Abstract
Mutations in several genes encoding transcription factors of the hepatocyte nuclear factor (HNF) cascade are associated with maturity-onset diabetes of the young (MODY), a monogenic form of early-onset diabetes mellitus. The ability of the orphan nuclear receptor small heterodimer partner (SHP, NR0B2) to modulate the transcriptional activity of MODY1 protein, the nuclear receptor HNF-4α, suggested SHP as a candidate MODY gene. We screened 173 unrelated Japanese subjects with early-onset diabetes for mutations in this gene and found five different mutations (H53fsdel10, L98fsdel9insAC, R34X, A195S, and R213C) in 6 subjects as well as one apparent polymorphism (R216H), all present in the heterozygous state. Interestingly, all of the subjects with the mutations were mildly or moderately obese at onset of diabetes, and analysis of the lineages of these individuals indicated that the SHP mutations were associated with obesity rather than with diabetes. Therefore, an additional group of 101 unrelated nondiabetic subjects with early-onset obesity was screened for mutations in the SHP gene. Two of the previously observed mutations (R34X and A195S) and two additional mutations (R57W and G189E) were identified in 6 subjects, whereas no mutations were identified in 116 young nondiabetic lean controls (P = 0.0094). Functional studies of the mutant proteins show that the mutations result in the loss of SHP activity. These results suggest that genetic variation in the SHP gene contributes to increased body weight and reveal a pathway leading to this common metabolic disorder in Japanese.
Keywords: nuclear receptor, maturity-onset diabetes of the young, insulin secretion, body weight, hepatocyte nuclear factor
Heterozygous mutations in genes encoding transcription factors in the hepatocyte nuclear factor (HNF) regulatory cascade (1) are associated with an early-onset autosomal dominant form of diabetes mellitus, maturity-onset diabetes of the young (MODY) (2). To date, diabetes-associated mutations have been found in three members of this regulatory network, HNF-1α, -1β, and -4α (MODY3, 5, and 1, respectively) (3–6). These forms of MODY are characterized primarily by defective insulin secretion with normal body weight (7–9). In contrast, forms of early-onset autosomal-dominant type 2 diabetes that are not linked to known MODY genes are often characterized by insulin resistance with high body weight, rather than by pure pancreatic β-cell defects (10). It is not known whether obesity-associated MODY genes or other common modifying factors are responsible for these phenotypic features.
The protein small heterodimer partner (SHP; also called NROB2 for nuclear receptor subfamily 0, group B, member 2), an atypical orphan nuclear receptor that lacks a conventional DNA-binding domain, interacts with a number of other nuclear receptors, including HNF-4α, and inhibits their transcriptional activity (11–17). SHP is expressed in the liver and has recently been suggested to regulate cholesterol homeostasis by an inhibitory effect on the expression of CYP7A, the rate-limiting enzyme in bile acid formation (18, 19). SHP is also found in other tissues, including pancreatic β cells (unpublished data), suggesting a role for this orphan receptor in the regulation of HNF-4α activity. Accordingly, the SHP gene is a plausible candidate MODY gene. We screened the SHP gene for mutations in Japanese subjects with early-onset nonketotic diabetes and identified six different mutations. Interestingly, five of the six mutations were associated with obesity rather than the diabetic phenotype in these families. An additional screen of 101 unrelated subjects with early-onset obesity revealed four mutations, two of which were not previously known and two of which appeared in the diabetic subjects. Functional analysis demonstrated that the mutant SHP proteins showed decreased ability to inhibit HNF-4α transactivation. The association of loss of SHP activity with increased body weight suggests an unexpected role for this orphan receptor in obesity.
Subjects and Methods
Mutation Analysis.
We studied 173 subjects with type 2 diabetes diagnosed before 15 years of age, because our previous study of MODY3 in young Japanese patients suggested that many of these patients have a monogenic form of diabetes (20). Of these subjects, 86 were obese and 87 were nonobese at diagnosis [obese vs. nonobese: body mass index (BMI) 28.4 ± 3.4 kg/m2 vs. 20.1 ± 2.0 kg/m2]. BMI >25 kg/m2 is the cut-off for obesity in this study, according to the criteria of the Japan Society for the Study of Obesity; there is no difference in BMI criteria by sex in Japanese (21). Appropriate informed consent was obtained from all of the subjects examined, including volunteer control subjects.
The two exons and flanking regions of the human SHP gene (GenBank accession no. AF044316) were screened for mutations by direct sequencing of the amplified polymerase chain reaction (PCR) products, using specific primer pairs and an ABI Prism dRhodamine Terminator Cycle Sequencing FS Ready Reaction Kit (Applied Biosystems). The sequencing reactions were analyzed by an Applied Biosystems DNA sequencer model 377. The presence or absence of each mutation in 190 unrelated nondiabetic and nonobese control subjects (hemoglobin A1c <5.6% and BMI <23 kg/m2) was first assessed by direct sequencing of the PCR products of the appropriate exon. For the association study, the two exons and flanking regions of the gene were also screened for mutations by direct sequencing in 101 young unrelated nondiabetic obese subjects (age 13.5 ± 5.1 years; BMI 31.0 ± 6.8 kg/m2) and 116 control subjects (age 11.9 ± 3.2 years; BMI 20.0 ± 2.7 kg/m2).
