Abstract
Systemic administration of δ-opioid receptor (DOR) agonists decreases immobility in the forced swim test (FST) and increases brain-derived neurotrophic factor (BDNF) mRNA expression in rats, indicating that DOR agonists may have antidepressant-like effects. The aim of this study was to investigate the effects of central administration of endogenous opioid peptides on behavior in the FST and on brain BDNF mRNA expression in rats. Effects of endogenous opioids were compared with those produced by intracerebroventricular administration of a selective non-peptidic DOR agonist (+)BW373U86. Antidepressant-like effects were measured by decreased immobility in the FST. BDNF mRNA expression was determined by in situ hybridization. Centrally administered (+)BW373U86 decreased immobility and increased BDNF mRNA expression in the frontal cortex through a DOR-mediated mechanism, because these effects were blocked by the DOR antagonist naltrindole, but not by the μ-opioid receptor (MOR) antagonist naltrexone (NTX) or the κ-opioid receptor antagonist nor-binaltorphimine. Of all the endogenous opioids tested, only leuand met-enkephalin produced behavioral effects like those of (+)BW373U86 in the FST. Unlike (+)BW373U86, the enkephalins upregulated BDNF mRNA expression in the hippocampus through DOR- and MOR-mediated mechanisms. β-Endorphin, endomorphin-1 and endomorphin-2 significantly increased BDNF mRNA levels in the frontal cortex, hippocampus and amygdala without reducing immobility; and most of these effects were reversed by NTX. This study is the first to provide evidence that endogenous opioids can upregulate BDNF mRNA expression through the DOR and MOR, and that leu- and met-enkephalin have similar pharmacological profiles to synthetic DOR agonists in producing antidepressant-like effects.
Keywords: amygdala, forced swim test, frontal cortex, hippocampus, in situ hybridization, neurotrophins
Introduction
Endogenous opioid peptides regulate many physiological functions, including mood and emotional responses (Bodnar & Klein, 2004). For example, abnormalities of β-endorphin levels have been found in the plasma and brain of patients suffering from depression (Scarone et al., 1990; Darko et al., 1992). Endogenous opioids such as endorphins and enkephalins have relatively high binding affinity for both δ-opioid receptors (DOR) and μ-opioid receptors (MOR) (Simon, 1991). Although early studies implicated the involvement of β-endorphin, leu-enkephalin and met-enkephalin in antidepressant actions, the roles of DOR and MOR in behavioral models of depression were not clearly verified (Kline et al., 1977; Kastin et al., 1978; Tejedor-Real et al., 1995). Nevertheless, RB101, which inhibits the degradation of enkephalins, produces antidepressant-like effects in the mouse forced swim test (FST), and this effect can be reversed by naltrindole (NTI), a DOR antagonist (Baamonde et al., 1992). These findings led to more research to elucidate the functional roles of DOR and MOR in affective states.
Genetic manipulations of endogenous opioids and opioid receptors indicate that activation of the DOR system may positively regulate mood (Roques, 2000; Gaveriaux-Ruff & Kieffer, 2002). For example, mice deficient in DOR display consistent anxiogenic- and depressive-like responses in behavioral assays (Filliol et al., 2000). In addition, preproenkephalin-knockout mice show more anxiety-like behaviors when they are exposed to fear or anxiety-provoking environments (Konig et al., 1996; Ragnauth et al., 2001). Pharmacological studies demonstrate that synthetic DOR-selective agonists produce antidepressant-like effects in the rat FST (Broom et al., 2002a; Jutkiewicz et al., 2004). However, it is unknown whether synthetic DOR agonists and endogenous opioids preferentially activating DOR share similar central sites of antidepressant-like actions.
More intriguing, a recent study shows that systemic administration of a DOR agonist (+)BW373U86 increases brain-derived neurotrophic factor (BDNF) mRNA expression in brain regions involved in stress responses and mood disorders (Torregrossa et al., 2004). BDNF belongs to the nerve growth factor family of neurotrophic factors that regulates neuronal survival, differentiation and plasticity (Binder & Scharfman, 2004). Several lines of evidence suggest that BDNF plays an important role in the pathophysiology of depression and in the mechanism of therapeutic actions of antidepressants (D'Sa & Duman, 2002; Castren, 2004; Hashimoto et al., 2004). For instance, patients with major depression have decreased serum BDNF levels (Shimizu et al., 2003), and preclinical studies demonstrate that infusion of BDNF into the midbrain and hippocampus produce antidepressant-like effects in behavioral models of depression (Siuciak et al., 1997; Shirayama et al., 2002). In addition, some studies have shown that chronic treatment with antidepressants can upregulate BDNF mRNA expression (Nibuya et al., 1995; Russo-Neustadt et al., 2004). Given that both BDNF and endogenous opioids are involved in neurocircuits regulating emotions, it is important to know whether endogenous opioids preferentially activating DOR can modulate BDNF gene expression.
Whereas increased DOR activity contributes to antidepressant-like behavioral effects and increases in BDNF mRNA expression (Broom et al., 2002a; Torregrossa et al., 2004), it is unknown how endogenous opioids that are not highly selective for each opioid receptor type can modulate these endpoints. Therefore, the aim of this study was to investigate whether central administration of endogenous opioids, including leu- and met-enkephalin, β-endorphin, endomorphin-1 and -2, and dynorphin A(1–17) at doses active in other behavioral endpoints can upregulate BDNF mRNA expression and decrease immobility in the rat FST in comparison to central administration of (+)BW373U86. In addition, antagonist studies were performed to verify the roles of DOR and MOR in both antidepressant-like behavioral effects and BDNF gene expression.
Materials and methods
Animals
Male Sprague–Dawley rats (250–275 g) were obtained from Harlan Sprague–Dawley (Indianapolis, IN, USA) and were housed in groups of three rats per cage. All animals were allowed ad libitum access to food and water, and were maintained on a 12 h light : dark cycle with lights on at 06.30 h in a room kept at a temperature of 21–22 °C. Experiments were performed in accordance with the Declaration of Helsinki and with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health. The experimental protocols were approved by the University Committee on the Use and Care of Animals at the University of Michigan.
Intracerebroventricular (i.c.v.) surgery
Rats were anesthetized by intramuscular injection of ketamine (100 mg/kg) and xylazine (10 mg/kg) and placed in a stereotaxic device. Each rat was prepared with a 23-gage stainless steel cannula (Small Parts, Miami Lakes, FL, USA) extending into the right lateral cerebral ventricle (coordinated from bregma, AP: 0.8 mm, ML: 1.5 mm, DV: 4.2 mm; Paxinos & Watson, 1986). The guide cannula was fixed in place with dental cement applied to the surface of the skull. Animals were allowed 6 or 7 days to recover from surgery. Each animal's i.c.v. cannula placement was verified after experiment by injecting methylene blue and checking for distribution. Only data obtained from animals with good i.c.v. cannula placement were used for data analysis.
