Abstract
Sterol synthesis is required for Sonic hedgehog (Shh) signal transduction. Errors in Shh signal transduction play important roles in the formation of human tumors, including medulloblastoma (MB). It is not clear which products of sterol synthesis are necessary for Shh signal transduction or how they act. Here we show that cholesterol or specific oxysterols are the critical products of sterol synthesis required for Shh pathway signal transduction in MB cells. In MB cells, sterol synthesis inhibitors reduce Shh target gene transcription and block Shh pathway-dependent proliferation. These effects of sterol synthesis inhibitors can be reversed by exogenous cholesterol or specific oxysterols. We also show that certain oxysterols can maximally activate Shh target gene transcription through the Smoothened (Smo) protein as effectively as the known Smo full agonist, SAG. Thus, sterols are required and sufficient for Shh pathway activation. These results suggest that oxysterols may be critical regulators of Smo, and thereby Shh signal transduction. Inhibition of Shh signaling by sterol synthesis inhibitors may offer a novel approach to the treatment of MB and other Shh pathway-dependent human tumors.
Keywords: Smoothened, Patched, sterol synthesis pathway, cholesterol, cancer
Mutations causing constitutive Sonic hedgehog (Shh) target gene transcription are associated with the formation of human tumors such as medulloblastoma (MB), which arises from cerebellar granule cell precursors (GCPs) (1). The most common of these mutations is loss of function of the PATCHED1 (PTCH) tumor suppressor gene, which occurs in sporadic and hereditary MB (2, 3). The protein encoded by PTCH, Patched1 (Ptc1), is the 12-transmembrane domain receptor for Shh (4). In the absence of Shh signal, Ptc1 inhibits the downstream transducers of the Shh pathway by blocking function of the seven-transmembrane protein, Smoothened (Smo). When Shh signal is present, it binds to and inhibits its receptor, Ptc1, allowing activation of Smo (5). Through an unknown mechanism, Smo initiates a series of downstream events culminating in the activation of the Gli family of transcription factors (Gli1, Gli2, and Gli3) that control expression of Shh target genes. Shh induces transcription of ptc1 and gli1, thus creating both negative and positive feedback effects (6, 7). The mechanism by which Ptc1 inhibits Smo is poorly understood and does not appear to involve binding of Ptc1 to Smo. Smo can be activated or inhibited by various small molecules (8), and it has been hypothesized that Ptc1, which is related to the resistance-nodulation-cell division family of bacterial pumps (9) and to the Niemann–Pick C1-like1 cholesterol transporter (10), modulates Smo activity by regulating the distribution of an endogenous small molecule Smo ligand (8, 9). However, the existence or identity of such an endogenous ligand remains unknown.
Previously, our lab generated a mouse model in which one copy of ptc1 was replaced with lacZ, such that lacZ is expressed from the endogenous ptc1 promoter of the knockout allele (11). Approximately 15% of these ptc1+/− mice develop MB. These tumors exhibit constitutive Shh target gene transcription because of inadequate Ptc1 function. Small molecule Shh pathway inhibitors markedly reduce tumor formation in ptc1+/− mouse models (12, 13), demonstrating the dependence of MB on Shh target gene expression. Identification of critical Shh targets involved in MB or of novel mechanisms of Shh pathway inhibition, therefore, would have important clinical applications. We conducted a microarray screen to identify transcripts differentially expressed in MB compared with normal GCPs. We found that a number of transcripts involved in sterol synthesis and uptake were enriched in MB, leading us to explore a possible role for sterols in MB.
Sterol synthesis is required for Shh signal transduction. The role of sterol synthesis in Shh signaling was first suggested by the finding that the Shh signal itself is covalently joined to cholesterol (14). Further evidence came from genetic defects in the sterol synthetic pathway (SSP) (Fig. 1A) that lead to holoprosencephaly (15), a phenotype associated with Shh deficiency (16). The SSP regulates synthesis of a range of molecules, including sterols, steroids, bile acids, vitamin D analogs, and isoprenoids. The critical SSP product required for Shh signaling has been hypothesized to be sterols because genetic errors associated with holoprosencephaly affect late steps of sterol synthesis. Two disorders of sterol synthesis associated with holoprosencephaly-like malformations, Smith–Lemli–Opitz syndrome and desmosterolosis, result from defects in the 7-dehydrocholesterol reductase and 3β-hydroxysterol-Δ24-reductase enzymes, respectively, which catalyze the final steps of cholesterol synthesis (15, 17). However, it is unclear whether impairment of Shh signaling results from deficient synthesis of a critical SSP product or from accumulation of an inhibitory upstream intermediate. Furthermore, these defects also block formation of SSP products synthesized from cholesterol such as oxysterols and steroids. Therefore, the exact mechanism by which disrupted sterol synthesis interferes with Shh signal transduction is unknown.
Fig. 1.
