Abstract
Tubulysin A (tubA) is a natural product isolated from a strain of myxobacteria that has been shown to depolymerize microtubules and induce mitotic arrest. The potential of tubA as an anticancer and antiangiogenic agent is explored in the present study. tubA shows potent antiproliferative activity in a panel of human cancer cell lines irrespective of their multidrug resistance properties. It induces apoptosis in cancer cells but not in normal cells and shows significant potential antiangiogenic properties in several in vitro assays. It is efficacious in initial animal studies using a hollow fibre assay with 12 different human tumour cell lines. This study suggests that both in vitro and preclinical profiles of tubA may translate into clinically useful anticancer properties.
Keywords: antiangiogenesis, anticancer agent, cytochrome P450, microtubule, tubulin, tubulysin A
Abbreviations: CA4 Prodrug®, combretastatin A4 prodrug; CYP, cytochrome P450 enzyme; DMEM, Dulbecco's modified Eagle's medium; GI50, 50% growth inhibition activity; HCT-116, human colorectal carcinoma; HL-60, human promyelocytic leukaemia; HUVEC, human umbilical-vein endothelial cells; 2ME2, 2-methoxyoestradiol; Pgp, P-glycoprotein; PtK2, Potorous tridactylis kidney epithelial cell; TGI, total growth inhibition; TNP-470, O-(chloroacetylcarbamoyl) fumagillol; tubA, tubulysin A; VEGF, vascular endothelial growth factor
INTRODUCTION
Most drugs currently available for the treatment of cancer are mechanistically based on the inhibition of cell proliferation and induction of apoptosis. These include cytotoxic agents [1,2] as well as drugs that are designed to inhibit or interfere with specific signal transduction pathways [3–5]. Agents interfering with microtubule assembly play an important role in currently available anticancer chemotherapy [1,6]. Microtubules are indispensable for cellular life, especially for cell growth and mitosis, maintenance of cell morphology and for the induction and progression of apoptosis, which makes them an attractive and intensively investigated anticancer target.
Microtubules are dynamic structures composed of α- and β-tubulin heterodimers that rapidly undergo both polymerization and depolymerization steps in a process referred to as polymerization dynamics. Microtubules show two types of non-equilibrium dynamics, treadmilling and dynamic instability, both crucial to cell division. In dividing cells, microtubules play a critical role in establishing the mitotic spindle apparatus [7]. A large number of natural compounds, including Taxol® (paclitaxel), colchicine, dolastatin, vinblastine, vinorelbine, Taxotere®, HTI-286 (N,β,β-trimethyl-L-phenylalanyl-N1-[(1S,2E)-3-carboxy-1-isopropylbut-2-enyl]-N1,3-dimethyl-L-valinamide), 2ME2 (2-methoxyoestradiol), CA4 Prodrug® (combretastatin A4 prodrug), discodermolide, eleutherobin, epothilones and cryptophycins, among others, have been shown to modulate microtubule dynamics in either the direction of polymerization or depolymerization [8–14]. This leads to cell cycle arrest at the metaphase/anaphase (G2/M phase) transition in the cell cycle and finally the induction of apoptosis. Therefore agents that target microtubules can be divided into two classes. One class includes agents like the vinca alkaloids (e.g. vinblastine and vinorelbine), colchicine and dolastatins, which inhibit the polymerization of microtubules in vitro. The second class consists of natural products like paclitaxel and epothilones that favour microtubule polymerization, thereby affecting the treadmilling process. The clinical success of paclitaxel and the vinca alkaloids as cancer therapeutics, as well as their disadvantages, has prompted the search for new anticancer compounds that target microtubules.
One of the greatest limitations of cancer chemotherapy is the development of multidrug resistance in patients. This enables the cancer cells to escape chemotherapy by developing a broad-spectrum resistance to anticancer drugs [15]. Multidrug resistance is often mediated by overexpression of transmembrane cellular pumps, such as Pgp (P-glycoprotein) and MDR (multidrug resistance) protein [16] along with other factors, including overexpression of key proteins involved in apoptosis, e.g. Bcl-2 (B-cell lymphocytic-leukaemia proto-oncogene 2), Bcl-xL, survivin, post-translational modifications and expression of different tubulin isotypes [1,17]. Long-term treatment with cancer drugs is also associated with severe side effects, e.g. colchicine has an unfavourable therapeutic index that precludes its clinical use as anticancer agent. Despite the success of several classes of tubulin-binding agents, they are generally limited by poor therapeutic indices, emerging drug resistance phenotypes, suboptimal pharmacokinetics due to formulation restrictions resulting from their poor aqueous solubility, and severe toxicity.
Among microorganisms producing biologically active natural products, the genus Myxobacterium is especially productive. The epothilones disorazol, chondramide B, apicularen, gephyronic acid and rhizopodin are bioactive natural products isolated from myxobacteria [18,19]. Recently, the family of tubAs (tubulysin A) was isolated for the first time from Archangium geophyra, a myxobacterial species, accessible via fermentation [20].
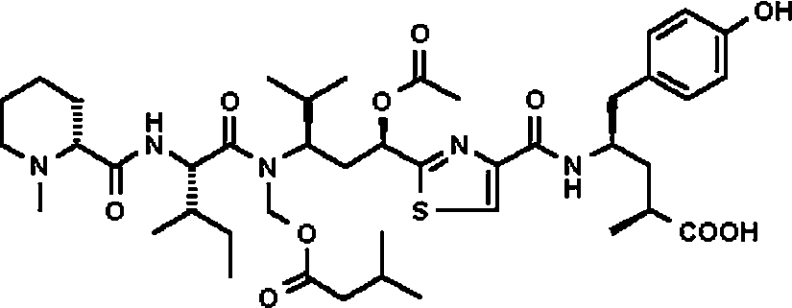
tubA, a member of this family, although peptidic in nature, contains mostly atypical amino acids and a central thiazole heterocycle. It also contains a basic N-terminal tertiary amine and an aromatic and acidic C-terminal end (Figure 1). The effect of tubA on microtubules was evaluated at the biochemical and cellular levels [21]. Biochemically, 1 μM of tubA affected the polymerization of tubulin by 46% in an in vitro assay using 10 μM purified tubulin. At the cellular level, tubA disrupted microtubule arrays in a time-dependent fashion in PtK2 cells (Potorous tridactylis kidney epithelial cells) treated with 50 ng/ml tubA [20] and induced a time-dependent mitotic cell cycle arrest in G2/M transition phase [8]. tubA affected the cell growth of L929 (mouse fibroblast) and KB-V1 cells (human cervix carcinoma, a multidrug resistance cell line) with the IC50 values of 0.07 and 1.4 ng/ml respectively [21]. Furthermore, it has been shown that nine different derivatives of tubA, namely tubulysins B–I (tubB–tubI), can also be isolated in small quantities from two different species of myxobacteria, Ar. geophyra and Angiococcus disciformis. The GI50 (50% growth inhibition activity) values of these derivatives range from 0.011 to 0.68 ng/ml with tubD being the most active one, but the ability to inhibit tubulin polymerization in an in vitro assay remains the same as tubA for all derivatives, which may reflect differences in cellular uptake. Further, the more lipophilicity among tubA–tubI correlated with better antiproliferative activity [21].