Drug administration
(+)BW373U86 2HCl was synthesized according to standard protocol (Bishop & McNutt, 1995), leu-enkephalin, met-enkephalin, β-endorphin, endomorphin-1, endomorphin-2, dynorphin A(1–17), naltrexone (NTX) HCl, NTI HCl (National Institute on Drug Abuse, Bethesda, MD, USA) and nor-binaltorphimine (Nor-BNI) 2HCl (Sigma-Aldrich, St. Louis, MO, USA) were dissolved in sterile water. All the peptides were stored at −20 °C. Drug was administered i.c.v. via the guide cannula in volumes of 10 μL using a 25-μL Hamilton syringe attached via a polyethylene PE20 tube (Plastics One, Roanoke, VA, USA) to a 30-gage needle (Becton Dickinson, Franklin Lakes, NJ, USA). Solution was injected over a period of 60 s and the needle was left within the guide cannula for an additional 30 s to prevent reflux. Sterile water was administered for the control injection. The doses of endogenous opioids were chosen based on previous studies showing that they were active in a variety of behavioral and physiological functional assays (Tseng et al., 1980; Hayes et al., 1983; Bhattacharya et al., 1992; Carrigan et al., 2000; Elhassan et al., 2000; Hung et al., 2002). For the antagonist study, animals were chosen randomly from the same batch and were injected with sterile water, NTX (0.1 mg/kg) or NTI (1 mg/kg) subcutaneously 15 min prior to an i.c.v. injection of sterile water vehicle or agonist. Nor-BNI (10 mg/kg) was injected subcutaneously 24 h prior to the i.c.v. injection of sterile water vehicle or agonist. The dose and pretreatment time for these antagonists were chosen based on previous studies showing that each antagonist produced selective functional antagonism for MOR, DOR and κ-opioid receptors (KOR) (Takemori et al., 1988; Chang et al., 1993; Walker et al., 1994).
FST
The modified FST (a one-time, 15-min swim session) was conducted as described by Broom et al. (2002a). This procedure was chosen in order to avoid potential impact of learning and adaptation of animals with pre-exposure to the apparatus. A series of studies from our laboratory have demonstrated that this procedure can be used to detect the antidepressant potential of compounds comprehensively (Broom et al., 2002a,b; Jutkiewicz et al., 2004; Torregrossa et al., 2004). Briefly, 30 min after agonist injection, rats were placed in a clear cylindrical Plexiglas container (46 cm tall × 20 cm diameter) filled with 30 cm of 25 °C (± 1 °C) water for a 15-min swim session. Cylinders were cleaned and fresh water added between each rat. The 15-min swim period was videotaped and behaviors were scored every 5 s by experimenters who were blinded to dosing conditions. Behaviors were classified as immobility, swimming or climbing, as defined by Broom et al. (2002a,b).
Locomotor activity measurements
To measure changes in locomotor activity, experimentally naïve rats that had recovered from i.c.v. cannula implantation surgery were prepared with transmitters (model ER-4000 E-Mitter; Mini Mitter, Bend, OR, USA), under ketamine (100 mg/kg, i.m.) and xylazine (10 mg/kg, i.m.) anesthesia. The transmitter was implanted inside the peritoneum of a rat at least 6 days before conducting an experiment. The transmitter broadcasts changes in locomotor activity to a receiver (model ER-4000 receiver, Mini Mitter) placed under the home cage of each rat. Data were collected and processed simultaneously by the Vital View data acquisition system (Mini Mitter). Immediately after agonist or vehicle injection, locomotor activity measurements were collected for each rat for 2 h. Locomotor activity counts during the 30–45th min after drug administration, which was the exact time frame as the FST was conducted, were compared and analysed for the significant difference among different dosing conditions.
In situ hybridization histochemistry
Three hours after agonist injection, experimentally naïve animals were killed by decapitation, and their brains were rapidly removed and frozen in isopentane at −40 °C, and were stored at −80 °C. Brains were sectioned at 20 μm on a cryostat and were thaw mounted onto poly-l-lysine-subbed slides. Slides were stored at −80 °C until they were processed for in situ hybridization, which was performed as described by Torregrossa et al. (2004). Briefly, BDNF mRNA expression was determined by a double label in situ hybridization with a [35S]-labeled BDNF cRNA probe (described by Isackson et al., 1991). The rat BDNF cDNA was donated by Drs Gall and Lauterborn (University of California, Irvine, USA). Sections were fixed in 4% paraformaldehyde for 1 h. Then, they were washed three times in 2 × standard saline citrate (SSC, 300 mm NaCl, 30 mm sodium citrate), placed in a solution consisting of 0.25% acetic anhydride in 0.1 m TEA, pH 8.0, at room temperature for 10 min, and dehydrated in progressively graded alcohols (50, 70, 80, 95, 100%). The sections were then labeled with the BDNF cRNA probe. The probe was radioactively labeled in a reaction containing 1 μg BDNF antisense linearized plasmid DNA, 5 × transcription buffer, 125 μCi each of [35S]UTP and [35S]CTP, 150 μm each of ATP and GTP, 12.5 mm dithiothreitol, 20 U RNase inhibitor and 6 U of T3 polymerase. The reaction was carried out for 90 min at 37 °C. The probes were separated from unincorporated nucleotides using BIORAD kit: MicroBioSpin 6 Chromatography Columns (Bio-Rad Laboratories, Hercules, CA, USA). The probes were diluted in a hybridization buffer to obtain 1.5 × 106 cpm/80 μL. The sections were then coverslipped and allowed to hybridize at 55 °C for 16 h. At this time, coverslips were removed and the slides were washed twice in 2 × SSC. Then, sections were incubated in RNase for 1 h at 37 °C, washed in decreasing concentrations of SSC (2 × ,1 × , 0.5 × , 0.25 × ), and washed in 0.1 × SSC at 65 °C for 1 h. Sections were then rinsed in distilled water and dehydrated through graded alcohols. The slides were exposed on Kodak XAR film (Eastman Kodak, Rochester, NY, USA) for 14 days. The rat BDNF probe is known to be selective for BDNF, as previous studies have shown that slides incubated in RNase prior to hybridization, and slides hybridized to the sense strand of the BDNF probe produce no specific binding (Isackson et al., 1991; Torregrossa et al., 2004).
Quantification of radioactive signal
BDNF mRNA levels were quantified using NIH Image software (Scion Image, Frederick, MD, USA). BDNF mRNA expression was examined in frontal cortex, CA1, CA3 and dentate gyrus (DG) regions of hippocampus and amygdala. Each brain region was analysed by creating an outline around the region and measuring both the left and right sides of the brain and from rostral–caudal sections 100–200 μm apart. At least six sections per region per rat were quantified. The signal measurements were corrected for background and were determined as the mean radioactive intensity per pixel for that region. These signal values for each section were then averaged to obtain the mean signal for each region in each rat. These data points were then averaged per group and compared statistically.
Statistical analysis
Behavioral data from the FST were expressed as mean ± SEM. BDNF signals from in situ hybridization were expressed as mean percent of vehicle ± SEM. Statistical analysis was performed using one-way anova with Dunnett's post hoc test to compare differences between groups where P < 0.05 was considered significant.
