Abstract
Background: Endothelial and smooth muscle cells were used as seeding cells and heterogeneous acellularized matrix was used as scaffold to construct the tissue-engineered graft. Methods: A 2 weeks piglet was selected as a donor of seeding cells. Two-centimetre length of common carotid artery was dissected. Endothelial cells and smooth muscle cells were harvested by trypsin and collagenase digestion respectively. The isolated cells were cultured and expanded using routine cell culture technique. An adult sheep was used as a donor of acellularized matrix. The thoracic aorta was harvested and processed by a multi-step decellularizing technique to remove the original cells and preserve the elastic and collagen fibers. The cultured smooth muscle cells and endothelial cells were then seeded to the acellularized matrix and incubated in vitro for another 2 weeks. The cell seeded graft was then transplanted to the cell-donated piglet to substitute part of the native pulmonary artery. Results: The cultured cells from piglet were characterized as endothelial cells by the presence of specific antigens vWF and CD31, and smooth muscle cells by the presence of specific antigen α-actin on the cell surface respectively with immunohistochemical technique. After decellularizing processing for the thoracic aorta from sheep, all the cellular components were extracted and elastic and collagen fibers kept their original morphology and structure. The maximal load of acellular matrix was decreased and 20% lower than that of untreated thoracic aorta, but the maximal tensions between them were not different statistically and they had similar load-tension curves. Three months after transplantation, the animal was sacrificed and the graft was removed for observation. The results showed that the inner surfaces of the graft were smooth, without thrombosis and calcification. Under microscopy, a great number of growing cells could be seen and elastic and collagen fibers were abundant. Conclusion: Cultured self-derived endothelial and smooth muscle cells could be used as seeding cells and heterogeneous acellularized matrix could be used as scaffold in constructing tissue-engineered graft.
Keywords: Tissue engineering, Self-derived cells, Heterogeneous acellularized matrix, Transplantation
INTRODUCTION
Vascular grafts have been used ubiquitously in surgeries for cardiovascular diseases including coronary artery bypass and many types of complicated congenital heart defects. Autologous blood vessel is no doubt the most preferable choice for the coronary artery bypass surgery, but it is not sufficient or suitable for the repair of most complicated congenital heart defects and multiple coronary bypass or repeated procedure. Homologous graft is another choice, and has been extensively used clinically. Theoretically, the cryopreserved homograft has viable endothelial cells, smooth muscle cells and fibroblasts, and is better than synthetic materials. However, studies have demonstrated that calcification and thrombogenesis of homograft are still problems postoperatively, which lead to the stenosis of the graft, and sometimes require re-operation several years after surgery to replace the damaged graft. These problems may be attributed partly to the immunological rejection of recipient to the homograft. Furthermore, the source of homografts is limited, especially small diameter homografts which are required for operation for infants or younger children. Another substitute is the graft made of synthetic materials. Although the compatibility of synthetic grafts has been improved dramatically, the small-diameter graft is still unsatisfactory due to the high incidence of calcification and thrombogenesis after operation. To try to solve these problems, there have been attempts to create tissue-engineered blood vessel (Langer and Vacanti, 1993; Vacanti and Langer, 1999). The aim of our research was to create a method for constructing a tissue-engineered graft with self-derived endothelial cells (ECs), smooth muscle cells (SMCs) and heterogeneous acellularized matrix, and to find out if this graft is suitable as a pulmonary vessel substitute.
MATERIALS AND METHODS
Animal preparation
Shanghai piglets and adult sheep were obtained from a local dealer for the trial. All animals received care in compliance with the “Guide for the Care and Use of Laboratory Animals” published by the National Institutes of Health after approval from our institutional animal protection board.
Culturing and identifying of ECs and SMCs
General anesthesia for a piglet was induced by fentanyl citrate infusion. A segment of 2 to 3 cm carotid artery was harvested. The piglet was sent to the pigsty after waking and was raised carefully for further experiment.
