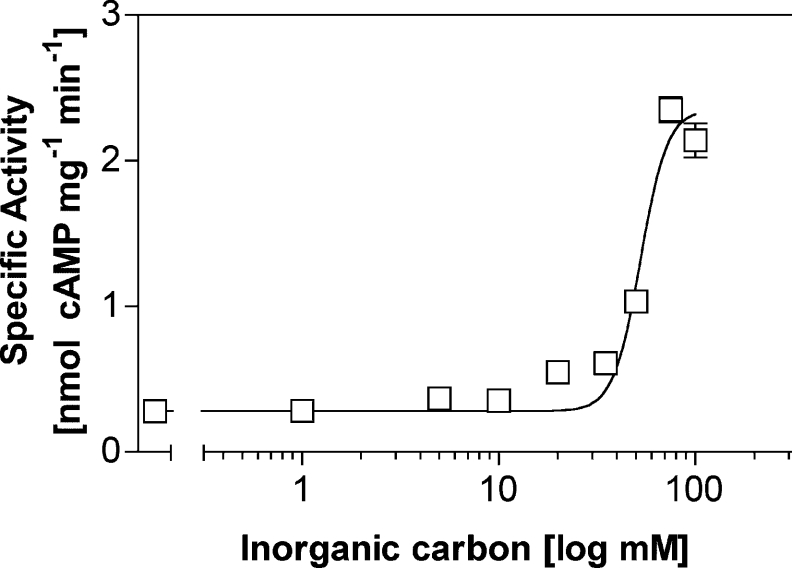
We determined the kinetics of activation of Slr1991120–337 by Ci (Table 1). Slr1991120–337 showed Michaelis–Menten kinetics in the presence of both Cl− and Ci. The Km value for Mn2+-ATP was greater in the presence of Ci than Cl−, but Vmax values were proportionately greater for Ci than Cl−. The overall result was that Ci increased turnover rate (kcat). A dose–response curve with increasing Ci was performed at a reduced pH (6.5) to eliminate problems with enzyme inhibition at >20 mM total Ci at pH 7.5 in the presence of Mn2+-ATP (Figure 2). The experiment revealed a maximum 8-fold stimulation with an apparent EC50 for Ci of 52.7±1.0 mM (n=6) (20.4 mM CO2/32.3 mM HCO3−).
Table 1. Kinetic parameters for Slr1991120–337.
Protein at 0.6 μM was assayed at pH 7.5 in the presence of 20 mM salt (n=6).
| Value | |||
|---|---|---|---|
| Parameter | Addition… | Cl− | HCO3− |
| Vmax (nmol of cAMP·min−1·mg−1) | 0.74±0.01 | 1.13±0.03 | |
| Km,ATP (μM) | 11.4±0.7 | 16.2±1.3 | |
| Hill slope | 1.01±0.01 | 1.03±0.03 | |
| kcat (min−1) | 0.018 | 0.027 | |
Figure 2. Response of wild-type Slr1991120–337 to Ci.
Slr1991120–337 specific activity (n=6) was plotted against increasing total Ci (‘inorganic carbon’). The assay mixture contained 1.5 μM protein and 20 μM Mn2+-ATP, pH 6.5, with Na+ as cation. The total salt concentration was adjusted to 200 mM with NaCl.