Results
Behavioral effects of i.c.v. administration of (+)BW373U86 and endogenous opioids
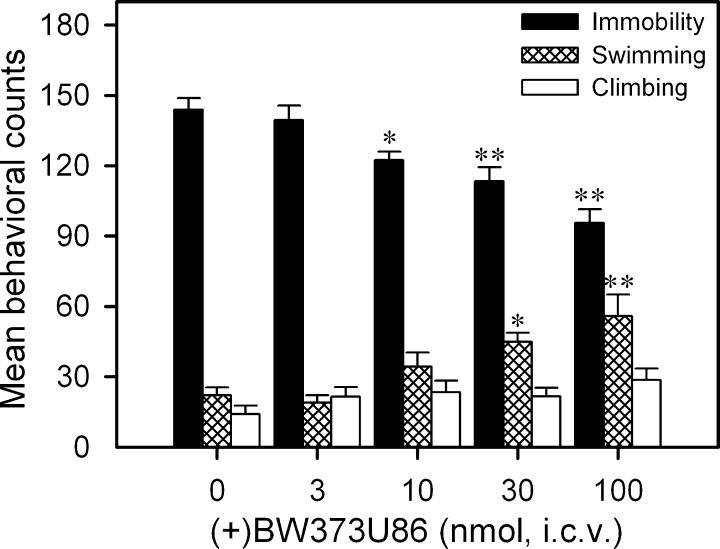
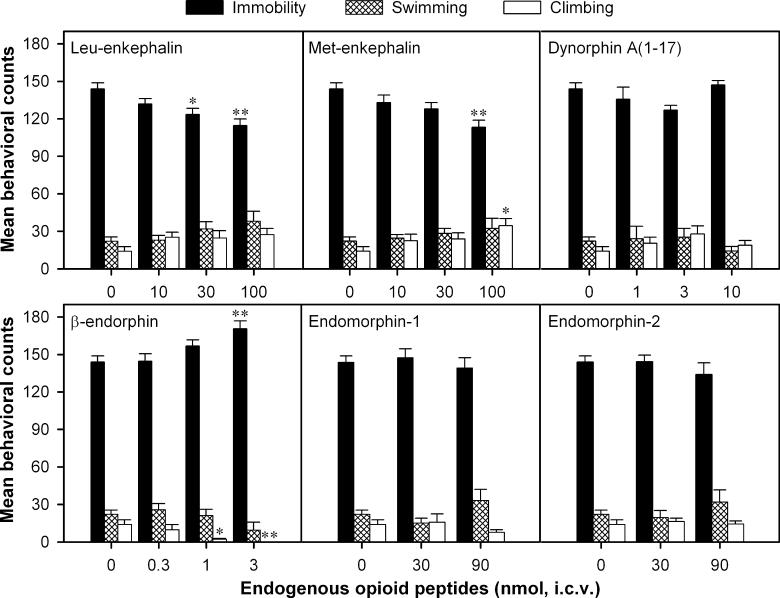
I.c.v. administration of (+)BW373U86 significantly decreased immobility and increased swimming in a dose-dependent manner in the rat FST (Fig. 1). Post hoc comparisons indicated that doses of 10 nmol and higher produced significant decreases in immobility. In addition, doses of 30 nmol and higher significantly increased swimming. I.c.v. administration of leu-enkephalin and met-enkephalin also significantly decreased immobility in a dose-dependent manner (Fig. 2). Post hoc comparisons indicated that leu-enkephalin at 30 and 100 nmol decreased immobility significantly. Met-enkephalin significantly decreased immobility and increased climbing, but only at the highest dose (i.e. 100 nmol) tested. In contrast, β-endorphin had no effect on immobility at lower doses, but significantly increased immobility and decreased climbing at a dose of 3 nmol. Endomorphin-1, endomorphin-2 and dynorphin A(1–17) did not alter behavior in the FST at the doses tested. In order to verify whether decreased immobility in the FST was associated with increased locomotor activity, effects of some agonists at doses active in the FST were tested on the locomotor activity. These included (+)BW373U86 (100 nmol), leu-enkephalin (100 nmol), met-enkephalin (100 nmol) and β-endorphin (3 nmol). The results, analysed by the time course and area under curve, indicated that none of the agonists produced a significant change in the locomotor activity during either the 30–45th min after drug administration or the entire 2-h test session (data not shown).
Fig. 1.
Effects of i.c.v. administration of (+)BW373U86 (nmol) on behavior in the FST in rats. Each value represents the mean ± SEM (n = 6). (+)BW373U86 was administered 30 min before the FST. *P < 0.05; **P < 0.01 compared with vehicle.
Fig. 2.
Effects of i.c.v. administration of endogenous opioid peptides (nmol) on behavior in the FST in rats. Each value represents the mean ± SEM (n = 6). Opioid peptide was administered 30 min before the FST. *P < 0.05; **P < 0.01 compared with vehicle.
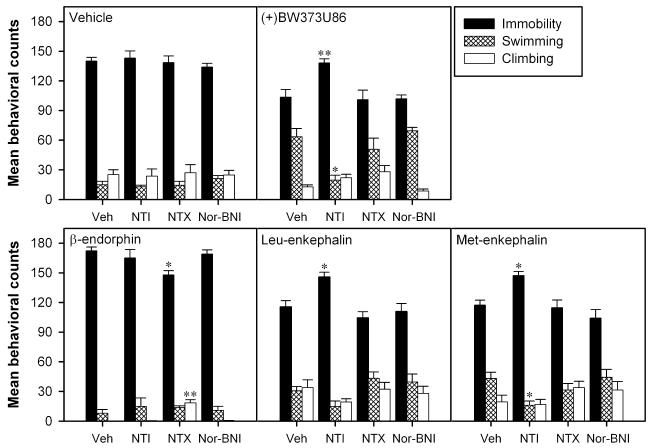
Effects of opioid antagonists on (+)BW373U86, enkephalins and β-endorphin activity in the FST
To determine what type of opioid receptor (DOR, MOR and KOR) mediated the effects of the agonists in the FST, the selective DOR antagonist NTI, MOR antagonist NTX, and KOR antagonist Nor-BNI were administrated 15 min (for NTI and NTX) or 24 h (for Nor-BNI) before i.c.v. administration of effective doses of each agonist. With vehicle pretreatments, (+)BW373U86 (100 nmol), leu-enkephalin (100 nmol) and met-enkephalin (100 nmol) decreased immobility, and β-endorphin (3 nmol) increased immobility significantly compared with vehicle control (Fig. 3). Subcutaneous administration of NTI (1 mg/kg), NTX (0.1 mg/kg) or Nor-BNI (10 mg/kg) alone had no effect in the FST. However, this dose of NTI blocked the antidepressant-like effects of (+)BW373U86 (100 nmol), leu-enkephalin (100 nmol) and met-enkephalin (100 nmol), while NTX and Nor-BNI had no effect on the changes in immobility produced by these agonists. In addition, NTX reversed the increase in immobility induced by β-endorphin (3 nmol). Neither NTI nor Nor-BNI significantly altered the β-endorphin-induced increase in immobility (Fig. 3).
Fig. 3.
Effects of opioid receptor antagonists on the behavioral activity of i.c.v. administration of vehicle (sterile water, 10 μL), (+)BW373U86 (100 nmol), β-endorphin (3 nmol), leu-enkephalin (100 nmol) and met-enkephalin (100 nmol) in the FST in rats (n = 6). Sterile water (Veh 1 mL/kg, s.c.), naltrindole (NTI 1 mg/kg, s.c.) or naltrexone (NTX 0.1 mg/kg, s.c.) was administered 15 min before agonist injection. Nor-binaltorphimine (Nor-BNI 10 mg/kg, s.c.) was administrated 24 h before agonist injection. *P < 0.05; **P < 0.01 compared with the vehicle-pretreated group.