After removing the outside fatty tissue, the harvested vessel was immersed in 0.25% trypsin plus 0.02% EDTA solution and incubated at 37 °C for 20 min for endothelial cells digestion. After incubation, the digestion was stopped by adding 10% fetal bovine serum (FBS). The ECs were removed from the inner surface of the vessel by gentle mechanical scratching. The remaining tissue was then minced into small pieces and incubated in a solution containing 0.2% collagenase and 0.02% EDTA for another 3 h for smooth muscle cells digestion. The digestion was also stopped by adding 10% FBS and SMCs were separated by washing several times. The ECs were suspended in DMEM culture solution with 10% FBS, 10 ng/ml vascular endothelial growth factor (VEGF), 1 ng/ml fibroblast growth factor-basic (bFGF) and antibiotics. While the SMCs were suspended in DMEM culture solution with 10% FBS and antibiotics. Both the cells were cultured in an incubator at temperature of 37 °C and atmosphere containing 5% carbon dioxide. After the cells expanded to a population covering 80% to 90% area of the culture flask, they were serially passed on to obtain sufficient cells for seeding. The cultured ECs were identified by specific monoclonal antibodies of vWF and CD31 while the SMCs were identified by monoclonal antibody of α-actin with immunohistochemical technique.
Making of acellularized matrix
Anesthesia for an adult sheep was induced by fentanyl citrate infusion. About 10 cm thoracic aorta was dissected. The aorta was incubated with 0.25% trypsin for 24 h at a temperature of 37 °C and then processed by an extractive technique using 0.5% SDS detergent under continuous shaking to remove the cells. After treatment, the decellularized vessel was frozen by putting it into a −80 °C refrigerator for 3 to 4 h and then quickly re-warming it. After 3 to 4 cycles of freezing-dissolving processing, it was dehydrated in a vacuum freeze dryer at temperature lower than −20 °C and then sterilized by epoxy ethane. The morphology was observed and mechanical tension was measured.
Seeding of cells and culturing in vitro
After 4 to 6 passages, the cultured cells were digested and suspended in the seeding medium. First the SMCs were seeded to the acellularized matrix by multiple-point injection. After 3 to 4 d culturing, the ECs were then seeded direct to the surface. Further 2 weeks culture was needed for cell expansion before they could be transplanted to the cell-donated piglet.
Transplantation of the graft
The cell-donated piglet was anesthetized by fentanyl citrate infusion and was intubated for mechanical ventilation. The chest wall was opened through the left third costal space. The main pulmonary artery was explored. The side of the pulmonary artery was clamped and some artery wall tissue was resected. The tissue-engineered graft was used to patch the defect of the pulmonary artery wall. After anastomosis, the chest wall was closed. After awaking, the animal was extubated and was raised carefully. Three months after operation the piglet was sacrificed to harvest the main pulmonary artery.
RESULTS
Cells
1. Endothelial cells (ECs)
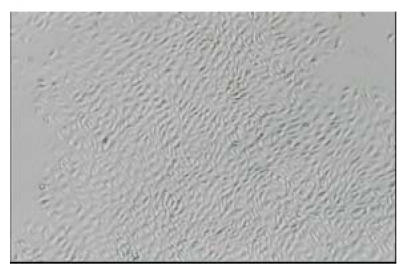
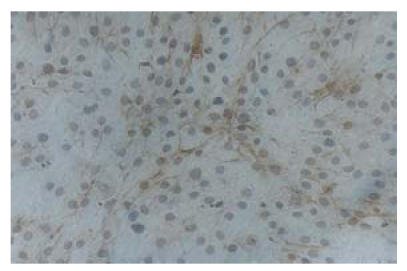
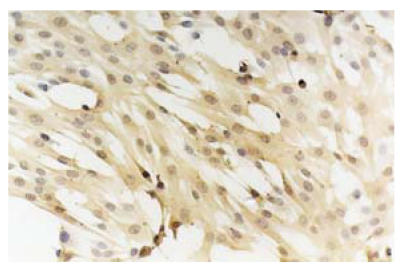
ECs were obtained from the carotid artery of piglet by trypsin digestion and mechanical scratching. After 48 h’s incubation in DMEM culture medium, the cells started to proliferate. The shapes of the cells were polygonal or round-like and extended outside. In 6 to 7 d, the cells formed a confluent layer and exhibited a cobblestone-like morphology characteristic of ECs (Fig.1). The plasma was transparent and the nucleus was round. Immunohistochemical analysis showed the positive reactions of the cells to the specific monoclonal antibodies vWF and CD31 (Figs.2 and 3). After six passages, about 98.8% of all the cultured cells were alive.