Figure 1. Chemical structure of tubA.
In the present study, the profile of tubA in a panel of human cancer cell lines, including the activity in various multidrug resistance cell lines, is described. Herein, we also report the induction of apoptosis by tubA in cancer cells but not in normal cells. Further, we demonstrate that tubA is neither a substrate for CYPs (cytochrome P450 enzymes) nor a substrate for drug efflux pumps. Moreover, this agent shows potential antiangiogenic activity as judged by both cell migration and cord formation using HUVEC (human umbilical-vein endothelial cells). tubA given via intraperitoneal route is active in an in vivo hollow-fibre assay in mice.
MATERIALS AND METHODS
Cell lines and compounds
All cells lines, except the 60-cancer cell line panel from the NCI, were purchased from A.T.C.C. (Manassas, VA, U.S.A.). Cell lines were maintained according to the instructions provided by A.T.C.C. in a 5% CO2 humidified incubator maintained at 37 °C temperature. Paclitaxel and vinblastine were purchased from Biomol (Plymouth Meeting, PA, U.S.A.) and tubA was isolated from fermentation broths of myxobacteria and subsequently purified using chromatographic techniques as described by Sasse et al. [20].
Proliferation assays
Cells were plated on to 96-well plates in 50 μl of media at a density of 70000 cells/well for HL-60 (human promyelocytic leukaemia) cells or 5000 cells/well for HCT-116 (human colorectal carcinoma) and HCT-15 cells.
Compounds were serially diluted into cell media as 2× stocks containing 1% DMSO and 50 μl of each dilution was added to the wells in triplicate. After 48 h of incubation with the compounds, cell survival was assessed by adding 10 μl of WST-1 [2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulphophenyl)-2H tetrazolium] reagent (Roche Applied Science) in each well of a 96-well plate. After incubation (3 h for HL-60 and 1 h for HCT-116 and HCT-15 cells), the plates were read in a plate reader (Spectramax 250; Molecular Devices, Sunnyvale, CA, U.S.A.) at 450 nm. The zero time for cells with only 0.5% DMSO was also assessed for each plate and was subtracted from the 48 h time points. The XLfit program (ID Business Solutions, Parsippany, NJ, U.S.A.) was used to obtain and calculate the GI50 curves for each compound using a nonlinear regression curve fit utilizing Lavenburg–Marquardt algorithm. Each experiment was done a minimum of six times.
The antiproliferative activity of tubA in a panel of 60 human cancer cell lines was performed at the NCI using a standard SRB (sulphorhodamine B) assay [22] and tubA was tested at five serial 1 log dilutions (concentrations 0.01–100 μM) using the standard 48 h incubation. All COMPARE analyses were performed at the NCI as described by Paull et al. [23].
Apoptosis assays
The DNA fragmentation assay [24] was conducted with HL-60 cells. Briefly, 106 cells/well were plated on to a 24-well plate. The cells were incubated with or without the compound in 0.5% DMSO for the time indicated. After incubation, the cells were collected, the DNA was extracted and subjected to gel electrophoresis as described in [24].
Quantification of apoptotic cells was performed using HL-60 (2×105 cells/well) plated on to a 6-well plate. After 24 h, drug or vehicle control was added at two times the final concentration in the plate. HUVEC were plated at 0.15×105 cells/10 ml of media in a 100 mm dish. After 2 days, media were replaced and drug was added to 70% confluent dishes. At indicated times, media and cells were collected, washed, centrifuged and analysed for the presence of apoptotic cells using the Annexin V staining kit (Guava Nexin™ kit) on Guava PCA (Guava Technologies, Hayward, CA, U.S.A.) using the instructions provided by the manufacturer. Results are shown as the percentage of apoptotic cells. Triplicates were used in all experiments and each experiment was repeated at least three times.
CYP inhibition
Microsomal preparations of insect cells (Gentest, Woburn, MA, U.S.A.), expressing recombinant human CYPs (CYP1A2, CYP3A4 and CYP2D6), were used in this assay. The assay was performed using fluorogenic substrates (7-ethoxy-3-cyanocoumarin for CYP1A2, dibenzylfluorescein for CYP3A4 and 7-methoxy-4-aminomethyl coumarin for CYP2D6) and standard positive controls (furafylline for CYP1A2, ketoconazole for CYP3A4 and quinidine for CYP2D6). The final DMSO concentration in all assays was maintained at 1% and the assay was performed according to the manufacturer's instructions. For each test, a blank signal was determined using vehicle-treated cells with no compounds and no fluorogenic substrate. The blank signal was subtracted from all sample signals. The IC50 values for furafylline (0.7 μM), ketoconazole (0.026 μM) and quinidine (0.016 μM) are consistent with the values reported in the literature [25].
In vitro angiogenesis assays
All assays were performed as described by Kaur et al. [26].
Proliferation assay
Briefly, HUVEC (1.5×103) were plated on to a 96-well plate in 100 μl of EBM-2 (endothelial basal medium-2; Clonetics, Walkersville, MD, U.S.A.). After 24 h (day 0), the test compound was added and cells were exposed to the drug for an additional 72 h. The day 0 absorbance was subtracted from the 72 h plates and data were plotted as a percentage of untreated control.