Effects of i.c.v. administration of (+)BW373U86 and enkephalins on BDNF mRNA expression
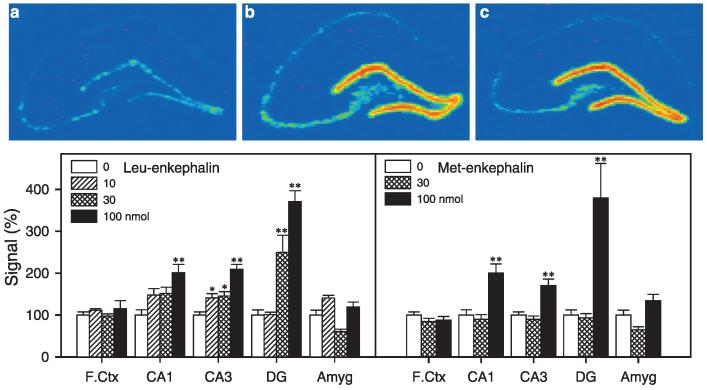
A recent study demonstrated that a systemic injection of (+)BW373U86 produced a maximum increase in BDNF mRNA expression 3 h after administration; therefore, this time-point was used for the present study (Torregrossa et al., 2004). In situ hybridization analysis demonstrated that 100 nmol (+)BW373U86 significantly increased BDNF mRNA expression in the frontal cortex (Fig. 4). Unlike (+)BW373U86, leu-enkephalin and met-enkephalin primarily increased BDNF mRNA expression in the subfields of the hippocampus, not in the frontal cortex (Fig. 5). Post hoc comparisons indicated that 10 nmol of leu-enkephalin slightly increased BDNF mRNA expression in the CA3, 30 nmol of leu-enkephalin moderately increased BDNF mRNA expression in the CA3 and DG; and 100 nmol of leu-enkephalin greatly upregulated BDNF mRNA expression in the CA1, CA3 and DG. In addition, a dose of 100 nmol met-enkephalin also significantly increased BDNF mRNA expression in the CA1, CA3 and DG. All of these ligands possessing DOR-binding affinity had no effect on BDNF mRNA expression in the amygdala (Fig. 5).
Fig. 4.

Representative autoradiograms of BDNF mRNA expression in the rat frontal cortex (a and b). BDNF mRNA expression was determined 3 h after i.c.v. administration of (+)BW373U86. Radiolabeled BDNF cRNA probes were used for in situ hybridization. (A) Section of frontal cortex taken from a vehicle-treated animal. (B) Section of frontal cortex taken from an animal treated with (+)BW373U86 (100 nmol, i.c.v.). Quantification of BDNF mRNA signals in different brain regions including frontal cortex (F.Ctx), CA1, CA3 and dentate gyrus (DG) of hippocampus, and amygdala (Amyg) are represented as mean percent of vehicle ± SEM (n = 4–6). **P < 0.01 compared with vehicle.
Fig. 5.
Representative autoradiograms of BDNF mRNA expression in the rat hippocampus (a–c). BDNF mRNA expression was determined 3 h after i.c.v. administration of enkephalins. (a) Section of hippocampus taken from a vehicle-treated animal. (b) Hippocampus of an animal treated with leu-enkephalin (100 nmol, i.c.v.). (c) Hippocampus section taken from an animal treated with met-enkephalin (100 nmol, i.c.v.). Quantification of BDNF mRNA signals in brain regions are represented as mean percent of vehicle ± SEM (n = 4–6). *P < 0.05; **P < 0.01 compared with vehicle.
Effects of opioid antagonists on (+)BW373U86- and enkephalin-induced increases in BDNF mRNA expression
Subcutaneous administration of NTI (1 mg/kg), NTX (0.1 mg/kg) and Nor-BNI (10 mg/kg) had no effect on BDNF mRNA expression alone (data not shown). Only NTI pretreatment blocked the increase in BDNF mRNA expression produced by i.c.v. (+)BW373U86, 100 nmol (Fig. 6). Both NTX and Nor-BNI failed to affect the increase in BDNF mRNA expression produced by (+)BW373U86. It was worth noting that i.c.v. dynorphin A(1–17) 10 nmol neither changed immobility nor increased BDNF mRNA expression, and Nor-BNI pretreatment did not attenuate effects of (+)BW373U86 on BDNF mRNA expression (data not shown), indicating the minimal role of KOR in both endpoints. Thus, we chose NTI and NTX for further antagonist studies of endogenous opioids.
Fig. 6.
Effects of opioid receptor antagonists on i.c.v. administered (+)BW373U86 (100 nmol), leu-enkephalin (100 nmol) and met-enkephalin (100 nmol) induced increases in BDNF mRNA expression in rats. Each value represents the mean percent of (veh + veh) ± SEM (n = 4–6). Levels of BDNF mRNA expression were determined 3 h after agonist administration. Subcutaneous administration of sterile water (Veh, 1 mL/kg), naltrindole (NTI, 1 mg/kg) or naltrexone (NTX, 0.1 mg/kg) was conducted 15 min before agonist administration. *P < 0.05 and **P < 0.01 compared with the veh + veh group; #P < 0.05 and ##P < 0.01 compared with the veh + agonist group.
Figure 6 illustrates that vehicle pretreatment did not change the upregulation of BDNF mRNA expression produced by i.c.v. leuenkephalin and met-enkephalin (100 nmol). In contrast, pretreatment with NTI significantly attenuated the increase in BDNF mRNA expression induced by leu-enkephalin in the CA3 and DG compared with animals that received vehicle pretreatment. NTI also prevented met-enkephalin-induced upregulation of BDNF mRNA expression in the CA1 and DG regions of the hippocampus. Due to the affinity of enkephalins for the MOR (Simon, 1991), we investigated whether the dose of NTX 0.1 mg/kg that is selective for MOR antagonism could reverse the increase in BDNF mRNA expression produced by leu- and met-enkephalin. Figure 6 illustrates that pretreatment with NTX blocked significantly the increase in BDNF mRNA expression produced by leu-enkephalin in the CA1, CA3 and DG regions of the hippocampus. In addition, NTX significantly blocked the increase in BDNF mRNA expression produced by met-enkephalin in the CA1 and DG compared with animals that received vehicle pretreatment.
Effects of i.c.v. administration of β-endorphin and endomorphins on BDNF mRNA expression
Figure 7 illustrates the effects of β-endorphin, endomorphin-1 and -2 on BDNF mRNA expressions in rats. A dose of 1 nmol β-endorphin had no effect on BDNF mRNA expression in any region we examined; however, 3 nmol β-endorphin significantly increased BDNF mRNA expression in the frontal cortex, CA1, CA3, DG and amygdala. Quantitative in situ hybridization for BDNF mRNA expression revealed that endomorphin-1 and -2 upregulated BDNF mRNA expression in a dose-dependent manner. Post hoc analysis showed that 10 nmol endomorphin-1 significantly increased BDNF mRNA expression in the DG of the hippocampus. Endomorphin-1, at doses of 30 and 90 nmol, increased BDNF mRNA expression significantly in the frontal cortex, CA1, CA3 and DG regions of the hippocampus, and in the amygdala. Endomorphin-2 showed a similar pattern to endomorphin-1. Doses of endomorphin-2 (i.e. 10 and 30 nmol) upregulated BDNF mRNA expression in the CA3 and DG. A higher dose of 90 nmol endomorphin-2 significantly increased BDNF mRNA expression in the frontal cortex, hippocampus and amygdala.