Wild-Type and Mutant Plasmid Constructs.
SHP genes carrying the mutations identified were generated by PCR-based site-directed mutagenesis and cloned in the expression vector pCMV-6b. The wild-type SHP and HNF-4α were also cloned in pCMV-6b and pcDNA3.1 to generate pCMV-SHP and pcDNA-HNF-4α, respectively. For luciferase reporter assay, the promoter region (nucleotide −129/+195) (22) of the human HNF-1α gene was inserted into the pGL3-Basic Reporter vector (Promega) to generate pGL3-HNF1.
Transient Transfection Assay.
Liver-derived HepG2 and insulin-producing MIN6-m9 cells (1 × 105) were grown in 24-well plates containing Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% or 15% fetal calf serum, respectively, and with 72 μM 2-mercaptoethanol for MIN6-m9 cells. The cells were transfected with 3 μl of liposomal Fugene 6 solution (Roche Molecular Biochemicals), 333 ng of pGL3-HNF-1α, 25 ng of pcDNA-HNF-4α, 0–625 ng of test DNA, and 17 ng of pRL (Renilla luciferase)-SV40. Luciferase reporter activity was measured by using a Dual-Luciferase Reporter Assay System (Promega) according to the manufacturer's instructions. Renilla luciferase activity was used to normalize transfection efficiencies among experiments.
Statistical Analyses.
Pairwise logarithm of odds (LOD) scores were estimated by using the linkage package and assuming an equal recombination frequency between males and females. The mode of inheritance was assumed to be dominant. The mutation frequency was assumed to be 0.005. When the quantity data were classified into two levels, exact P values for the simple linear rank statistics based on Wilcoxon scores were obtained. When the quality data were classified into two levels, Fisher's exact test was used. BMI and weight at birth were adjusted by two-way analysis of variance (ANOVA). Individual ages were classified into three groups; 19 years and below, 20–49 years, and 50 years and over. All computations were performed with the Statistical Analysis System (SAS) version 6.12 (23). Data obtained by luciferase reporter assay were analyzed by the Student t test, using statview 4.51 (Abacus Concepts, Berkeley, CA).
Results
Identification of SHP Mutations.
The human SHP gene (gene symbol, NROB2) has been localized to chromosome 1p36.1 (14). It consists of two exons and spans approximately 2 kb. To test the possibility that it could be a MODY gene, 173 unrelated Japanese subjects with early-onset nonketotic diabetes were examined for SHP gene mutations. The two exons and flanking sequences were screened for mutations by direct sequencing of PCR products. This analysis revealed six different mutations, all in a heterozygous state, in seven subjects. The mutations include two frameshift mutations, H53fsdel10 (deletion of 10 bases starting at codon 53 for His) and L98fsdel9insAC (deletion of 9 bases and insertion of a dinucleotide AC at codon 98 for Leu), one nonsense mutation R34X (amino acid replacement of Arg codon 34 by terminator) that was found in two subjects, and three missense mutations, R213C, R216H, and A195S (replacements of Arg codons 213 and 216 by Cys and His codons, respectively, and Ala codon 195 by Ser). The H53fsdel10, L98fsdel9insAC, and R34X mutations are truncations that would completely inactivate the putative ligand-binding function and also delete C-terminal sequences associated with transcriptional repression (15, 16). The residues affected by the missense mutations, Arg-213, Ala-195, and Arg-216, are conserved in mammalian SHP sequences. The H53fsdel10, L98fsdel9insAC, R34X, R213C, and A195S mutations were not found on screening 190 control subjects (380 alleles). However, the R216H allele was present in 2 of 190 control subjects, suggesting that it may be a polymorphism that is not pathogenic. This suggestion was confirmed by the lack of effect of this alteration on SHP function (see below).