Cord formation assay
The test compound (500 μl of two times the final concentration) was mixed with 500 μl of cells (2×105 cells/ml) and 200 μl of this suspension was placed into duplicate wells containing polymerized Matrigel®. After 24 h, triplicate pictures were taken for each well using a Bioquant Image Analysis system. Drug effects were assessed by measuring the length of cords (mm) and the number of junctions formed. The analysis was done using the XLfit program as described in the Proliferation assays section.
Cell migration assay
Migration was assessed using 8 μm pore size 96-well Neuroprobe disposable chambers (Neuroprobe, Gaithersburg, MD, U.S.A.). The bottom microplate wells received DMEM (Dulbecco's modified Eagle's medium) (baseline) or DMEM containing the chemoattractant, VEGF (vascular endothelial growth factor; 10 ng/ml). The top chambers received HUVEC cell suspension (1×106 cells/ml) prepared in DMEM containing 1% BSA with or without test compound. After 5 h incubation at 37 °C, the filter was processed as described in [26]. Unstimulated control values (no VEGF) were subtracted from the stimulated control (VEGF) and the drug-treated values. The data were plotted as the mean migrated cells and the IC50 was calculated from the plotted data using the XLfit program as described in the Proliferation assays section. The viability of the cells after tubA treatment was assessed in both cell migration and cord formation assays using Trypan Blue.
Hollow fibre assay
Early animal studies at the NCI were conducted with 12 different human cancer cell lines in a hollow fibre assay in mice [27,28]. Briefly, each mouse received three intraperitoneal implants (one of each tumour cell line) and three subcutaneous implants (one of each tumour cell line as in intraperitoneal implants). For each experiment, three mice/dose were used. Vehicle controls consisted of six mice that received only the vehicle, 10% (v/v) DMSO in saline/0.05% Tween 80® (Sigma, St. Louis, MO, U.S.A.). Evaluation of 12 tumour cell lines required a total of four experiments, each containing three tumour cell lines. tubA was administered at two doses, 0.04 and 0.06 mg/kg, via the intraperitoneal route once daily for 4 days. Twenty four hours after the last treatment, the fibres were collected, assessed for viable cell mass, using a stable endpoint MTT (2,3-bis-(2-methoxy-4-nitro-5-sulphophenyl)-2H-tetrazolium-5-carboxanilide, disodium salt) cell viability assay, and percentage net cell growth and the scores were calculated by comparing with the zero time viable cell mass as described by the NCI [27,28].
RESULTS
tubA is a potent antiproliferative agent
The growth inhibitory activity of tubA was assessed with a 48 h incubation in the NCI 60-human cancer cell line screen as described in the Materials and methods section. Endpoint parameters, including the GI50 and TGI (total growth inhibition), were calculated for each cell line tested. The TGI is defined as the drug concentration that is needed to completely inhibit cell growth (cytostasis). The results shown in Supplementary Figure S1 (at http://www.BiochemJ.org/bj/396/bj3960235add.htm) are an average of two independent experiments and clearly show that tubA is extremely potent in this screen. Thus it was not possible to calculate the GI50 for this compound in the concentration range used in this assay. The average GI50 for all 60 cell lines was calculated as 12 nM. This is an estimate since, for most cell lines, the minimum test concentration of tubA was not low enough to calculate a GI50. Thus the average GI50 was calculated using the lowest concentration (10 nM) evaluated for those cell lines. Although we were not able to see any distinct pattern in GI50, tubA shows a distinct pattern for TGI in the NCI 60-cancer cell line screen for a panel of melanoma, leukaemia and prostate cancers suggesting specificity for certain tumour types. In a COMPARE analysis, the TGI profile of tubA correlated with other tubulin-binding agents (results not shown).
Since in the NCI screen we were unable to calculate the GI50 value for tubA, we decided to examine its ability to inhibit tumour cell growth and compare the growth inhibitory effect with other anti-microtubule agents in a few cell lines. Cell growth was assessed in HL-60, and HCT-116 and HCT-15 cells following a 2 day continuous exposure to candidate agents. tubA inhibited the cell growth at subnanomolar concentrations as shown in Table 1 and shows more potent antiproliferative activity than paclitaxel and vinblastine in these cell lines.
Table 1. tubA is a potent antiproliferative agent and is not a substrate for Pgp.
| GI50 (nM) | |||
|---|---|---|---|
| Compound | HL-60 | HCT-116 | HCT-15 |
| tubA | 0.059±0.03 | 0.007±0.004 | 0.10±0.12 |
| Paclitaxel | 1.95±1.01 | 0.066±0.064 | 139±40 |
| Vinblastine | 0.94±0.51 | 0.072±0.08 | 14.4±7.8 |
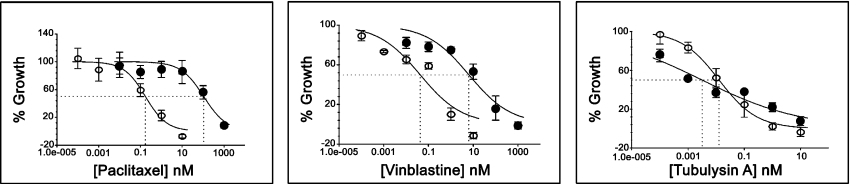
It is clear from Supplementary Figure S1 (at http://www.BiochemJ.org/bj/396/bj3960235add.htm) that tubA is active in multidrug resistant cell lines at a similar concentration to that of non-multidrug resistant cell lines (e.g. HCT-116), but the lack of GI50 data in this screen prompted us to further investigate the effect of tubA on cell proliferation using HCT-15, a colon cancer cell line that has very high expression of Pgp compared with HCT-116 (a colon cancer cell line that has very low expression of Pgp). The GI50 values for tubA in these two cell lines were also compared with the activities of paclitaxel and vinblastine (Table 1). It is clear from Table 1 and Figure 2 that the GI50 value of tubA shows only approx. 15-fold difference in both cell lines (GI50 of 0.1 versus 0.007 nM). In comparison, paclitaxel shows approx. 2000-fold less activity (GI50 of 139 versus 0.066 nM) and vinblastine shows approx. 200-fold less activity (GI50 of 14 versus 0.07 nM) in HCT-15 cells compared with HCT-116 cells. Furthermore, the compounds that are substrates for Pgp show a strong negative correlation in COMPARE analysis using molecular targets [29–31]. The Pearson correlation coefficient with multidrug resistance activity by rhodamine efflux for tubA using TGI values is only −0.263 and the correlation with MDR1 expression is −0.194.