Fig. 7.
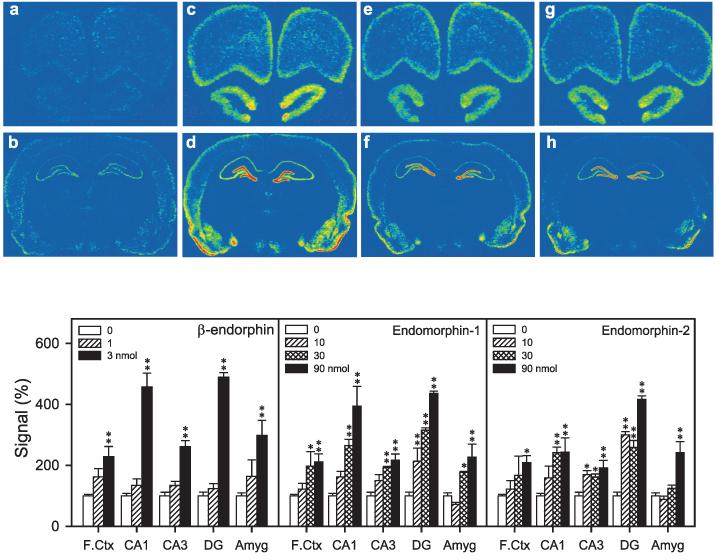
Representative autoradiograms of BDNF mRNA expression in the rat brain regions (a–h). Levels of BDNF mRNA were determined 3 h after agonist injection. (a and b) Sections taken from a vehicle-treated animal. (c and d) Sections taken from an animal treated with β-endorphin (3 nmol, i.c.v.). (e and f) Sections taken from an animal treated with endomorphin-1 (90 nmol, i.c.v.). (g and h) Sections taken from an animal treated with endomorphin-2 (90 nmol, i.c.v.). Quantification of BDNF mRNA signals in brain regions are represented as mean percent of vehicle ± SEM (n = 4–6). *P < 0.05; **P < 0.01 compared with vehicle.
Effects of opioid antagonists on β-endorphin- and endomorphin-induced increases in BDNF mRNA expression
Figure 8 illustrates that vehicle pretreatment did not change the upregulation of BDNF mRNA expression produced by i.c.v. β-endorphin (3 nmol), endomorphin-1 (90 nmol) and endomorphin-2 (90 nmol). Pretreatment with NTX (0.1 mg/kg) completely blocked the increases in BDNF mRNA expression in the frontal cortex, CA1, CA3, DG and amygdala induced by β-endorphin and endomorphin-1; and in the frontal cortex produced by endomorphin-2. NTX partially blocked endomorphin-2-induced increases in BDNF mRNA expression in the DG, while having no effect on BDNF mRNA expression in the CA1 and CA3. In contrast, pretreatment with NTI (1 mg/kg) had no effect on the effect of endomorphin-1, but partially blocked the increases in BDNF mRNA expression produced by β-endorphin in the CA1, CA3 and DG. NTI also partially attenuated endomorphin-2-induced increase in BDNF mRNA expression in the DG of the hippocampus.
Fig. 8.
Effects of opioid receptor antagonists on i.c.v. administered β-endorphin (3 nmol), endomorphin-1 (EM-1, 90 nmol) and endomorphin-2 (EM-2, 90 nmol) induced increases in BDNF mRNA expression in rats. Each value represents the mean percent of (veh + veh) ± SEM (n = 4–6). Levels of BDNF mRNA expression were determined 3 h after agonist administration. Subcutaneous administration of sterile water (Veh, 1 mL/kg), naltrindole (NTI, 1 mg/kg) or naltrexone (NTX, 0.1 mg/kg) was conducted 15 min before agonist administration. *P < 0.05 and **P < 0.01 compared with the veh + veh group; #P < 0.05 and ##P < 0.01 compared with the veh + agonist group.
Discussion
The results of the present study provide the first functional evidence that central administration of endogenous opioid peptides significantly upregulate BDNF mRNA expression in the frontal cortex, hippocampus and amygdala, through activation of the DOR and MOR. However, only the enkephalins, which preferentially activate the DOR endogenously, have a similar pharmacological profile to the synthetic DOR agonist in producing antidepressant-like behavioral effects in rats.
The FST is widely used for screening novel potential antidepressants and is sensitive to the major classes of known antidepressants (Porsolt et al., 1977; Cryan et al., 2002). The localization of DOR in large concentrations in cortical and limbic brain regions involved in mood and stress response suggests that DOR agonists could regulate mood (Cahill et al., 2001). Indeed, systemic administration of (+)BW373U86 and SNC80, selective non-peptidic DOR agonists, produce a fast-onset antidepressant-like effect (i.e. decreased immobility) in the rat FST (Broom et al., 2002a). In this study, we further found that i.c.v. administration of (+)BW373U86 dose-dependently decreased immobility in the FST. In addition, the antidepressant-like effect of centrally administered (+)BW373U86 was blocked by the selective DOR antagonist NTI, but not by the MOR antagonist NTX, or the KOR antagonist Nor-BNI. These results confirm the notion that activation of central DOR can produce behavioral antidepressant-like effects.
Although leu- and met-enkephalin are thought to be the endogenous ligands for the DOR, dynorphin A(1–17) is an endogenous ligand for the KOR, and β-endorphin, endomorphin-1 and -2 are endogenous ligands for the MOR, most of the endogenous opioids have affinity for all three opioid receptor types (Simon, 1991). Nevertheless, the present results demonstrated that only leu- and met-enkephalin dose-dependently decreased immobility in the FST; and β-endorphin, endomorphin-1 and -2, and dynorphin A(1–17) did not decrease immobility at any of the doses tested. More important, the antidepressant-like effects of the enkephalins were blocked by NTI, and were not affected by a MOR or KOR antagonist. These findings clearly indicate that antidepressant-like effects of enkephalins were mediated through activation of central DORs. In addition, centrally administered (+)BW373U86 and the enkephalins did not affect locomotor activity at the doses that produced antidepressant-like effects. These findings, along with other pharmacological and genetic studies, suggest that the increased DOR activity positively regulates mood, and dysregulation of the enkephalin release may partially contribute to the occurrence of depression (Filliol et al., 2000; Ragnauth et al., 2001; Broom et al., 2002a).
Given that central administration of DOR agonists produced antidepressant-like effects and that increased BDNF gene expression is important for the therapeutic actions of antidepressants, we further investigated whether centrally administered DOR agonists could lead to an increase in BDNF mRNA expression in different brain regions. Interestingly, we found that i.c.v. administration of (+)BW373U86 (100 nmol) elevated BDNF mRNA expression in the frontal cortex; however, it had no effect on BDNF mRNA expression in other brain regions. This finding appears to agree with recent studies demonstrating that the largest increases in BDNF mRNA expression produced by systemic administration of (+)BW373U86 are most consistently observed in the frontal cortex (Torregrossa et al., 2004). Furthermore, the DOR antagonist naltrindole blocked (+)BW373U86-mediated increase in BDNF mRNA expression, while MOR and KOR antagonists had no effect. These results seem to support the idea that central DOR activation can rapidly upregulate BDNF gene expression.