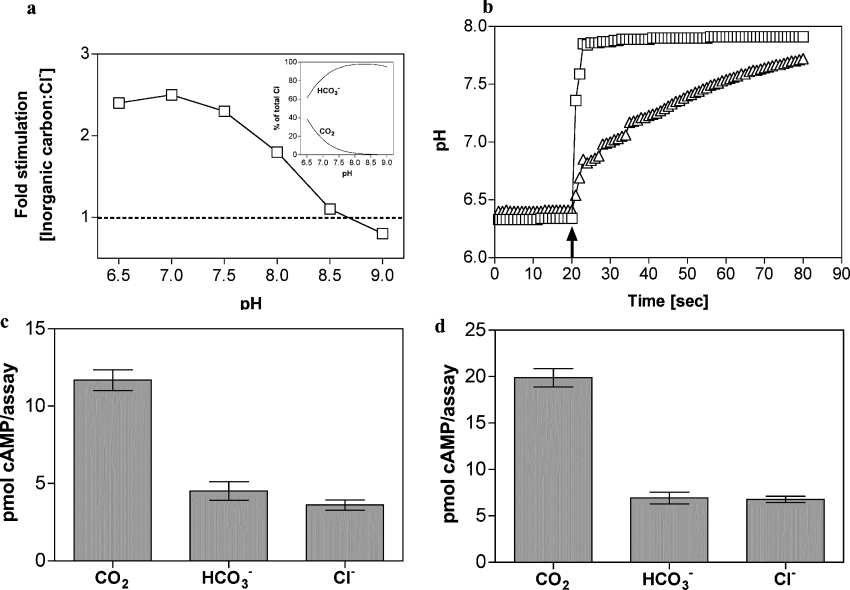
We investigated the response of Slr1991120–337 to total Ci at various pH values to gain an insight into whether the enzyme is responsive to CO2 and/or HCO3−. The experiment was performed using Mg2+-ATP as substrate since Mg2+ cofactor is more soluble than Mn2+ in the presence of Ci at alkaline pH. Intriguingly, relative stimulation (Ci/NaCl) varied from 1.1 at pH 8.5 (0.3 mM CO2/39.1 mM HCO3−/0.6 mM CO32−) to 2.4 at pH 6.5 (15.5 mM CO2/24.5 mM HCO3−) (Figure 3a). This is consistent with a role for CO2 as opposed to HCO3− as the activating carbon species, but may also be due to the altered protonation status of the enzyme limiting the ability of Slr1991 to respond to HCO3− at elevated pH. We therefore sought direct evidence for regulation of Slr1991120–337 by CO2 and/or HCO3− by analysis under conditions of Ci disequilibrium when a single predominant carbon species, CO2 or HCO3−, is present at a defined pH. We exploited the fact that acquisition of the equilibrium between CO2 and HCO3− is significantly lowered at reduced temperature in the absence of carbonic anhydrase and is a well established method for identifying the Ci substrate for CO2/HCO3−-fixing enzymes [14]. We followed the acquisition of the CO2/HCO3− equilibrium by measuring the pH of a weakly buffered (5 mM) Mes solution on addition of 20 mM CO2 or 20 mM NaHCO3 in the presence or absence of carbonic anhydrase at 0 °C (Figure 3b). On the basis of these results we defined conditions for assaying AC under conditions of disequilibrium using 20 mM CO2 or HCO3− as a 10 s assay period at 0 °C after addition of Ci. Under these conditions, Ci is predominantly in the form added to the assay (CO2 or HCO3−) and has not significantly advanced toward the equilibrium determined by assay pH (held with 100 mM Mes). Control experiments demonstrated that, under the conditions used for the assay, the final pH was equivalent when CO2, HCO3− or Cl− were added, demonstrating that any observed stimulation was due to addition of Ci and not a change in assay pH (results not shown).
Figure 3. Activation of AC by CO2.
(a) Ratio of the specific activities of Slr1991120–337 when assayed in the presence of 40 mM total Ci or NaCl at various pH values (8 μM protein, 1 mM Mg2+-ATP and 20 mM Mg2+). The inset shows the percentage of total Ci made up by CO2 and HCO3− over the pH range tested. (b) Change in pH of a 5 mM Mes solution (starting pH 6.4) on addition of 20 mM NaHCO3 (↑) in the presence (□) or absence (△) of 132 units of carbonic anhydrase at 0 °C. (c) cAMP produced by Slr1991120–337 under conditions of Ci disequilibrium (50 μM Slr1991120–337 protein, 0 °C, 10 s, 20 mM CO2/NaHCO3/NaCl, 100 mM Mes, pH 6.5, 150 μM Mn2+-ATP). (d), cAMP produced by CyaB1595–859 under conditions of Ci disequilibrium (38 μM CyaB1595–859 protein, 0 °C, 10 s, 20 mM CO2/NaHCO3/NaCl, 100 mM Mes, pH 6.5, 150 μM Mn2+-ATP, n=6).
We assayed Slr1991120–337 under conditions of Ci disequilibrium and observed, surprisingly, that CO2, but not HCO3−, stimulated the enzyme (Figure 3c). We investigated whether this highly significant result was unique to Slr1991 or of more general significance. The CyaB1595–859 protein of Anabaena PCC 7120 was previously shown to respond to HCO3−/CO2, but the activating species was not demonstrated [7]. Consistent with the findings for Slr1991120–337, CyaB1595–859 was also stimulated by CO2, but not HCO3−, under conditions of Ci disequilibrium (Figure 3d).
These results demonstrate that, for at least two randomly selected prokaryotic ACs, Slr1991 and CyaB1, the activating carbon species is dissolved CO2 and not the more ably binding HCO3− species. These enzymes therefore represent the first identified signalling molecules demonstrated to respond directly to CO2. HCO3− regulation of AC has been proposed, but not proven, for enzymes from species as diverse as the cyanobacterium Spirulina platensis, the encapsulated yeast-like fungus Cryptococcus neoformans, the yeast Candida albicans, the photosynthetic bacterium Chloroflexus aurantiacus and mammals [6,9,15,16]. An urgent examination of these systems is required to prove whether the ACs defined from these species respond to HCO3− or to CO2 as described here.