Figure 2. tubA is not a substrate for Pgp.
GI50 curves for paclitaxel, vinblastine and tubA in HCT-15 (●) and HCT-116 (○) cell lines. Each error bar represents the standard deviation for three data points. The intersected line in each graph represents GI50.
tubA induces apoptosis
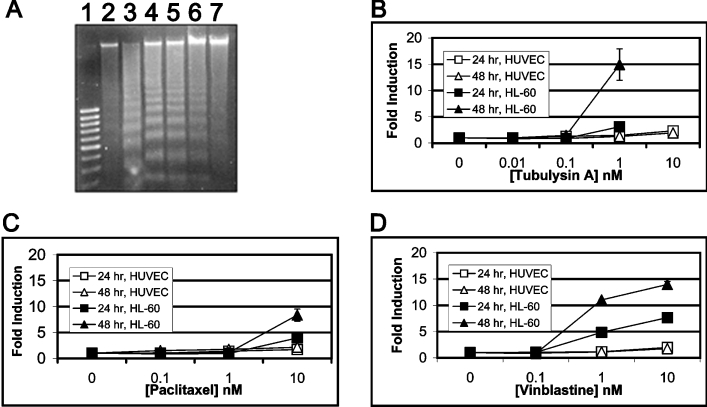
All anti-microtubule agents identified to date are known to induce apoptotic cell death in cancer cells. Therefore, to determine the ability of tubA to affect the induction of apoptosis, we used a DNA fragmentation assay with camptothecin as a positive control [24]. The activity of tubA was assessed against HL-60 cells after a 24 h exposure to four different concentrations (0.1–100 nM). As shown in Figure 3(A), tubA induces DNA laddering, a hallmark of apoptosis, in the nanomolar concentration range in these cells.
Figure 3. tubA induces apoptosis in HL-60 cells but not in HUVEC.
(A) DNA fragmentation. Lane 1, 100 bp DNA marker; lane 2, vehicle control; lane 3, 1.25 μM camptothecin; lanes 4–7, 100, 10, 1 and 0.1 nM tubA respectively. (B–D) Time- and dose-dependent induction of annexin V-positive cells treated with tubA (B), paclitaxel (C) or vinblastine (D) in both normal (HUVEC; open symbols) or cancer (HL-60; filled symbols) cells. GI50 curves for 24 h (square) and 48 h (triangle) endpoints for both cell lines. Each error bar represents the standard deviation for three data points.
Consistent with an induction of DNA laddering, we also observed an increase in annexin V-positive cells when treated with tubA, paclitaxel or vinblastine as compared with the untreated cells in a time- and dose-dependent manner (Figures 3B–3D). Annexin V-positive cells are indicative of induction of apoptosis. There is at least 10-fold difference in the induction of apoptosis in cancer (HL-60) versus normal (HUVEC) cells as shown in Figures 3(B)–3(D) for all three agents. tubA at a very high concentration of 10 nM does not show any significant apoptosis in HUVEC. However, in HL-60 tumour cell line, a 15-fold increase in apoptosis is observed even at 1 nM (Figure 3B), and at 10 nM all the cells had undergone apoptosis (results not shown). In contrast, both paclitaxel and vinblastine show 10–15-fold apoptosis only at 10 nM in HL-60 cells as compared with HUVEC (Figures 3C and 3D).
tubA is not a substrate for CYPs
The CYPs constitute a superfamily of isoforms that play an important role in the oxidative metabolism of drugs. Each CYP isoform possesses a characteristic broad spectrum of catalytic activities on substrates. The ability of a single CYP to metabolize multiple substrates is responsible for a large number of documented drug interactions associated with CYP inhibition [32,33]. From the viewpoint of avoiding potential drug interactions, it is desirable to develop a new drug candidate that is not a potent CYP inhibitor. Thus careful evaluation of potential drug interactions of a new drug candidate during early stages of development is beneficial. To evaluate the inhibitory effect of tubA on three major CYPs (CYP1A2, CYP3A4 and CYP2D6), we have used microsomal preparations of insect cells (Gentest) expressing recombinant human CYPs. In general, the potential of a drug to induce drug–drug interactions is classified as low if IC50>100 μM, moderate if IC50=10–100 μM, high if IC50=1–10 μM, and very high if IC50<1 μM. The activity of tubA was compared with paclitaxel and vinblastine in this assay and the results, presented in Table 2, indicate that like paclitaxel and vinblastine, tubA shows a low potential for interaction with three major CYP.
Table 2. tubA is not a substrate for key CYPs.
| IC50 (μM) | ||||
|---|---|---|---|---|
| Compound | Enzyme… | CYP1A2 | CYP2D6 | CYP3A4 |
| tubA | >100 | >100 | 82 | |
| Paclitaxel | >100 | >100 | >100 | |
| Vinblastine | >100 | >100 | >100 | |
tubA shows potential antiangiogenic activity
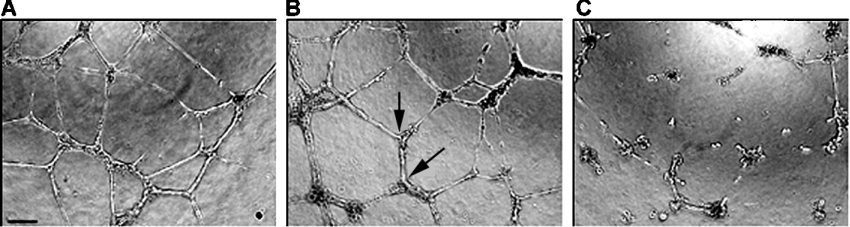
Angiogenesis is the phenomenon by which new blood vessels are generated in vivo [34]. The newly generated blood vessels are needed for the growth of cancer cells at the tumour site. Tubulin-binding agents like paclitaxel, 2ME2 and CA4 Prodrug® have been shown to have excellent antiangiogenic properties [35] and are currently being evaluated in several clinical trials. Therefore we evaluated tubA for its potential antiangiogenic activity as predicted by in vitro assays of proliferation, cord formation and cell migration assays using HUVEC. These studies used HUVEC and compared tubA with TNP-470 [O-(chloroacetylcarbamoyl) fumagillol] and paclitaxel. TNP-470 is a small molecule with antiangiogenic properties that is often used as a standard when evaluating other potential small molecule inhibitors [34]. As shown in Figures 4(A)–4(C) and Table 3, it is clear that the treatment of cells with tubA resulted in a dramatic decrease in both the total number of cord junctions (IC50 of 2.07 nM) as well as the average length of the cords (IC50 of 2.97 nM). This behaviour is similar to that of the controls, TNP-470 and paclitaxel. The cell migration was also severely impaired by the 5 h treatment with tubA (IC50 value of 2.26 nM; Table 3) in a concentration range that did not affect the cell growth in 5 h treatment as estimated by counting the total number of live cells for each concentration and comparing it with the vehicle control (results not shown). For cell growth inhibition, HUVEC were incubated in the presence of tubA for 72 h, resulting in a GI50 value of 0.34 nM. The cumulative results of all three assays, presented in Table 3, indicate that tubA is far more potent than the positive controls, TNP-470 or paclitaxel. These results suggest that tubA may have potential antiangiogenic activity.