Central administration of leu- and met-enkephalin both produced behavioral antidepressant-like effects and increased BDNF mRNA expression in rats, in a manner similar to the non-peptidic DOR agonist. However, unlike (+)BW373U86, the enkephalins primarily increased BDNF mRNA expression in the subfields of the hippocampus, not in the frontal cortex. One explanation might be differences in the stability of these ligands and their distributions in the brain after i.c.v. administration. The other explanation is that the enkephalins have affinity for the MOR that is highly expressed in the hippocampus compared with the DOR (Mansour et al., 1988; Zastawny et al., 1994; Jenab et al., 1995). The efficacy of ligands can vary greatly across brain subregions expressing different receptor densities (Maher et al., 2000; Ko et al., 2003). In particular, the upregulation of BDNF mRNA expression produced by the enkephalins was partially blocked by the DOR antagonist, but it was completely blocked by the MOR antagonist. These results may indicate that the increases in BDNF mRNA expression produced by the enkephalins in some areas were primarily mediated through the MOR, with a moderate contribution from activation of the DOR. It would be important to explore whether activation of central DOR and MOR can increase BDNF mRNA expression in MOR- vs. DOR-knockout mice. These findings also led to the question of whether central administration of endogenous opioids preferentially activating MORs could upregulate BDNF mRNA expression.
Although β-endorphin had been thought to be an endogenous MOR ligand, it has almost equal affinity for the DOR (Simon, 1991). Endomorphin-1 and -2 are peptides that showed the highest affinity and selectivity for the MOR of all known endogenous opioids (Zadina et al., 1997). Our findings revealed that centrally administered β-endorphin, endomorphin-1 and -2 significantly increased BDNF mRNA expression in the frontal cortex, hippocampus and amygdala in a dose-dependent manner. The MOR antagonist NTX completely blocked the increase in BDNF mRNA expression produced by β-endorphin and endomorphin-1 in all brain regions, and blocked the effect of endomorphin-2 in the frontal cortex. In contrast, the DOR antagonist NTI only produced a moderate blockade of β-endorphin-induced increased BDNF mRNA expression, but it showed little or no antagonist effect against endomorphin-1 and -2. Taken together, these endogenous opioids can upregulate BDNF mRNA expression in the rat brain through activation of the central MOR without producing antidepressant-like behavioral effects. It is worth noting that BDNF might play a prodepressive role in the ventral tegmental area–nucleus accumbens pathway (Eisch et al., 2003), which is opposite to the antidepressive role of BDNF in the hippocampus (Siuciak et al., 1997; Shirayama et al., 2002). In addition, morphine can upregulate BDNF mRNA in brain areas that project to the nucleus accumbens (Le Foll et al., 2005). These findings collectively indicate that an increase in BDNF gene expression alone may not predict the antidepressant-like behavioral effect of an opioid ligand.
Previous studies have demonstrated that the antidepressant-like effect of DOR agonists in the rat FST and increases in BDNF mRNA expression are not dependent on the convulsions produced by DOR agonists (Broom et al., 2002b; Torregrossa et al., 2004). In this study, we did not observe overt convulsions after i.c.v. administration of (+)BW373U86, leu- or met-enkephalin at the doses producing antidepressant-like effects. In contrast, the highest doses of β-endorphin (3 nmol), endomorphin-1 (90 nmol) and -2 (90 nmol) produced overt convulsions, but did not decrease immobility in the FST. These observations provide further evidence that the antidepressant-like effects of DOR agonists do not depend on a convulsion. Nevertheless, it cannot yet be ruled out that such ligands produce seizure-like EEG changes, which in turn increase BDNF gene expression. For example, low doses of non-peptidic DOR agonists produce some paroxysmal activity, but do not produce non-convulsive seizures in the absence of overt convulsions (Jutkiewicz et al., unpublished results). These acute EEG changes may be responsible for the increased BDNF gene expression. However, activation of BDNF mRNA expression by ligands causing convulsions was an ‘all-or-none’ type of response because subconvulsive doses of pentylenetetrazol and kainic acid failed to alter neurotrophin gene expression (Humpel et al., 1993; Heese et al., 2000). Seizure-induced increases in BDNF mRNA expressions were often observed in widespread brain regions, including the hippocampus, amygdala and other cortical areas (Ernfors et al., 1991; Isackson et al., 1991; Bengzon et al., 1993), but rats exhibiting weak seizures did not show increased BDNF mRNA expression in any brain regions (Hashimoto et al., 1998). In the present study, enkephalins did not produce convulsions, but enkephalins increased BDNF mRNA expression only in the hippocampus. Future studies are needed to qualitatively compare seizures provoked by central DOR and MOR activation, and to clarify the role of central DOR and MOR in the regulation of BDNF mRNA expression.
The present finding that endogenous opioids upregulated BDNF mRNA expression through activation of the DOR and MOR may have physiological significance beyond that of mood regulation. Both DOR and MOR antagonists alone did not change BDNF mRNA expression, suggesting no role of opioid receptors in the tonic regulation of BDNF gene expression. Nevertheless, it will be important to determine whether stress-induced release of endogenous opioids alters BDNF signaling. It is well known that BDNF participates in synaptic plasticity mechanisms such as long-term potentiation, learning and memory (Huang & Reichardt, 2001; Malcangio & Lessmann, 2003). The finding that centrally administered β-endorphin, endomorphin-1 and -2 increased BDNF mRNA expressions in the hippocampus and amygdala may imply a potential link between MOR and BDNF in learning and memory or opioid drug-conditioning effect. It will be valuable to further elucidate the relationship between opioid receptors and BDNF following acute and chronic stress or opioid drug-taking behaviors in a broader context of physiological functions.
In summary, this study provides the first functional evidence that endogenous opioid peptides active at MOR or DOR, but not KOR, can acutely upregulate BDNF mRNA expression in the rat brain. Endogenous opioids such as leu- and met-enkephalin have actions similar to the synthetic DOR agonist, indicating that regulation of the enkephalins is important for emotional responses. Although β-endorphin and the endomorphins do not appear to produce antidepressant-like behavioral effects, future studies are required to investigate the involvement of endogenous opioids active at MOR or exogenous MOR agonists in BDNF-related physiological functions.
Acknowledgements
This work was supported by US National Institutes of Health grants DA00254, DA07281, DA13685 and MH42251.