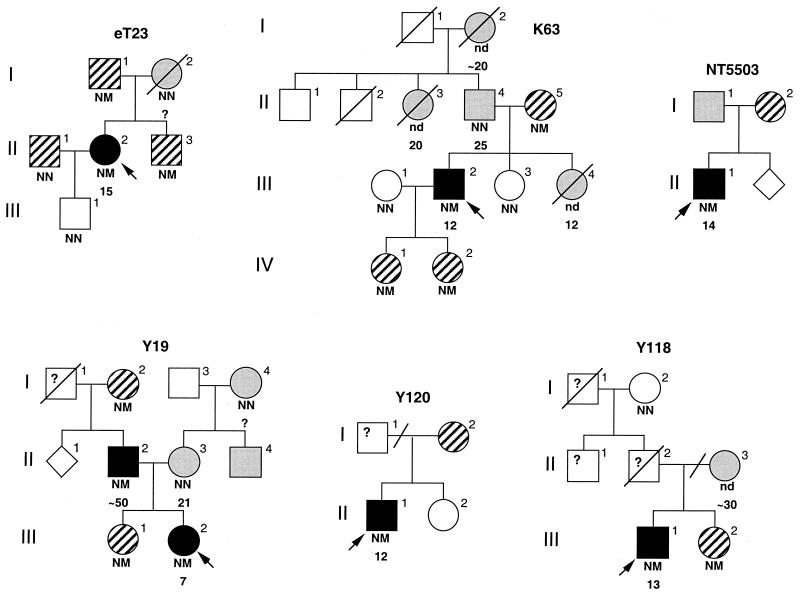
Genotyping of other family members showed that the mutations found in NROB2 did not cosegregate with early-onset diabetes (Fig. 1). In fact, the combined LOD score for early-onset diabetes was −∞ at θ = 0. Strikingly, however, all subjects with the mutations were mildly or moderately obese, with a combined LOD score for obesity of 2.31 at θ = 0.
Figure 1.
Families with mutations in NROB2. Circles represent females; squares, males; rotated squares, sex unspecified. Individuals with both type 2 diabetes and obesity are indicated by black symbols, those with diabetes and no obesity are indicated by shaded symbols, and those with obesity and/or overweight at birth without diabetes are indicated by diagonal symbols. Arrow indicates the proband of each family. The NROB2 genotype is indicated below the symbol: N, normal; M, mutant; nd, not determined. Mutations (family): H53fsdel10 (Y19), L98fsdel9insAC (eT23), R34X (Y118 and Y120), R213C (K63), and A195S (NT5503). Age (years) at diagnosis of diabetes is indicated below the genotype. The oblique lines present between subjects II-2 and II-3 in Y118 and between subjects I-1 and I-2 in Y120 mean divorce.
On the basis of the potential linkage of the SHP gene with obesity, an additional association study of young unrelated nondiabetic individuals with (101 subjects) or without (116 subjects) obesity was carried out. Screening the gene for mutations by direct sequencing in these subjects resulted in the identification of four different mutant alleles in 6 subjects (5.9%) in the obese group and none (0%) in the nonobese group (P = 0.0094). Two of the mutant alleles had already been identified in the diabetic subjects (R34X and A195S), whereas two (R57W and G189E) were novel. Because the LOD score for diabetes was indicative of no linkage (−∞ at θ = 0), the diabetic and nondiabetic subjects were pooled. The overall frequency of the NROB2 mutations in early-onset obesity in this study was 6.3% (12/187) and 0% (0/203) in nonobese subjects (P = 0.00012). These results demonstrate a clear association of SHP gene mutations with obesity.
The available clinical features of the six diabetic subjects with the mutations are summarized in Table 1. All of them were obese at the time of diagnosis of diabetes. Interestingly, the birth weights of these subjects also were also at least 1 standard deviation higher than the mean birth weight in Japanese at each corresponding gestational age (24), including that of subject Y118, who was born prematurely at ≈35 weeks because of the mother's preeclampsia. The clinical records of subjects eT23 and NT5503 at birth were not available. Comparisons of genotype with BMI and birth weight of family members indicate that the SHP mutations are associated significantly with both high birth weight (P = 0.0082) and obesity (P = 0.0036) in these lineages (Table 2). Because many genetic and environmental factors are shared among family members, the significant association of the SHP genotype with these phenotypes strongly suggests that the mutations contribute to the increase of body weight in the affected subjects. Clinical records indicate that none of the carrier mothers had gestational diabetes. The subject with the polymorphism R216H had normal birth weight (3,110 g, 39 weeks, multipara, mean = 3,080 g).
Table 1.