Figure 4. tubA shows significant potential antiangiogenic effect in HUVEC in in vitro assays.
(A–C) cord formation. (A) Vehicle (1% DMSO) treatment; (B, C) 1 and 10 nM tubA respectively. The two arrows in (B) represent two cord junctions and the distance between these two arrows is the length of a cord. Cord junctions and lengths were calculated from six images per data point. The scale bar in (A) indicates the magnification of ×10.
Table 3. Potential antiangiogenic effects of tubA in HUVEC.
| IC50 (nM) | ||||
|---|---|---|---|---|
| GI50 (nM) | Cord formation | |||
| Compound | Growth inhibition | Junctions | Length | Cell migration |
| tubA | 0.34 | 2.07 | 2.97 | 2.26 |
| TNP-470 | 3.16 | 1000 | 1000 | 500 |
| Paclitaxel | 1.65 | 50 | 50 | 100 |
In vivo efficacy of tubA in hollow fibre assays
tubA was evaluated at the NCI in the hollow fibre assay in mice using 12 different human cancer cell lines. Briefly, the hollow fibres were implanted in mice at both intraperitoneal and subcutaneous sites [27]. tubA was administered intraperitoneally at two doses, 0.04 and 0.06 mg/kg, given once daily for 4 days. The results are presented in Table 4. In this assay, tubA inhibited cell growth in 33% of the samples tested, producing a hollow fibre score of 32.
Table 4. tubA is effective in a short-term in vivo assay.
The assessment of tubA in hollow fibre xenograft assays in 12 different tumour cell lines. i.p., intraperitoneal; s.c., subcutaneous; NSCLC, non-small-cell lung cancer; CNS, central nervous system.
| Net growth (%)* | ||||
|---|---|---|---|---|
| Tumour type | Cell line | Dose (mg/kg) | i.p. | s.c. |
| Lung/NSCLC | NCI-H23 | 0.06 | 100 | 71 |
| 0.04 | 98 | 77 | ||
| NCI-H522 | 0.06 | 62 | >100 | |
| 0.04 | 60 | 89 | ||
| Colon | SW-620 | 0.06 | 83 | 78 |
| 0.04 | >100 | >100 | ||
| COLO205 | 0.06 | 63 | 80 | |
| 0.04 | 100 | >100 | ||
| Ovarian | OVCAR-5 | 0.06 | 40 | 86 |
| 0.04 | 47 | 50 | ||
| OVCAR-3 | 0.06 | 45 | 76 | |
| 0.04 | 59 | >100 | ||
| Melanoma | LOX IMVI | 0.06 | 66 | >100 |
| 0.04 | 98 | >100 | ||
| UACC-62 | 0.06 | 64 | >100 | |
| 0.04 | >100 | 96 | ||
| CNS | U251 | 0.06 | 52 | 80 |
| 0.04 | 57 | 82 | ||
| SF-295 | 0.06 | 30 | 97 | |
| 0.04 | 53 | >100 | ||
| Breast | MDA-MB435 | 0.06 | 26 | 80 |
| 0.04 | 30 | >100 | ||
| MDA-MB231 | 0.06 | 57 | 82 | |
| 0.04 | 48 | 87 | ||
*Percentage treated/control.
DISCUSSION
Microtubules are a well-established drug target for the treatment of cancer. Tubulin-binding agents affect the growth of solid tumours in two different ways; first, inhibiting tumour cell growth by disrupting the microtubule dynamics causing cell cycle arrest, and secondly, through a more recently observed inhibitory activity on neovascularization. Angiogenesis is one of the important biological requirements for the growth of solid tumours beyond a few millimetres [34]. An angiogenesis inhibitor in combination with an antimitotic agent may provide improved therapy for combating various cancers [36] as evidenced by recent approval of Avastin® (antiangiogenic) in combination with 5-Fluorouracil® (RNA/DNA antimetabolite) for the treatment of colorectal cancer. Further, several clinical trials are currently ongoing for the combination of Squalamine® (antiangiogenic agent) [37] with paclitaxel or cisplatin for ovarian and lung cancers. Before this study, tubA was known to inhibit microtubule formation and inhibit cell growth, but its potential anticancer activity as judged by its antiproliferative activity in various cancer cell types and, most importantly, its in vivo activity and potential antiangiogenic properties were not investigated previously. In the present study, we demonstrated the potential utility of the tubAs as a new class of anticancer compounds by investigating its in vitro and preliminary in vivo efficacy. We used tubA as a prototype for this novel class of peptidic compounds to demonstrate its potent antiproliferative and potential antiangiogenic properties.