Acknowledgments
We thank Professor Alistair Hetherington, Department of Biological Sciences, Lancaster University, Lancaster, U.K., for helpful discussions. The BBSRC (Biotechnology and Biological Sciences Research Council) supported this work.
References
- 1.Badger M. R., Price G. D. CO2 concentrating mechanisms in cyanobacteria: molecular components, their diversity and evolution. J. Exp. Bot. 2003;54:609–622. doi: 10.1093/jxb/erg076. [DOI] [PubMed] [Google Scholar]
- 2.Adrogue H. E., Adrogue H. J. Acid–base physiology. Respir. Care. 2001;46:328–341. [PubMed] [Google Scholar]
- 3.Smith K. S., Ferry J. G. Prokaryotic carbonic anhydrases. FEMS Microbiol. Rev. 2000;24:335–366. doi: 10.1111/j.1574-6976.2000.tb00546.x. [DOI] [PubMed] [Google Scholar]
- 4.Hetherington A. M., Raven J. A. The biology of carbon dioxide. Curr. Biol. 2005;15:R406–R410. doi: 10.1016/j.cub.2005.05.042. [DOI] [PubMed] [Google Scholar]
- 5.Buck J., Sinclair M. L., Schapal L., Cann M. J., Levin L. R. Cytosolic adenylyl cyclase defines a unique signaling molecule in mammals. Proc. Natl. Acad. Sci. U.S.A. 1999;96:79–84. doi: 10.1073/pnas.96.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y., Cann M. J., Litvin T. N., Iourgenko V., Sinclair M. L., Levin L. R., Buck J. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science. 2000;289:625–628. doi: 10.1126/science.289.5479.625. [DOI] [PubMed] [Google Scholar]
- 7.Cann M. J., Hammer A., Zhou J., Kanacher T. A defined subset of adenylyl cyclases is regulated by bicarbonate ion. J. Biol. Chem. 2003;278:35033–35038. doi: 10.1074/jbc.M303025200. [DOI] [PubMed] [Google Scholar]
- 8.Cann M. Bicarbonate stimulated adenylyl cyclases. IUBMB Life. 2004;56:529–534. doi: 10.1080/15216540400013861. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi M., Buck J., Levin L. R. Conservation of functional domain structure in bicarbonate-regulated “soluble” adenylyl cyclases in bacteria and eukaryotes. Dev. Genes Evol. 2004;214:503–509. doi: 10.1007/s00427-004-0432-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanacher T., Schultz A., Linder J. U., Schultz J. E. A GAF-domain-regulated adenylyl cyclase from Anabaena is a self-activating cAMP switch. EMBO J. 2002;21:3672–3680. doi: 10.1093/emboj/cdf375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal. Biochem. 1974;58:541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- 12.Kaneko T., Sato S., Kotani H., Tanaka A., Asamizu E., Nakamura Y., Miyajima N., Hirosawa M., Sugiura M., Sasamoto S., et al. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 1996;3:109–136. doi: 10.1093/dnares/3.3.109. [DOI] [PubMed] [Google Scholar]
- 13.Masuda S., Ono T. A. Adenylyl cyclase activity of Cya1 from the cyanobacterium Synechocystis sp. strain PCC 6803 is inhibited by bicarbonate. J. Bacteriol. 2005;187:5032–5035. doi: 10.1128/JB.187.14.5032-5035.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper T. G., Filmer D. The active species of “CO2” utilized by ribulose diphosphate carboxylase. J. Biol. Chem. 1969;244:1081–1083. [PubMed] [Google Scholar]
- 15.Klengel T., Liang W. J., Chaloupka J., Ruoff C., Schroppel K., Naglik J. R., Eckert S. E., Mogensen E. G., Haynes K., Tuite M. F. Fungal adenylyl cyclase integrates CO2 sensing with cAMP signaling and virulence. Curr. Biol. 2005;15:2021–2026. doi: 10.1016/j.cub.2005.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steegborn C., Litvin T. N., Levin L. R., Buck J., Wu H. Bicarbonate activation of adenylyl cyclase via promotion of catalytic active site closure and metal recruitment. Nat. Struct. Mol. Biol. 2005;12:32–37. doi: 10.1038/nsmb880. [DOI] [PMC free article] [PubMed] [Google Scholar]