Clinical profiles of probands with NROB2 mutations
| Family ID mutation | Y19 H53fsdel10 | eT23 L98fsdel9insAC | K63 R213C | Y118 R34X | Y120 R34X | NT5503 A195S | Reference ranges |
|---|---|---|---|---|---|---|---|
| Age at diagnosis, yr | 7 | 15 | 12 | 13 | 12 | 14 | — |
| Current age, yr | 14 | 46 | 35 | 17 | 18 | 19 | — |
| BMI at diagnosis, kg/m2 | 25.1 | 39.5 | 25.0 | 33.8 | 25.7 | 33.2 | <23 |
| Weight at birth,* g | 5,005 | ? | 4,000 | 3,200 | 4,500 | ? | Ref. 24 |
| OGTT† | |||||||
| 0 min | 13.2 (66) | 13.6 (78) | 7.0 (68) | 7.2 (309) | 7.5 (150) | 6.3 (220) | 3.3–6.2 (<60) |
| 30 min | 23.2 (108) | 18.6 (162) | 12.2 (264) | 11.9 (540) | 14.4 (294) | 10.6 (966) | (138–462) |
| 120 min | 23.2 (84) | 21.8 (380) | 10.5 (386) | 14.3 (1,080) | 18.6 (372) | 12.6 (1,170) | 6.7> (66–198) |
| AST, IU⋅liter−1 | 11 | 12 | 26 | 96 | 16 | 208 | 10–40 |
| ALT, IU⋅liter−1 | 14 | 7 | ND | 148 | 17 | 357 | 5–40 |
| LDH, IU⋅liter−1 | 404 | 303 | 353 | 464 | 380 | 667 | 230–420 |
| γ-GTP, IU⋅liter−1 | 9 | 6 | ND | 163 | 4 | 70 | 6–14 |
| T-Chol, mg⋅dl−1 | 164 | 190 | 233 | 149 | 152 | 38 | 120–219 |
| TG, mg⋅dl−1 | 66 | 132 | ND | 170 | 182 | 76 | 35–195 |
| NEFA, mEq⋅liter−1 | ND | 0.17 | ND | 0.86 | 0.79 | 0.87 | 0.15–0.55 |
| HDL, mg⋅dl−1 | 68 | 94 | 43 | 54 | 36 | 32 | 35–80 |
| LH, mIU⋅ml−1 | 2.5 | 4.4 | 5.1 | 0.81 | 1.6 | ND | 0.6–16.8 |
| FSH, mIU⋅ml−1 | 10.3 | 4.2 | 7.0 | 1.96 | 4.54 | ND | 1.6–19 |
| Estradiol, pg⋅ml−1 | <10 | 21.1 | ND | 31.2 | 10.1 | ND | 8–60 |
| Testosterone, ng⋅ml−1 | ND | 23.2 | ND | 2.48 | 2.27 | ND | <86 |
| Cortisol, μg⋅dl−1 | ND | 5.4 | 5.5 | 13.2 | 12.6 | 7.2 | 5–15 |
| ACTH, pg⋅ml−1 | ND | 30 | ND | ND | 28 | ND | <60 |
| Free T4, ng⋅dl−1 | 1.32 | 0.8 | 1.0 | 1.01 | 1.01 | ND | 0.8–1.7 |
| Leptin, ng⋅ml−1 | 8.8 | 22.4 | 5.5 | 16 | 6.5 | ND |
IU, international unit; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDH, lactate dehydrogenase; γ-GTP, γ-glutamyl transpeptidase; T-Chol, total cholesterol; TG, triglyceride; HDL, high density lipoprotein cholesterol; NEFA, nonesterified fatty acid; LH, luteinizing hormone; FSH, follicle-stimulating hormone; ACTH, adrenocorticotropic hormone; T4, thyroxine; ND, not done. Biochemical data in subjects Y19, Y118, Y120, and NT5503 were obtained at diagnosis of diabetes. Plasma leptin levels were recently measured (5–30 years after the diagnosis).
Subject Y118 was born immaturely due to mother's preeclampsia (birth weight > mean + 1.0 SD). The birth weight of subject NT5503 is unknown. The birth weight of the other subjects was higher than the mean + 1.5 SD. Reference ranges for weight at birth in Japanese are described by Nishida et al. (24).
OGTT, oral glucose tolerance test; the first numbers are plasma glucose in mmol⋅liter−1, and the numbers in parentheses are immunoreactive insulin in pmol⋅liter−1. Reference values for immunoreactive insulin are in the range of mean ± SD.
Table 2.
Genotype association with BMI and birth weight in MODY families
| Genotype | n | BMI, kg/m2 | P | n | Weight at birth, g | P |
|---|---|---|---|---|---|---|
| NN | 9 | 22.76 ± 2.42 | 0.0036 | 8 | 2,707.1 ± 299.7 | 0.0082 |
| NM | 15 | 28.83 ± 1.18 | 8 | 3,899.6 ± 228.9 |
In Japan, each pregnant woman is expected to report her pregnancy to the municipal government and receive a Maternal and Child Health Handbook, a health record from pregnancy through child delivery. Data are from these records. Results are mean ± SD. Statistical analyses were performed as described in Subjects and Methods. N, normal SHP; M, mutant SHP.
Plasma insulin levels in these diabetic subjects at onset were at least 1 standard deviation higher than the mean value in controls. The affected individuals also appear to have decreased insulin sensitivity as estimated from the fasting insulin and glucose levels by using homeostasis model assessment (HOMA) (25, 26). As shown in Table 1, the plasma levels of growth-related steroid hormones in the subjects examined were within normal limits. The levels of leptin also were appropriate to the degree of obesity. There were no consistent alterations in serum lipids. Liver dysfunction and fatty liver were observed at diagnosis in subjects Y118 and NT5503. However, subject Y120, who has the same mutation (R34X) as Y118, had normal liver function. Although one might expect that the nonsense or frameshift mutations would cause more severe symptoms in the affected subjects than do missense mutations, there is no obvious correlation between the type of mutation and the degree of obesity. All subjects with the NROB2 mutations had normal intellectual development and fertility. The clinical features of the six nondiabetic volunteer children with elevated BMI who were found to have mutations are not available because consent for further examinations was not obtained.