tubA was shown previously to depolymerize microtubules followed by cell cycle arrest in PtK2 and L929 cells [21]. It showed activity in multidrug resistant cell lines using engineered human cervix carcinoma cell lines [21]. In the present study, we profiled tubA for its antiproliferative activity in the NCI 60-cell line screen and used the TGI profile in COMPARE analysis, which correlated with other tubulin-binding agents. As shown previously, we also observed that the antiproliferative activity of tubA is in the subnanomolar range irrespective of the cell's Pgp expression level and the maximum difference in two cell lines observed was approx. 15-fold, which is not a significant difference [Supplementary Figure S1 (at http://www.BiochemJ.org/bj/396/bj3960235add.htm); Figure 2 and Table 1]. Both vinblastine and paclitaxel are 200- and 2000-fold respectively less active in the HCT-15 cell line, a very high Pgp expresser, compared with HCT-116 cells, which have low Pgp expression. In the correlation studies with multidrug resistance activity as well as with MDR1 expression, tubA scored a very low negative value, suggesting that it is not likely to be a substrate for Pgp. Thus tubA may have a better antiproliferative profile than currently available drugs in this class, such as paclitaxel and vinblastine that are known to be a substrate for Pgp, although it remains to be seen. However, from day to day experiments, we have observed difference in GI50 values for all three compounds as evident by rather large S.D. in Table 1, but the trend remained the same among different experiments. Figure 3 represents one such experiment. One explanation for the large S.D. may be the potent effect of these compounds on cells. Thus it may not be possible to generate very reproducible GI50 values. Further, tubA showed 1–2 log more potent TGI activity as compared with the average TGI value for a panel of melanoma cells from the 60-cancer cell line screen from the NCI, suggesting its selectivity for certain tumour types. However, the real potential of this agent as an anticancer agent will be revealed by its careful future evaluation in in vivo xenograft assays.
Often, if not always, cancer treatment involves combinations of several drugs. Thus it is beneficial to evaluate the possibility of drug–drug interactions for any potential drug agents to determine their clinical utility. tubA was checked for this by evaluating its inhibitory activity on three major CYP enzymes in an in vitro assay. As shown in Table 2, tubA does not interact with any of these enzymes, indicating that this class of compounds should not alter the metabolism of other drugs when used in combination for the treatment of cancer, as is the case with paclitaxel.
To date, several anti-microtubule agents, like paclitaxel, CA4 Prodrug®, 2ME2 and the dolastatins, were shown to have antiangiogenic activity in vivo. Herein, we report the potential antiangiogenic properties of tubA in HUVEC-based in vitro assays and compare the data with paclitaxel and a known small molecule inhibitor, TNP-470. tubA shows similar IC50 values for both cord formation and cell migration (Figure 4 and Table 3). Inhibition of new vessel formation, inhibition of metastasis and antiproliferative activity may prove an added advantage for this class of compounds. These results only predict the potential use of tubA as an antiangiogenic agent, but its true potential as an antiangiogenic agent needs further evaluation in preclinical models. Whether potent antiangiogenic activity will be an added advantage along with its antiproliferative activity for potential clinical use in the treatment of solid cancers remains to be seen.
Furthermore, tubA at 1 nM concentration shows 15-fold less sensitivity in the induction of apoptosis in normal cells (HUVEC) compared with cancer cells (HL-60) at the 48 h time point, but at 10 nM all cancer cells underwent apoptosis (results not shown), while in normal cells there was practically no induction of apoptosis at this concentration (Figure 3B). Based on the in vitro data on apoptosis, we hypothesize that most likely the in vitro data will translate into the animal and clinical settings and that tubA would be less likely to be more toxic than currently used paclitaxel or vinblastine.
The in vivo efficacy of tubA at both 0.04 and 0.06 mg/kg doses in the hollow fibre assay indicates a clinical potential for this class of compounds. The 12 cancer cell lines used in the assay include a total of six cancer types. tubA inhibited cell growth in 33% of the samples tested, which falls among the top 5% of 3290 compounds tested in a similar fashion at the NCI [38]. The formulation of tubA included 10% DMSO in saline/0.05% Tween 80®, similar to 3290 other drugs tested in this assay at the NCI so that the sensitivity of this agent can be compared with others. However, unlike other drugs in this category such as paclitaxel and vinblastine, tubA is soluble in polar solvents like water and ethanol, which can be used to further evaluate the effects of this agent in future animal studies and to develop this compound for clinical use.
It has been observed previously that all xenograft-positive agents are also positive in the hollow fibre assay [39]. Furthermore, it has been recently shown that this assay can be successfully used as a short-term in vivo model system for studying pharmacodynamic endpoints of both standard and novel compounds designed for microtubules [40]. Thus it is important to screen all compounds that affect microtubule dynamics first in hollow fibre assays before testing them in xenograft models. tubA scored very highly in hollow fibre assays, but whether it will perform similarly in xenograft models remains to be seen.
In conclusion, both in vitro and in vivo data suggest that the natural product tubA is a potent antiproliferative compound with in vivo activity and potential antiangiogenic properties. It is effective at subnanomolar concentrations in a wide variety of human cancer cells including those with multidrug resistance. It is soluble in polar solvents, which will be a great advantage in developing this class of compounds as compared with other drugs in this category that are not easily formulated due to insolubility. Further work on semi-synthetic derivatives of tubA will provide a structure–activity relationship as well as desired pharmacokinetic properties; such studies will be useful in advancing this class of compounds for potential clinical use in the treatment of a variety of solid tumours. A study of various natural derivatives of tubA indicates that lipophilicity is important for cell-based activity [21]; thus this will provide the basis for synthesis of semi-synthetic derivatives of tubA for future studies. Most tubulin-binding agents induce neurotoxicity, despite their success in clinical settings. Thus further use of animal models to investigate the neurotoxicity of tubA and its derivatives is an absolute requirement for determining the clinical potential for this class of compounds. Furthermore, it is clear that total synthesis of this class of compounds might not be possible due to structural constraints; however, a recent attempt to assemble genetic tools to produce tubA biosynthetically [41] gives a hope for the development of this class of compounds in future as potential anticancer agents.
Online data
Acknowledgments
We thank Nicole Brusis from Morphochem AG for providing technical assistance. We extend special thanks to Dr Gerhard Höfle and his laboratory at German Research Center for Biotechnology (Germany) for providing tubA used in this study. We also thank Professor David Stern (Yale University), Dr Maureen Gilmore-Hebert (Yale University) and Dr Sukumar Sakakmuri (Morphochem Inc.) for a critical reading of this paper. A portion of the studies reported here was supported under the NCI contract NO1-CO-12400.