Abbreviations
- BDNF
brain-derived neurotrophic factor
- DG
dentate gyrus
- DOR
δ-opioid receptors
- FST
forced swim test
- KOR
κ-opioid receptors
- MOR
μ-opioid receptors
- Nor-BNI
nor-binaltorphimine
- NTI
naltrindole
- NTX
naltrexone
- SSC
standard saline citrate
References
- Baamonde A, Dauge V, Ruiz-Gayo M, Fulga IG, Turcaud S, Fournie-Zaluski MC, Ronques BP. Antidepressant-type effects of endogenous enkephalins protected by systemic RB101 are mediated by opioid delta and dopamine D1 receptor stimulation. Eur. J. Pharmacol. 1992;216:157–166. doi: 10.1016/0014-2999(92)90356-9. [DOI] [PubMed] [Google Scholar]
- Bengzon J, Kokaia Z, Ernfors P, Kokaia M, Leanza G, Nilsson OG, Persson H, Lindvall O. Regulation of neurotrophin and trkA, trkB and trkC tyrosine kinase receptor messenger RNA expression in kindling. Neuroscience. 1993;53:433–446. doi: 10.1016/0306-4522(93)90207-v. [DOI] [PubMed] [Google Scholar]
- Bhattacharya SK, Saraswati M, Sen AP. Effect of centrally administered enkephalins on carrageenan-induced paw oedema in rats. Res. Exp. Med. 1992;192:443–449. doi: 10.1007/BF02576302. [DOI] [PubMed] [Google Scholar]
- Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth Factors. 2004;22:123–131. doi: 10.1080/08977190410001723308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop MJ, McNutt RW. An efficient synthesis of the benahydrylpiperazine delta opioid agonist (+)-BW373U86. Bioorg. Med. Chem. Lett. 1995;5:1311–1314. [Google Scholar]
- Bodnar RJ, Klein GE. Endogenous opiates and behavior: 2003. Peptides. 2004;25:2205–2256. doi: 10.1016/j.peptides.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Broom DC, Jutkiewicz EM, Folk JE, Traynor JR, Rice KC, Woods JH. Nonpeptidic delta-opioid receptor agonists reduce immobility in the forced swim assay in rats. Neuropsychopharmacology. 2002a;26:744–755. doi: 10.1016/S0893-133X(01)00413-4. [DOI] [PubMed] [Google Scholar]
- Broom DC, Jutkiewicz EM, Folk JE, Traynor JR, Rice KC, Woods JH. Convulsant activity of a non-peptidic delta-opioid receptor agonists is not required for its antidepressant effects in Sprague-Dawley rats. Psychopharmacology. 2002b;164:42–48. doi: 10.1007/s00213-002-1179-y. [DOI] [PubMed] [Google Scholar]
- Cahill CM, McClellan KA, Morinville A, Hoffert C, Hubatsch D, O'Donnell D, Beaudet A. Immunohistochemical distribution of opioid receptors in the rat central nervous system: evidence for somatodendritic labeling and antigen-specific cellular compartmentalization. J. Comp. Neurol. 2001;440:65–84. doi: 10.1002/cne.1370. [DOI] [PubMed] [Google Scholar]
- Carrigan KA, Nelson CJ, Lysle DT. Endomorphin-1 induces antinociception without immunomodulatory effects in the rat. Psychopharmacology. 2000;151:299–305. doi: 10.1007/s002130000487. [DOI] [PubMed] [Google Scholar]
- Castren E. Neurotrophins as mediators of drug effects on mood, addiction, and neuroprotection. Mol. Neurobiol. 2004;29:289–302. doi: 10.1385/MN:29:3:289. [DOI] [PubMed] [Google Scholar]
- Chang KJ, Rigdon GC, Howard JL, McNutt RW. A novel, potent and selective nonpeptidic delta opioid receptor agonist BW373U86. J. Pharmacol. Exp. Ther. 1993;267:852–857. [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol. Sci. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- D'Sa C, Duman RS. Antidepressants and neuroplasticity. Bipolar. Disord. 2002;4:183–194. doi: 10.1034/j.1399-5618.2002.01203.x. [DOI] [PubMed] [Google Scholar]
- Darko DF, Risch SC, Gillin JC, Golshan S. Association of beta-endorphin with specific clinical symptoms of depression. Am. J. Psychiatry. 1992;149:1162–1167. doi: 10.1176/ajp.149.9.1162. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Bolanos CA, de Wit J, Simonak RD, Pudiak CM, Barrot M, Verhaagen J, Nestler EJ. Brain-derived neurotrophic factor in the ventral midbrain-nucleus accumbens pathway: a role in depression. Biol. Psychiatry. 2003;54:994–1005. doi: 10.1016/j.biopsych.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Elhassan AM, Papadogiannakis N, Adem A, Suliman I, Gad A, Lindgren JU. Intracerebroventricular met-enkephalin administration modulates adjuvant arthritis. Brain Res. 2000;879:23–28. doi: 10.1016/s0006-8993(00)02686-x. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Bengzon J, Kokaia Z, Persson H, Lindvall O. Increased levels of messenger RNAs for neurotrophic factors in the brain during kindling epileptogenesis. Neuron. 1991;7:165–176. doi: 10.1016/0896-6273(91)90084-d. [DOI] [PubMed] [Google Scholar]
- Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, Befort K, Gaveriaux-Ruff C, Dierich A, LeMeur M, Valverde O, Maldonado R, Kieffer BL. Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat. Genet. 2000;25:195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- Gaveriaux-Ruff C, Kieffer BL. Opioid receptor genes inactivated in mice: the highlights. Neuropeptides. 2002;36:62–71. doi: 10.1054/npep.2002.0900. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Shimizu E, Iyo M. Critical role of brain-derived factor in mood disorders. Brain Res. Rev. 2004;45:104–114. doi: 10.1016/j.brainresrev.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Watanabe K, Nishimura T, Iyo M, Shirayama Y, Minabe Y. Behavioral changes and expression of heat shock protein hsp-70 mRNA, brain-derived neurotrophic factor mRNA, and cyclooxygenase-2 mRNA in rat brain following seizures induced by systemic administration of kainic acid. Brain Res. 1998;804:212–223. doi: 10.1016/s0006-8993(98)00708-2. [DOI] [PubMed] [Google Scholar]
- Hayes AG, Skingle M, Tyers MB. Antinociceptive profile of dynorphin in the rat. Life Sci. 1983;33:657–660. doi: 10.1016/0024-3205(83)90588-x. [DOI] [PubMed] [Google Scholar]
- Heese K, Otten U, Mathivet P, Raiteri M, Marescaux C, Bernasconi R. GABA(B) receptor antagonists elevate both mRNA and protein levels of the neurotrophins nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) but not neurotrophin-3 (NT-3) in brain and spinal cord of rats. Neuropharmacology. 2000;39:449–462. doi: 10.1016/s0028-3908(99)00166-5. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humpel C, Wetmore C, Olson L. Regulation of brain-derived neurotrophic factor messenger RNA and protein at the cellular level in pentylenetetrazol-induced epileptic seizures. Neuroscience. 1993;53:909–918. doi: 10.1016/0306-4522(93)90476-v. [DOI] [PubMed] [Google Scholar]
- Hung KC, Wu HE, Mizoguchi H, Tseng LF. Acute antinociceptive tolerance and unidirectional cross-tolerance to endomorphin-1 and endomorphin-2 given intraventricularly in the rat. Eur. J. Pharmacol. 2002;448:169–174. doi: 10.1016/s0014-2999(02)01984-2. [DOI] [PubMed] [Google Scholar]