Functional Analysis of Mutant Proteins.
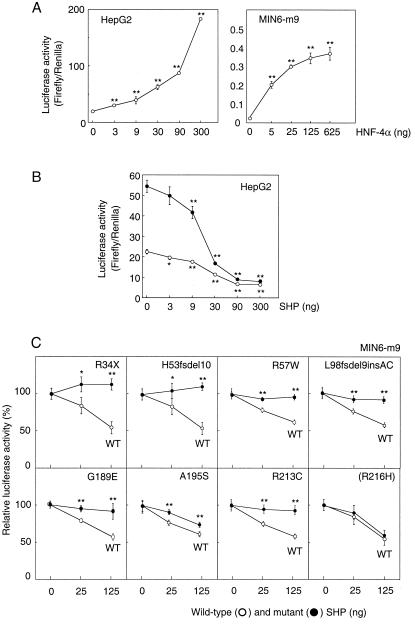
To determine whether the mutations identified in MODY families alter the function of the SHP protein, the effect of the wild-type and mutant proteins on HNF-4α-mediated transactivation of HNF-1α gene transcription was examined. HNF-4α efficiently increased reporter gene activity directed by transcription from the HNF-1α gene promoter in liver HepG2 and insulin-producing MIN6-m9 cells (Fig. 2A). Expression of wild-type SHP significantly decreased both endogenous and exogenous HNF-4α transactivation activities in HepG2 cells, confirming that SHP is a negative regulator of HNF-4α (ref. 15 and Fig. 2B). Because the expression of endogenous HNF-4α is low in MIN6-m9 cells, the effects of the SHP mutant proteins on exogenous HNF-4α activity can be readily monitored in this cell line. When the H53fsdel10, L98fsdel9insAC, R34X, A195S, and R213C mutant proteins were expressed with HNF-4α in MIN6-m9 cells, a significant reduction in suppressive activity of the mutant proteins was observed. The R216H protein showed no significant reduction in activity, consistent with this alteration being a polymorphism (Fig. 2C). The two additional mutations identified in nondiabetic subjects with obesity also showed significantly reduced ability to inhibit HNF-4α transactivation.
Figure 2.
Inhibition of transactivation activity of HNF-4α by wild-type and mutant SHP. (A) HNF-4α generated significant increases in luciferase reporter activity directed by the HNF-1α gene promoter in HepG2 and MIN6-m9 cells. (B) Wild-type SHP significantly decreased transactivation activity of both exogenous (25 ng, ●) and endogenous (○) HNF-4α in HepG2 cells. (C) The relative luciferase activity (firefly/Renilla) of each construct at 0, 25, and 125 ng of SHP was measured in MIN6-m9 cells (n = 5 in each experiment). Percent activity in relation to basic HNF-4α activity is shown as mean ± SD. *, P < 0.05; **, P < 0.01.
Discussion
The identification of genes responsible for monogenic forms of obesity has led to a better understanding of the molecular and physiological basis of this common metabolic condition. In humans, five such genes have been described to date. Mutations in the genes for leptin, the leptin receptor, prohormone convertase 1 (PC1), and pro-opiomelanocortin (POMC) lead to a distinct phenotype of morbid obesity and various hypothalamic and pituitary disorders with a recessive inheritance (27–31). Mutations in the melanocortin-4 receptor (MC4-R) gene cause a nonsyndromic phenotype of morbid obesity with dominant inheritance (refs. 32 and 33 and Table 3). The proteins encoded by these genes are functionally related in the central signaling system of energy homeostasis and feeding behavior (34). Leptin, a cytokine secreted from adipose tissues, interacts with its receptor in the hypothalamus to modulate the expression of central signaling proteins including POMC, which in turn regulates both energy expenditure and feeding. α-Melanocyte-stimulating hormone (MSH), processed from the precursor POMC by the proteolytic enzymes PC1 and carboxypeptidase E, also influences energy expenditure and food intake by activation of MC4-R in the hypothalamus. In contrast, the subjects with SHP gene mutations exhibited no endocrine abnormalities in pituitary or hypothalamus and showed a distinct, relatively mild obesity. Thus, these NROB2 mutations reveal a pathway to increased weight that is apparently independent of the hypothalamic pathway. This pathway could be related to effects of SHP on one or more of a number of nuclear receptors associated with metabolic regulation. These include the steroid, retinoid, and thyroid hormone receptors, the orphan HNF-4α, and particularly the peroxisome proliferator-activated receptors (PPARs), which play a key role in adipogenesis (35).