Fig. 1.
Seven days primary cultured ECs. The cells were polygonal or round-liked and formed a confluent layer, exhibiting a slabstone or cobblestone-liked morphology (LM×40)
Fig. 2.
Immunohistochemical analysis showed the positive reaction of the cultured cells to CD31 monoclonal antibody which is specific to ECs (LM×100)
Fig. 3.
Immunohistochemical analysis showed the positive reaction of the cultured cells to vWf monoclonal antibody which is specific to ECs (LM×200)
2. Smooth muscle cells (SMCs)
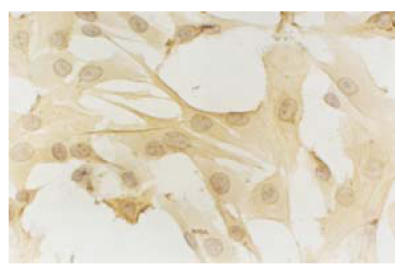
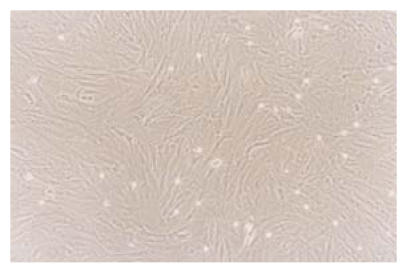
SMCs were obtained by collagenase digestion and then cultured in DMEM culture medium. After 4 to 5 d’s incubation, the cells reached 60% area of culture flask and exhibited different shapes, such as fusiform, triangle and star-like. The plasma was bright and nucleus was round or elliptic. In 7 to 8 d, nuclear fission occurred and cell population reached 90% of the culture flask. The cells became fusiform and formed multiple layers, showing “hills and valleys” phenotype (Fig.4). Immunohistochemical analysis showed that the cells positively reacted to the monoclonal antibody of α-actin that was specific to SMCs (Fig.5). After six passages, about 98.2% of all the cultured cells were alive.
Fig. 4.
Seven days primary cultured SMCs. The cells were fusiform-liked and showed the “hills and valleys” phenotype (LM×100)
Fig. 5.
Immunohistochemical analysis showed the positive reaction of the cultured cells to α-actin monoclonal antibody which is specific to SMCs (LM×100)
Acellular matrix
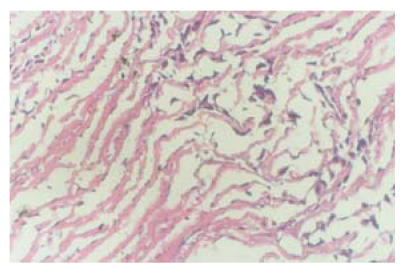
Under microscopy no cells remained in the decellularized matrix, but the collagen and elastic fibers kept their original porous morphology and structure (Fig.6). Mechanical measurement confirmed that the maximal load of acellular matrix was 20% lower than that of fresh thoracic artery of the sheep, but the maximal tension was not different from that statistically. The acellular matrix and fresh thoracic artery had similar load-tension curves under single axis load. Gel graphic analysis found that the acellular matrix, fresh thoracic artery and type 3 fibroprotein as control have almost the same strains at the same molecular level. Further analysis confirmed that there was no statistical difference between the volumes of type 3 fibroprotein in gel strains of acellular matrix and fresh thoracic artery.