References
- 1.Jordan M. A., Wilson L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 2.Cozzi P., Mongelli N., Suarato A. Recent anticancer cytotoxic agents. Curr. Med. Chem. Anticancer Agents. 2004;4:93–121. doi: 10.2174/1568011043482061. [DOI] [PubMed] [Google Scholar]
- 3.Reed J. C. Apoptosis-targeted therapies for cancer. Cancer Cell. 2003;3:17–22. doi: 10.1016/s1535-6108(02)00241-6. [DOI] [PubMed] [Google Scholar]
- 4.Miles D. W. Update on HER-2 as a target for cancer therapy: herceptin in the clinical setting. Breast Cancer Res. 2001;3:380–384. doi: 10.1186/bcr326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tiseo M., Loprevite M., Ardizzoni A. Epidermal growth factor receptor inhibitors: a new prospective in the treatment of lung cancer. Curr. Med. Chem. Anticancer Agents. 2004;4:139–148. doi: 10.2174/1568011043482106. [DOI] [PubMed] [Google Scholar]
- 6.Prinz H. Recent advances in the field of tubulin polymerization inhibitors. Expert Rev. Anticancer Ther. 2002;2:695–708. doi: 10.1586/14737140.2.6.695. [DOI] [PubMed] [Google Scholar]
- 7.Wilson L., Jordan M. A. New microtubule/tubulin-targeted anticancer drugs and novel chemotherapeutic strategies. J. Chemother. 2004;16(Suppl. 4):83–85. doi: 10.1179/joc.2004.16.Supplement-1.83. [DOI] [PubMed] [Google Scholar]
- 8.Hamel E. Interactions of antimitotic peptides and depsipeptides with tubulin. Biopolymers. 2002;66:142–160. doi: 10.1002/bip.10255. [DOI] [PubMed] [Google Scholar]
- 9.Cruz-Monserrate Z., Vervoort H. C., Bai R., Newman D. J., Howell S. B., Los G., Mullaney J. T., Williams M. D., Pettit G. R., Fenical W., Hamel E. Diazonamide A and a synthetic structural analog: disruptive effects on mitosis and cellular microtubules and analysis of their interactions with tubulin. Mol. Pharmacol. 2003;63:1273–1280. doi: 10.1124/mol.63.6.1273. [DOI] [PubMed] [Google Scholar]
- 10.Loganzo F., Discafani C. M., Annable T., Beyer C., Musto S., Hari M., Tan X., Hardy C., Hernandez R., Baxter M., et al. HTI-286, a synthetic analogue of the tripeptide hemiasterlin, is a potent antimicrotubule agent that circumvents P-glycoprotein-mediated resistance in vitro and in vivo. Cancer Res. 2003;63:1838–1845. [PubMed] [Google Scholar]
- 11.Ngan V. K., Bellman K., Panda D., Hill B. T., Jordan M. A., Wilson L. Novel actions of the antitumor drugs vinflunine and vinorelbine on microtubules. Cancer Res. 2000;60:5045–5051. [PubMed] [Google Scholar]
- 12.Gerth K., Bedorf N., Hofle G., Irschik H., Reichenbach H. Epothilons A and B: antifungal and cytotoxic compounds from Sorangium cellulosum (Myxobacteria). Production, physico-chemical and biological properties. J. Antibiot. (Tokyo) 1996;49:560–563. doi: 10.7164/antibiotics.49.560. [DOI] [PubMed] [Google Scholar]
- 13.Long B. H., Carboni J. M., Wasserman A. J., Cornell L. A., Casazza A. M., Jensen P. R., Lindel T., Fenical W., Fairchild C. R. Eleutherobin, a novel cytotoxic agent that induces tubulin polymerization, is similar to paclitaxel (Taxol) Cancer Res. 1998;58:1111–1115. [PubMed] [Google Scholar]
- 14.Mabjeesh N. J., Escuin D., LaVallee T. M., Pribluda V. S., Swartz G. M., Johnson M. S., Willard M. T., Zhong H., Simons J. W., Giannakakou P. 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell. 2003;3:363–375. doi: 10.1016/s1535-6108(03)00077-1. [DOI] [PubMed] [Google Scholar]
- 15.Dumontet C., Sikic B. I. Mechanisms of action of and resistance to antitubulin agents: microtubule dynamics, drug transport, and cell death. J. Clin. Oncol. 1999;17:1061–1070. doi: 10.1200/JCO.1999.17.3.1061. [DOI] [PubMed] [Google Scholar]
- 16.Pajeva I. K., Wiese M. Pharmacophore model of drugs involved in P-glycoprotein multidrug resistance: explanation of structural variety (hypothesis) J. Med. Chem. 2002;45:5671–5686. doi: 10.1021/jm020941h. [DOI] [PubMed] [Google Scholar]
- 17.Bhalla K. N. Microtubule-targeted anticancer agents and apoptosis. Oncogene. 2003;22:9075–9086. doi: 10.1038/sj.onc.1207233. [DOI] [PubMed] [Google Scholar]
- 18.Gerth K., Bedorf N., Hofle G., Irschik H., Reichenbach H. Epothilons A and B: antifungal and cytotoxic compounds from Sorangium cellulosum (Myxobacteria). Production, physico-chemical and biological properties. J. Antibiot. (Tokyo) 1996;49:560–563. doi: 10.7164/antibiotics.49.560. [DOI] [PubMed] [Google Scholar]
- 19.Reichenbach H., Höfle G. Myobacteria as producers of secondary metabolites. In: Grabley S., Thiericke R., editors. Drug Discovery from Nature. New York: Springer; 2000. pp. 149–178. [Google Scholar]
- 20.Sasse F., Steinmetz H., Heil J., Hofle G., Reichenbach H. Tubulysins, new cytostatic peptides from myxobacteria acting on microtubuli. Production, isolation, physico-chemical and biological properties. J. Antibiot. (Tokyo) 2000;53:879–885. doi: 10.7164/antibiotics.53.879. [DOI] [PubMed] [Google Scholar]
- 21.Steinmetz H., Glaser N., Herdtweck E., Sasse F., Reichenbach H., Hofle G. Isolation, crystal and solution structure determination, and biosynthesis of tubulysins – powerful inhibitors of tubulin polymerization from myxobacteria. Angew. Chem. Int. Ed. Engl. 2004;43:4888–4892. doi: 10.1002/anie.200460147. [DOI] [PubMed] [Google Scholar]
- 22.Monks A., Scudiero D., Skehan P., Shoemaker R., Paull K., Vistica D., Hose C., Langley J., Cronise P., Vaigro-Wolff A., et al. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J. Natl. Cancer Inst. 1991;83:757–766. doi: 10.1093/jnci/83.11.757. [DOI] [PubMed] [Google Scholar]