- Isackson PJ, Huntsman MM, Murray KD, Gall CM. BDNF mRNA expression is increased in adult rat forebrain after limbic seizures: temporal patterns of induction distinct from NGF. Neuron. 1991;6:937–948. doi: 10.1016/0896-6273(91)90234-q. [DOI] [PubMed] [Google Scholar]
- Jenab S, Kest B, Franklin SO, Inturrisi CE. Quantitative distribution of the delta opioid receptor mRNA in the mouse and rat CNS. Life Sci. 1995;56:2343–2355. doi: 10.1016/0024-3205(95)00228-x. [DOI] [PubMed] [Google Scholar]
- Jutkiewicz EM, Eller EB, Folk JE, Rice KC, Traynor JR, Woods JH. Delta-opioid agonists: differential efficacy and potency of SNC80 and its 3-OH (SNC86) and 3-desoxy (SNC162) derivatives in Sprague-Dawley rats. J. Pharmacol. Exp. Ther. 2004;309:173–181. doi: 10.1124/jpet.103.061242. [DOI] [PubMed] [Google Scholar]
- Kastin AJ, Scollan EL, Ehrensing RH, Schally AV, Coy DH. Enkephalin and other peptides reduce passiveness. Pharmacol. Biochem. Behav. 1978;9:515–519. doi: 10.1016/0091-3057(78)90051-5. [DOI] [PubMed] [Google Scholar]
- Kline NS, Li CH, Lehmann HE, Lajtha A, Laski E, Cooper T. β-Endorphin-induced changes in schizophrenic and depressed patients. Arch. Gen. Psychiatry. 1977;34:1111–1113. doi: 10.1001/archpsyc.1977.01770210125012. [DOI] [PubMed] [Google Scholar]
- Ko MCH, Lee H, Harrison C, Clark MJ, Song HF, Naughton NN, Woods JH, Traynor JR. Studies of μ-, κ-, and δ-opioid receptor density and G protein activation in the cortex and thalamus of monkeys. J. Pharmacol. Exp. Ther. 2003;306:179–186. doi: 10.1124/jpet.103.050625. [DOI] [PubMed] [Google Scholar]
- Konig M, Zimmer AM, Steiner H, Holmes PV, Crawley JN, Brown-stein MJ, Zimmer A. Pain responses, anxiety and aggression in mice deficient in pre-proenkephalin. Nature. 1996;383:535–538. doi: 10.1038/383535a0. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Diaz J, Sokoloff P. A single cocaine exposure increases BDNF and D3 receptor expression: implications for drug-conditioning. Neuroreport. 2005;16:175–178. doi: 10.1097/00001756-200502080-00022. [DOI] [PubMed] [Google Scholar]
- Maher CE, Selley DE, Childers SR. Relationship of μ opioid receptor binding to activation of G-proteins in specific rat brain regions. Biochem. Pharmacol. 2000;59:1395–1401. doi: 10.1016/s0006-2952(00)00272-0. [DOI] [PubMed] [Google Scholar]
- Malcangio M, Lessmann V. A common thread for pain and memory synapses? Brain-derived neurotrophic factor and trkB receptors. Trends Pharmacol. Sci. 2003;24:116–121. doi: 10.1016/S0165-6147(03)00025-7. [DOI] [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Anatomy of CNS opioid receptors. Trends Neurosci. 1988;11:308–314. doi: 10.1016/0166-2236(88)90093-8. [DOI] [PubMed] [Google Scholar]
- Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J. Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2nd Academic Press; San Diego: 1986. [Google Scholar]
- Porsolt RD, Pichon M, Jalfre M. Depression: a new animal model to antidepressant treatment. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Ragnauth A, Schuller A, Morgan M, Chan J, Ogawa S, Pintar J, Bodnar RJ, Pfaff DW. Female preproenkephalin-knockout mice display altered emotional response. Proc. Natl. Acad. Sci. USA. 2001;98:1958–1963. doi: 10.1073/pnas.041598498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roques BP. Novel approaches to targeting neuropeptides system. Trends Pharmacol. Sci. 2000;21:475–483. doi: 10.1016/s0165-6147(00)01571-6. [DOI] [PubMed] [Google Scholar]
- Russo-Neustadt AA, Alejandre H, Garcia C, Ivy AS, Chen MJ. Hippocampal brain-derived neurotrophic factor expression following treatment with reboxetine, citalopram, and physical exercise. Neuropsychopharmacology. 2004;29:2189–2199. doi: 10.1038/sj.npp.1300514. [DOI] [PubMed] [Google Scholar]
- Scarone S, Gambini O, Calabrese G, Sacerdote P, Bruni M, Carucci M, Panerai AE. Asymmetrical distribution of beta-endorphin in cerebral hemispheres of suicides: preliminary data. Psychiatry Res. 1990;32:159–166. doi: 10.1016/0165-1781(90)90082-g. [DOI] [PubMed] [Google Scholar]
- Shimizu E, Hashimoto K, Okamura N, Koike K, Komatsu N, Kumakiri C, Nakazato M, Watanabe H, Shinoda N, Okada S, Iyo M. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol. Psychiatry. 2003;54:70–75. doi: 10.1016/s0006-3223(03)00181-1. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral model of depression. J. Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon EJ. Opioid receptors and endogenous opioid peptides. Med. Res. Rev. 1991;11:357–374. doi: 10.1002/med.2610110402. [DOI] [PubMed] [Google Scholar]
- Siuciak JA, Lewis DR, Wiegand SJ, Lindsay RM. Antidepressant-like effect of brain-derived neurotrophic factor (BDNF) Pharmacol. Biochem. Behav. 1997;56:131–137. doi: 10.1016/S0091-3057(96)00169-4. [DOI] [PubMed] [Google Scholar]
- Takemori AE, Ho BY, Naeseth JS, Portoghese PS. Nor-binaltorphimine, a highly selective kappa-opioid antagonist in analgesic and receptor binding assays. J. Pharmacol. Exp. Ther. 1988;246:255–258. [PubMed] [Google Scholar]
- Tejedor-Real P, Mico JA, Maldonado R, Roques BP, Gibert-Rahola J. Implication of endogenous opioid system in the learned helplessness model of depression. Pharmacol. Biochem. Behav. 1995;52:145–152. doi: 10.1016/0091-3057(95)00067-7. [DOI] [PubMed] [Google Scholar]
- Torregrossa MM, Isgor C, Folk JE, Rice KC, Watson SJ, Woods JH. The δ-opioid receptor agonist (+)BW373U86 regulates BDNF mRNA expression in rats. Neuropsychopharmacology. 2004;29:649–659. doi: 10.1038/sj.npp.1300345. [DOI] [PubMed] [Google Scholar]
- Tseng LF, Wei ET, Loh HH, Li CH. β-Endorphin: central sites of analgesia, catalepsy and body temperature changes in rats. J. Pharmacol. Exp. Ther. 1980;214:328–332. [PubMed] [Google Scholar]
- Walker EA, Makhay MM, House ID, Young AM. In vivo apparent pA2 analysis for naltrexone antagonism of discriminative stimulus and analgesic effects of opiate agonists in rats. J. Pharmacol. Exp. Ther. 1994;271:959–968. [PubMed] [Google Scholar]
- Zadina JE, Hackler L, Ge LJ, Kastin AJ. A potent and selective endogenous agonist for the μ-opiate receptor. Nature. 1997;386:499–502. doi: 10.1038/386499a0. [DOI] [PubMed] [Google Scholar]
- Zastawny RL, George SR, Nguyen T, Cheng R, Tsatsos J, Briones-Urbina R, O'Dowd BF. Cloning, characterization, and distribution of a mu-opioid receptor in rat brain. J. Neurochem. 1994;62:2099–2105. doi: 10.1046/j.1471-4159.1994.62062099.x. [DOI] [PubMed] [Google Scholar]