Fig. 6.
The decellularized matrix, no cells remained, but the collagen and elastic fibers kept their original porous morphology and structure, HE staining (LM×40)
Cells seeding and culturing
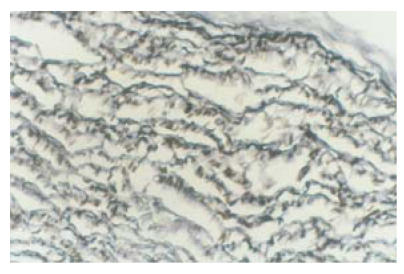
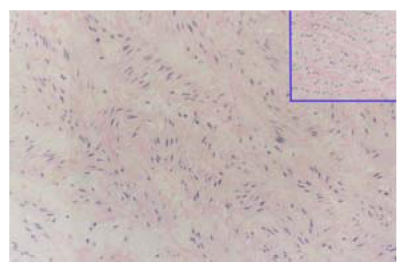
The SMCs and ECs were seeded into the acellular matrix respectively and incubated in culture medium. The pH of the culture medium decreased after 1 to 2 d’s seeding, which showed the vigorous metabolism and progressive proliferation of seeded cells. In one week, many SMC-like fusiform cells could be seen in the matrix, and EC-like round cells could be seen at the surface of the matrix (Figs.7 and 8).
Fig. 7.
Seven days after cells seeding, many SMC-liked cells could be seen in the decellularized matrix, but the arrangement of cells were not very regular, HE staining (LM×40)
Fig. 8.
Seven days after cells seeding, ECs-liked cells could be seen at the surface of the decellularized matrix. Silver nitrate staining (LM×40)
Transplantation
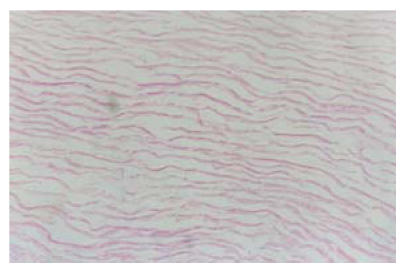
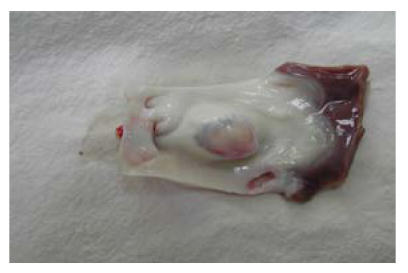
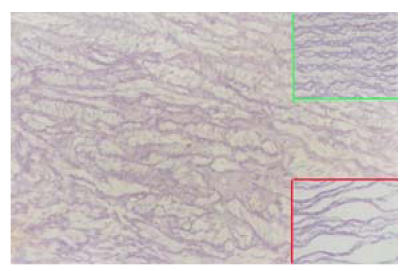
After transplantation of constructed grafts, the piglet was raised carefully and grew healthily. Three months later it was sacrificed and the transplanted graft was dissected for observation. Under naked eyes, the inner surface of the graft was smooth and was laied with endothelial-like tissue. Neither thrombosis nor calcification occurred (Fig.9). Under microscopy, many growing cells could be seen (Fig.10). Elastic fibers were abundant and similar to the native pulmonary artery, as shown by elastic staining (Fig.11). Also load-tension curves were similar to those of native pulmonary vessels.
Fig. 9.
Three months after transplantation, the inner surface of the graft was smooth and was laied with endothelial-like tissue. Neither thrombosis nor calcification could be seen
Fig. 10.
Three months after transplantation, many growing cells could be seen in the graft. Right upper graph was the neighboring pulmonary artery tissue. HE staining (LM×40)
Fig. 11.
Three months after transplantation, elastic fibers in the graft were abundant. Right upper graph was the neighboring pulmonary artery tissue and right lower graph was the acellularized matrix. Elastic staining (LM×40)
DISCUSSION
Construction of a tissue-engineered graft includes three stages: cell harvesting and culturing, scaffold making and cells seeding (Griffith and Naughton, 2002; Fuchs et al., 2001).