- 23.Paull K. D., Shoemaker R. H., Hodes L., Monks A., Scudiero D. A., Rubinstein L., Plowman J., Boyd M. R. Display and analysis of patterns of differential activity of drugs against human tumor cell lines: development of mean graph and COMPARE algorithm. J. Natl. Cancer Inst. 1989;81:1088–1092. doi: 10.1093/jnci/81.14.1088. [DOI] [PubMed] [Google Scholar]
- 24.Wang J. L., Zhang Z. J., Choksi S., Shan S., Lu Z., Croce C. M., Alnemri E. S., Korngold R., Huang Z. Cell permeable Bcl-2 binding peptides: a chemical approach to apoptosis induction in tumor cells. Cancer Res. 2000;60:1498–1502. [PubMed] [Google Scholar]
- 25.Crespi C. L., Miller V. P., Penman B. W. Microtiter plate assays for inhibition of human, drug-metabolizing cytochromes P450. Anal. Biochem. 1997;248:188–190. doi: 10.1006/abio.1997.2145. [DOI] [PubMed] [Google Scholar]
- 26.Kaur G., Belotti D., Burger A. M., Fisher-Nielson K., Borsotti P., Riccardi E., Thillainathan J., Hollingshead M., Sausville E. A., Giavazzi R. Antiangiogenic properties of 17-(dimethylaminoethylamino)-17-demethoxy-geldanamycin: an orally bioavailable heat shock protein 90 modulator. Clin. Cancer Res. 2004;10:4813–4821. doi: 10.1158/1078-0432.CCR-03-0795. [DOI] [PubMed] [Google Scholar]
- 27.Hollingshead M. G., Alley M. C., Camalier R. F., Abbott B. J., Mayo J. G., Malspeis L., Grever M. R. In vivo cultivation of tumor cells in hollow fibers. Life Sci. 1995;57:131–141. doi: 10.1016/0024-3205(95)00254-4. [DOI] [PubMed] [Google Scholar]
- 28.Alley M. C., Pacula-Cox C. M., Hursey M. L., Rubinstein L. R., Boyd M. R. Morphometric and colorimetric analyses of human tumor cell line growth and drug sensitivity in soft agar culture. Cancer Res. 1991;51:1247–1256. [PubMed] [Google Scholar]
- 29.Alvarez M., Paull K., Monks A., Hose C., Lee J. S., Weinstein J., Grever M., Bates S., Fojo T. Generation of a drug resistance profile by quantitation of mdr-1/P-glycoprotein in the cell lines of the National Cancer Institute Anticancer Drug Screen. J. Clin. Invest. 1995;95:2205–2214. doi: 10.1172/JCI117910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee J. S., Paull K., Alvarez M., Hose C., Monks A., Grever M., Fojo A. T., Bates S. E. Rhodamine efflux patterns predict P-glycoprotein substrates in the National Cancer Institute drug screen. Mol. Pharmacol. 1994;46:627–638. [PubMed] [Google Scholar]
- 31.Bates S. E., Fojo A. T., Weinstein J. N., Myers T. G., Alvarez M., Pauli K. D., Chabner B. A. Molecular targets in the National Cancer Institute drug screen. J. Cancer Res. Clin. Oncol. 1995;121:495–500. doi: 10.1007/BF01197759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benet L. Z., Cummins C. L., Wu C. Y. Transporter–enzyme interactions: implications for predicting drug–drug interactions from in vitro data. Curr. Drug Metab. 2003;4:393–398. doi: 10.2174/1389200033489389. [DOI] [PubMed] [Google Scholar]
- 33.Tracy T. S. Atypical enzyme kinetics: their effect on in vitro–in vivo pharmacokinetic predictions and drug interactions. Curr. Drug Metab. 2003;4:341–346. doi: 10.2174/1389200033489280. [DOI] [PubMed] [Google Scholar]
- 34.Tonini T., Rossi F., Claudio P. P. Molecular basis of angiogenesis and cancer. Oncogene. 2003;22:6549–6556. doi: 10.1038/sj.onc.1206816. [DOI] [PubMed] [Google Scholar]
- 35.Sridhar S. S., Shepherd F. A. Targeting angiogenesis: a review of angiogenesis inhibitors in the treatment of lung cancer. Lung Cancer. 2003;42(Suppl. 1):S81–S91. doi: 10.1016/s0169-5002(03)00308-8. [DOI] [PubMed] [Google Scholar]
- 36.Retter A. S., Figg W. D., Dahut W. L. The combination of antiangiogenic and cytotoxic agents in the treatment of prostate cancer. Clin. Prostate Cancer. 2003;2:153–159. doi: 10.3816/cgc.2003.n.023. [DOI] [PubMed] [Google Scholar]
- 37.Schiller J. H., Bittner G. Potentiation of platinum antitumor effects in human lung tumor xenografts by the angiogenesis inhibitor squalamine: effects on tumor neovascularization. Clin. Cancer Res. 1999;5:4287–4294. [PubMed] [Google Scholar]
- 38.Plowman J., Dykes D. J., Hollingshead M., Simpson-Herren L., Alley M. C. Human tumor xenograft models in NCI drug development. In: Teicher B., editor. Anticancer Drug Development Guide: Preclinical Screening, Clinical Trials and Approval. Totowa, NJ: Human Press; 1997. pp. 101–125. [Google Scholar]
- 39.Hollingshead M., Roberson J., Decker W., Buckheit R., Jr, Elder C., Malspeis L., Mayo J., Grever M. In vivo drug screening applications of HIV-infected cells cultivated within hollow fibers in two physiologic compartments of mice. Antiviral Res. 1995;28:265–279. doi: 10.1016/0166-3542(95)00055-q. [DOI] [PubMed] [Google Scholar]
- 40.Suggitt M., Swaine D. J., Pettit G. R., Bibby M. C. Characterization of the hollow fiber assay for the determination of microtubule disruption in vivo. Clin. Cancer Res. 2004;10:6677–6685. doi: 10.1158/1078-0432.CCR-04-0855. [DOI] [PubMed] [Google Scholar]
- 41.Sandmann A., Sasse F., Muller R. Identification and analysis of the core biosynthetic machinery of tubulysin, a potent cytotoxin with potential anticancer activity. Chem. Biol. 2004;11:1071–1079. doi: 10.1016/j.chembiol.2004.05.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.