Table 3.
Comparison of phenotypic features of monogenic forms of obesity
| Gene | Obesity | Birth weight | Endocrine abnormalities | Hyperphagia | Inheritance | Chromosome |
|---|---|---|---|---|---|---|
| LEP | Severe | Normal | Low
leptin Hypogonadism High thyroid stimulating hormone High insulin |
+ | Recessive | 7q31.3 |
| LEPR | Severe | ? |
High
leptin Pituitary dysfunction Hypogonadotrophic hypogonadism Hypothalamic hypothyroidism Sympathetic dysfunction High insulin |
+ | Recessive | 1p31 |
| POMC | Severe | Normal | Red hair pigmentation ACTH deficiency, hypocortisolism Low α-MSH |
+ | Recessive | 2p23.3 |
| PC1 | Severe | ? | Hypogonadotrophic
hypogonadism Hypocortisolism High proinsulin, low insulin Postprandial hypoglycemia High POMC |
? | Recessive | 5q1.5–2.1 |
| MC4-R | Severe | Normal | Not observed | + | Dominant | 18q22 |
| NROB2 | Mild | High | Mild hyperinsulinemia | − | Dominant | 1p36.1 |
LEP, leptin; LEPR, leptin receptor; POMC, pro-opiomelanocortin; PC1, prohormone convertase 1; MC4-R, melanocortin-4 receptor; ACTH, adrenocorticotropic hormone; α-MSH, α-melanocyte-stimulating hormone.
Mutations in NROB2 are associated with a dominantly inherited form of early-onset obesity similar to that in patients with the most common form of obesity (25–30 kg/m2) in Japanese (21, 36). Most studies examining the complications associated with obesity have been based on data from Western populations, and it has been well recognized that the standard World Health Organization (WHO) criteria classifying obesity and preobesity by using BMI (obesity >30 kg/m2; preobesity >25 kg/m2) is not appropriate in the Asia–Pacific region.n For example, only ≈2% of Japanese, Korean, and Chinese populations are classified as obese when the WHO criteria are used, and increased morbidity risks occur in people with much lower BMIs in the Asia–Pacific region (footnote n; refs. 36–40). It is tempting to speculate, therefore, that NROB2 mutations are a component of the genetic background of obesity in Japanese, although neither the significance of such mutations in the development of adult-onset obesity in this population nor the prevalence of mutations in Western populations is known at present.
Interestingly, NROB2 mutations are also associated with high birth weight. Because SHP inhibits HNF-4α, the loss of SHP function should lead to increased activity of HNF-4α, resulting in increased insulin secretion. Because insulin is one of the key hormones in adipogenesis, increased insulin secretion occurring in the fetus might well increase intrauterine growth (41–43). Such increased fetal growth could also underlie the postnatal obesity in the affected subjects.
Finally, it is clear that the activity of SHP could be regulated by yet-unidentified ligands, and the results described here suggest that such SHP agonists or antagonists could have a significant effect on body weight. Accordingly, identification and characterization of possible SHP ligands and the partner proteins that mediate its effects should provide new insight into the mechanisms of fetal growth and energy expenditure.
Acknowledgments
We are grateful to all of the patients with early-onset diabetes and/or obesity cooperating in this study and to their referring physicians, and we are grateful for the encouragement of Dr. T. Takeuchi (Gunma University) and the Meeting of Active Pediatric Endocrinologists. We also thank the volunteer control subjects and Dr. T. Yanagawa at Nerima General Hospital (Tokyo) for participating in this study. We thank Drs. S. Maeda, Z. Zhang, and T. Nishigori for excellent experimental help and Mss. T. Ishikawa and N. Sakurai for technical services. This study was supported by Grants-in-Aid for Scientific Research and for Creative Basic Research from the Japanese Ministry of Science, Education, Sports and Culture, the Mitsubishi Foundation, the Ono Medical Research Foundation, the Korea Science and Engineering Foundation and Hormone Research Center, and the National Institutes of Health (P01 DK57743).
Abbreviations
- HNF
hepatocyte nuclear factor
- MODY
maturity-onset diabetes of the young
- SHP
small heterodimer partner
- BMI
body mass index
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.021544398.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.021544398
World Health Organization (Western Pacific Region) and IASO (The International Association for the Study of Obesity)/IOTF (The International Obesity Task Force) (2000) Web site: http://www.idi.org.au/obesityreport.hm.