Most reports cited use of self-derived cells as the seeding cells. Usually small arterial or venous vessels were used and the cells were harvested by enzymatic digestions. The isolated cells were cultured in vitro to expand to sufficient number for seeding (Kim et al., 1998). ECs and SMCs were two main cell components for in vitro graft construction. In this report, a piglet was selected as cell donator. The carotic artery was dissected and the ECs and SMCs were harvested and isolated by trypsin and collagenase from the vessel separately. They were cultured and passed on by routine methods. Immunohistochemical methods were used to determine if the cells expressed the specific antigen with monoclonal antibodies. The results showed that the cultured ECs were positive to vWF and CD31 monoclonal antibodies, which were two types of specific antigens in ECs. The cultured SMCs were positive to α-actin monoclonal antibody that was believed existed specifically in the SMCs.
Because the harvesting of the cells from autologous small artery or vein will injure the body, some authors have directed their attention to research of stem cells to find a new source of seeding cells (Wu and Huang, 2004; Luttun et al., 2002). Stem cells are types of undifferentiated primary cells in the body. They could differentiate into many types of mature peripheral cells in vitro by controlling the culture environment. Embryo stem cells are no doubt the most advisable origin of seeding cells for tissue engineered graft construction. Owing to ethics and technical problems, the use of embryo stem cells is problematic. Researchers found that stem cells also exist in marrow and peripheral blood. We had already obtained endothelial progenitor cell, which is considered a type of undifferentiated primary cell, from peripheral blood of piglet and successfully expanded and differentiated to mature ECs shown by presence of vWF, CD31, FLK-1/KDR/VEGFR-2, and VE-cadherin. All these are specific antigens to mature ECs.
Scaffold making is another important aspect in constructing a tissue-engineered graft. The materials selected for scaffold making should have good biocompatibility with the seeding cells and recipients. They should also have desirable mechanical features of blood flow dynamics. Biodegradable polymer scaffolds have been studied recently (Xue and Greisler, 2003). Most extensively used biodegradable polymers are polyglycolic acid (PGA), polylactic acid (PLA) and their component poly(lactic-co-glycolic acid) (PLGA). Major disadvantages of biodegradable polymer scaffolds are that natural three-dimensional structure matrix has to be reconstructed and that important extracellular matrix proteins, such as natural ligand of cellular attachment for integrin receptor, are not present in synthetic polymers. Acellular matrix is another prospective choice of scaffold and has been studied recently. The acellular matrix used in constructing tissue engineered grafts is usually obtained from pig, sheep or other animals. Decellularized by enzymatic digestion, almost all the cells were extracted and then the immunogenicity was decreased greatly, but the natural three-dimensional structure and extracellular matrix composition were preserved. The recognition sites of the acellular matrix, which were suitable for the adhesion of seeding cells, were also preserved. In this report, multiple-stage processing was used to make the acellular matrix (Huang et al., 2001). The results showed that the cells in the sheep aorta were completely extracted after treatment. The remaining matrix was net-like. Although the maximal load of the matrix was 20% lower than untreated vessel, the maximal tensions of matrix and untreated vessel were not different statistically and were similar under single axis load. Theoretically, the mechanical feature of the acellular matrix treated by our method should be strong enough for sustaining blood flow dynamics in vivo.
We used two-step seeding method in this report. First, the SMCs were seeded by multipoint injection of cell suspension to the acelullarized matrix in tissue culture dishes. The ECs were then seeded to the graft surface three days later. The seeded graft was incubated in static condition for cell growing and extending. Many SMC-like fusiform cells and EC-like round cells still could be seen in the matrix in two weeks. The constructed graft was transplanted to the cell-donor piglet as a substitute of part of the pulmonary artery. By this method, the cells seeded to the graft could be recognized by recipient as autologous cells and immunological reactions avoided. Three months after surgery, the piglet was sacrificed and the transplanted graft was dissected to appraise the results. We saw that the inner surface of the graft was smooth without thrombosis and calcification. Under microscope a great number of growing cells could be seen in the graft. Elastic fiber was abundant as demonstrated by elastic staining, which was the mark of the secretive function of SMCs. The cells found in the graft might have two origins, either proliferated from seeded cells or migrated from the neighboring vessel cells. To determine the cells’ origination, some authors marked seeded cell before transplantation. They found that both marked cells and unmarked cells could be found several months after operation and suggested that both proliferation of seeded cells and migration of neighboring vessels existed.
Some authors suspected the static seeding method and proposed a dynamic seeding condition (Sodian et al., 2002; Hoerstrup et al., 2001). They seeded cells either by stirring matrix and the cell suspension in spinner flasks or by agitating matrix and the cell suspension in tubes with an orbital shaker. They found that the dynamic cell seeding was preferable to the static seeding in cell density and distribution. To achieve better seeding result, we have designed a bioreactor and some primary inspiring results have already been obtained.
Footnotes
Project (No. 99ZB14018) supported by the Natural Science Foundation of Shanghai, China
References
- 1.Fuchs JR, Nasseri BA, Vacanti JP. Tissue engineering: a 21st century solution to surgical reconstruction. Ann Thorac Surg. 2001;72(2):577–591. doi: 10.1016/S0003-4975(01)02820-X. [DOI] [PubMed] [Google Scholar]
- 2.Griffith LG, Naughton G. Tissue engineering—current challenges and expanding opportunities. Science. 2002;295(5557):1009–1014. doi: 10.1126/science.1069210. [DOI] [PubMed] [Google Scholar]
- 3.Hoerstrup SP, Zund G, Sodian R, Schnell AM, Grunenfelder J, Tunina MI. Tissue engineering of small caliber vascular grafts. Eur J Cardio-Thorac Surg. 2001;20(1):164–169. doi: 10.1016/S1010-7940(01)00706-0. [DOI] [PubMed] [Google Scholar]
- 4.Huang HM, Ren H, Liu ZY. Making of acellularized matrix and seeding of smooth muscle cells. Biomedical Engineering and Clinic. 2001;5(2):181–184. [Google Scholar]
- 5.Kim BS, Putnam AJ, Kulik TJ, Moomey DJ. Optimizing seeding and culture methods to engineer smooth muscle on biodegradable polymer matrices. Biotechnol Bioeng. 1998;57(1):46–54. doi: 10.1002/(SICI)1097-0290(19980105)57:1<46::AID-BIT6>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 6.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260(5110):920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 7.Luttun A, Carmeliet G, Carmeliet P. Vascular progenitors: from biology to treatment. Trends Cardiovasc Med. 2002;12(2):88–96. doi: 10.1016/S1050-1738(01)00152-9. [DOI] [PubMed] [Google Scholar]
- 8.Sodian R, Lemke T, Fritsche C, Hoerstrup SP, Fu P, Potapov EV, Hausmann H, Hetzer R. Tissue-engineering bioreactors: a new combined cell-seeding and perfusion system for vascular tissue engineering. Tissue Eng. 2002;8(5):863–870. doi: 10.1089/10763270260424222. [DOI] [PubMed] [Google Scholar]
- 9.Vacanti JP, Langer R. Tissue engineering: the design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet. 1999;354(Suppl 1):SI32–SI34. doi: 10.1016/s0140-6736(99)90247-7. [DOI] [PubMed] [Google Scholar]
- 10.Wu SF, Huang HM. In-vitro culturing and differentiating of peripheral endothelial progenitor cell. J Shanghai Second Medical University. 2004;24(2):159–161. [Google Scholar]
- 11.Xue L, Greisler HP. Biomaterials in the development and future of vascular grafts. J Vasc Surg. 2003;37(2):472–480. doi: 10.1067/mva.2003.88. [DOI] [PubMed] [Google Scholar]