References
- 1.De Simone V, Cortese R. Biochim Biophys Acta. 1992;1132:119–126. doi: 10.1016/0167-4781(92)90001-g. [DOI] [PubMed] [Google Scholar]
- 2.Fajans S S. Diabetes Metab Rev. 1989;5:579–606. doi: 10.1002/dmr.5610050705. [DOI] [PubMed] [Google Scholar]
- 3.Yamagata K, Oda N, Kaisaki P J, Menzel S, Furuta H, Vaxillaire M, Southam L, Cox R D, Lathrop G M, Boriraj V V, et al. Nature (London) 1996;384:455–458. doi: 10.1038/384455a0. [DOI] [PubMed] [Google Scholar]
- 4.Yamagata K, Furuta H, Oda N, Kaisaki P J, Menzel S, Cox N J, Fajans S S, Signorini S, Stoffel M, Bell G I. Nature (London) 1996;384:458–460. doi: 10.1038/384458a0. [DOI] [PubMed] [Google Scholar]
- 5.Horikawa Y, Iwasaki N, Hara M, Furuta H, Hinokio Y, Cockburn B N, Linder T, Yamagata K, Ogata M, Tomonaga O, et al. Nat Genet. 1997;17:384–385. doi: 10.1038/ng1297-384. [DOI] [PubMed] [Google Scholar]
- 6.Nishigori H, Yamada S, Kohama T, Tomura H, Sho K, Horikawa Y, Bell G I, Takeuchi T, Takeda J. Diabetes. 1998;47:1354–1355. doi: 10.2337/diab.47.8.1354. [DOI] [PubMed] [Google Scholar]
- 7.Byrne M M, Sturis J, Fajans S S, Ortiz F J, Stoltz A, Stoffel M, Smith M J, Bell G I, Halter J B, Polonsky K S. Diabetes. 1995;44:699–704. doi: 10.2337/diab.44.6.699. [DOI] [PubMed] [Google Scholar]
- 8.Byrne M M, Sturis J, Menzal S, Yamagata K, Fajans S S, Dronsfield M J, Bain S C, Hattersley A T, Velho G, Froguel P, et al. Diabetes. 1996;45:1503–1510. doi: 10.2337/diab.45.11.1503. [DOI] [PubMed] [Google Scholar]
- 9.Iwasaki M, Ogata M, Tomonaga O, Kuroki H, Kasahara T, Yano N, Iwamoo Y. Diabetes Care. 1998;21:2144–2148. doi: 10.2337/diacare.21.12.2144. [DOI] [PubMed] [Google Scholar]
- 10.Doria A, Yang Y, Malecki M, Dreyfus J, O'Keeffe C, Orran T, Warram J H, Krolewski A S. Diabetes Care. 1999;22:253–261. doi: 10.2337/diacare.22.2.253. [DOI] [PubMed] [Google Scholar]
- 11.Seol W, Choi H-S, Moore D D. Science. 1996;272:1336–1339. doi: 10.1126/science.272.5266.1336. [DOI] [PubMed] [Google Scholar]
- 12.Masuda N, Yasumo H, Tamura T, Hashiguchi N, Furusawa T, Tsukamoto T, Sadano H, Osumi T. Biochim Biophys Acta. 1997;1350:27–32. doi: 10.1016/s0167-4781(96)00196-0. [DOI] [PubMed] [Google Scholar]
- 13.Johansson L, Thomsen J S, Damdimopoulos A E, Spyrou G, Gustafsson J-A, Treuter E. J Biol Chem. 1999;274:345–353. doi: 10.1074/jbc.274.1.345. [DOI] [PubMed] [Google Scholar]
- 14.Lee H-K, Lee Y-K, Park S-H, Kim Y-S, Park S H, Lee J W, Kwon H-B, Jaemog S, Moore D D, Choi H-S. J Biol Chem. 1998;273:14398–14402. doi: 10.1074/jbc.273.23.14398. [DOI] [PubMed] [Google Scholar]
- 15.Lee Y-K, Dell H, Dowhan D H, Hadzopoulou-Cladaras M, Moore D D. Mol Cell Biol. 2000;20:187–195. doi: 10.1128/mcb.20.1.187-195.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seol W, Chung M, Moore D D. Mol Cell Biol. 1997;17:7126–7131. doi: 10.1128/mcb.17.12.7126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seol W, Hanstein B, Brown M, Moore D D. Mol Endocrinol. 1998;12:1551–1557. doi: 10.1210/mend.12.10.0184. [DOI] [PubMed] [Google Scholar]
- 18.Lu T T, Makishima M, Repa J J, Schoonjans K, Kerr T A, Auwerx J, Mangelsdorf D J. Mol Cell. 2000;6:507–515. doi: 10.1016/s1097-2765(00)00050-2. [DOI] [PubMed] [Google Scholar]
- 19.Goodwin B, Jones S A, Price R R, Watson M A, McKee D D, Moore L B, Galaedi C, Wilson J G, Lewis M C, Roth M E, et al. Mol Cell. 2000;6:517–526. doi: 10.1016/s1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- 20.Yamada S, Tomura H, Nishigori H, Sho K, Mabe H, Iwatani N, Takumi T, Kito Y, Moriya N, Muroya K, et al. Diabetes. 1999;48:645–649. doi: 10.2337/diabetes.48.3.645. [DOI] [PubMed] [Google Scholar]
- 21.Japanese Society for the Study of Obesity. J Jpn Soc Study Obes. 2000;6:18–28. [Google Scholar]
- 22.Gragnoli C, Linder T, Cockburn B N, Kaisaki P J, Gragnoli F, Marozzi G, Bell G I. Diabetes. 1997;46:1648–1651. doi: 10.2337/diacare.46.10.1648. [DOI] [PubMed] [Google Scholar]
- 23.Goodnight J H, Harvey W R. SAS Technical Report R-103. Cary, NC: SAS Institute; 1978. [Google Scholar]
- 24.Nishida H, Sakamoto S, Sakanoue M. Acta Pediatr Scand. 1985;319:62–67. doi: 10.1111/j.1651-2227.1985.tb10111.x. [DOI] [PubMed] [Google Scholar]
- 25.Matthews D R, Hosker J P, Rudenski A S, Naylor B A, Treacher D F, Turner R C. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 26.Tomono S, Kawazu S, Utsugi T, Kato N, Ohno T, Ohyama Y, Uchiyama T, Ito H, Ito Y, Nagai R. J Jpn Diabetes Soc. 1999;42:895–902. [Google Scholar]
- 27.Jackson R S, Creemers J W M, Ohagi S, Raffin-Sanson M-L, Sanders L, Montague C T, Hutton J C, O'Rahilly S. Nat Genet. 1997;16:303–306. doi: 10.1038/ng0797-303. [DOI] [PubMed] [Google Scholar]
- 28.Montague C T, Farooqi I S, Whitehead J P, Soos M A, Rau H, Wareham N J, Sewter C P, Digby J E, Mohammed S N, Hurst J A, et al. Nature (London) 1997;387:903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 29.Krude H, Biebermann H, Luck W, Horn R, Brabant G, Gruters A. Nat Genet. 1998;19:155–157. doi: 10.1038/509. [DOI] [PubMed] [Google Scholar]
- 30.O'Rahilly S, Gray H, Humphreys P J, Krook A, Polonsky K S, White A, Gibson S, Taylor K, Carr C. N Engl J Med. 1995;333:1386–1390. doi: 10.1056/NEJM199511233332104. [DOI] [PubMed] [Google Scholar]
- 31.Clement K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, Gourmelen M, Dina C, Chambaz J, Lacorte J-M, et al. Nature (London) 1998;392:398–401. doi: 10.1038/32911. [DOI] [PubMed] [Google Scholar]
- 32.Vaisse C, Clement K, Guy-Grand B, Froguel P. Nat Genet. 1998;20:113–114. doi: 10.1038/2407. [DOI] [PubMed] [Google Scholar]
- 33.Yao G S H, Farooqi I S, Aminian S, Halsall D J, Stanhope R G, O'Rahilly S. Nat Genet. 1998;20:111–112. doi: 10.1038/2404. [DOI] [PubMed] [Google Scholar]
- 34.Chen D, Garg A. J Lipid Res. 1999;40:1735–1746. [PubMed] [Google Scholar]
- 35.Tontnoz P, Hu E, Spiegelman B M. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 36.Yoshiike N, Matsumura Y, Zaman M M, Yamaguchi M. Int J Obes. 1998;22:684–687. doi: 10.1038/sj.ijo.0800651. [DOI] [PubMed] [Google Scholar]
- 37.Ito K, Murano M. Jpn J Pediatr. 1999;52:1182–1186. [Google Scholar]
- 38.Ge L. Asia Pacific J Clin Nutr. 1997;6:175–179. [Google Scholar]
- 39.Ko G T C, Chan J C N, Woo J, Lau E, Yeung V T F, Chow C-C, Wai H P S, Li J K Y, So W-Y, Cockram C S. Int J Obes. 1997;21:995–1001. doi: 10.1038/sj.ijo.0800508. [DOI] [PubMed] [Google Scholar]
- 40.Report of a WHO Consultation on Obesity. Obesity: Preventing and Managing the Global Epidemic. Geneva: World Health Organization; 1997. [PubMed] [Google Scholar]
- 41.Hoegsberg B, Gruppuso P A, Coustan D R. Diabetes Care. 1993;16:32–36. doi: 10.2337/diacare.16.1.32. [DOI] [PubMed] [Google Scholar]
- 42.Akinbi H T, Gerdes J S. J Pediatr. 1995;127:481–484. doi: 10.1016/s0022-3476(95)70087-0. [DOI] [PubMed] [Google Scholar]
- 43.Otonkoski T, Ammala C, Huopio H, Cote G J, Chapman J, Cosgrove K, Ashfield R, Huang E, Komulainen J, Ashcroft F M, et al. Diabetes. 1999;48:408–415. doi: 10.2337/diabetes.48.2.408. [DOI] [PubMed] [Google Scholar]