Sterol synthesis is required for MB cell proliferation. (A) The SSP. Sites of action of SSPIs are shown by colored bars. Steps catalyzed by enzymes deficient in Smith–Lemli–Opitz syndrome (∗) and desmosterolosis (∗∗) are shown. Multiple enzymatic steps not shown are indicated by double arrows. (B) Sterol synthesis inhibitors block MB cell proliferation. PZp53MED cells were treated with the indicated inhibitors for 72 h, and cell titer was determined. Relative cell titer values represent cell titer of treated cells relative to untreated control. (C) ZGA inhibits proliferation of MB cells selectively compared with normal cell controls (GCP and Hs68). Ptc1−/− fibroblasts, which also show constitutive Shh target gene transcription, were modestly inhibited by ZGA. (D) WSC can restore proliferation in the presence of 10 μM ZGA and 3 μM TPL. (E) Unmodified cholesterol (Chol) and 25-OHC delivered in ethanol vehicle (EtOH) can also restore proliferation in the presence of 10 μM ZGA, whereas 7-OHC cannot.
Despite covalent modification of Shh ligand by cholesterol, the block in signaling when sterol synthesis is impaired comes from a decreased response to Shh by the receiving cells (18). Shh autoprocessing proceeds normally in cells with genetic deficiencies in sterol synthesis (19). Furthermore, Shh does not absolutely require the cholesterol adduct for signaling activity, although this modification can affect the range of action of the signal (20). Cells with defects in sterol biosynthesis exhibit a diminished response to exogenous Shh ligand. Cells depleted of lipids and sterols by treatment with the drug cyclodextrin, which binds and sequesters hydrophobic molecules, and with a statin drug have a reduced response to exogenous Shh. Cyclodextrin and statin treatment can inhibit Shh target gene transcription in ptc1−/− fibroblasts but not in cells expressing a constitutively active form of Smo (20). Therefore, the putative requirement for sterols in Shh signal transduction occurs downstream of Ptc, likely at the level of Smo. However, because cyclodextrin nonspecifically binds and sequesters a multitude of lipids and hydrophobic molecules, and because statins inhibit one of the earliest steps in the SSP, the identity and function of the SSP products required for Shh signaling remains unclear.
Here we report that cholesterol or specific derivatives of cholesterol, notably oxysterols, are required for Shh signal transduction in MB cells and that increased concentrations of specific oxysterols activate the Shh pathway. These results suggest a previously unrecognized role for oxysterols as mediators of Shh signaling and implicate sterol synthesis inhibitors as potential therapeutic agents for the treatment of MB.
Results
Sterol Synthesis Is Required for Proliferation of MB Cells.
Transcripts belonging to the sterol regulatory element-binding protein gene family were enriched in mouse MB RNA compared with GCP RNA by microarray analysis (data not shown). The sterol regulatory element-binding protein gene family encodes transcription factors controlling expression of >30 genes involved in lipid and sterol synthesis and processing. Expression of other sterol-related transcripts was also increased in MB, including hydroxymethylglutaryl-CoA reductase (which catalyzes an important initial step in sterol synthesis and is the target of statins), low-density lipoprotein-receptor (the major protein involved in sterol uptake) (21), and apolipoprotein E (a major sterol carrier of the central nervous system) (22). Enrichment of sterol-related transcripts in MB suggests that these genes and their effects on sterol or lipid metabolism might play a role in MB tumorigenesis.
To examine the possible importance of sterol synthesis in MB growth, we explored whether pharmacologic inhibition of sterol synthesis could influence proliferation of a MB cell line, PZp53MED, derived from MB arising in ptc1+/−p53−/− mice (12). By using inhibitors that block the SSP (Fig. 1A) at various points, we could ascertain whether SSP inhibitors (SSPIs) affect MB cell proliferation and gain information as to what the vital product of the pathway might be. Inhibitors that block the SSP upstream of the putative key product should have an inhibitory effect on MB cells, whereas drugs that block the SSP downstream of the key product should not.
We used five different SSPIs: (i) simvastatin is a hydroxymethylglutaryl-CoA reductase inhibitor that blocks an early step in the SSP, preventing synthesis of all sterols, steroids, and isoprenoids (23); (ii) zaragozic acid A (ZGA) is a squalene synthesis inhibitor that blocks conversion of isoprenoids into sterols, leaving only isoprenoid synthesis intact (24); (iii) ketoconazole allows synthesis of an early sterol, lanosterol, but prevents synthesis of downstream sterols (25); (iv) triparanol (TPL) blocks the final step of cholesterol synthesis, allowing production of sterols other than cholesterol and its derivatives (26); and (v) aminoglutethimide blocks conversion of cholesterol to pregnenelone and other steroids, leaving sterol synthesis unaffected (27).
All SSPIs except aminoglutethimide reduced PZp53MED cell proliferation relative to untreated control (Fig. 1B), indicating that cholesterol or a cholesterol derivative is required for MB cell proliferation, whereas steroids are not.
To determine whether the antiproliferative effect of SSPIs is specific to MB cells, we tested the effects of ZGA on other cell types. ZGA was chosen because it is one of the most potent inhibitors of MB cell proliferation. Also, ZGA is an inhibitor of the most proximal step in the sterol-specific portion of the SSP, thus, it is expected to have more nonspecific effects than distal inhibitors. Three cell types were used as controls for the selectivity of ZGA. GCPs are the normal cell type most closely related to MB. We also used a normal fibroblast line, Hs68, and a fibroblast line derived from ptc1−/− embryos, which exhibits constitutive Shh target gene transcription, like PZp53MED cells. ZGA reduced PZp53MED cell number compared with untreated control by ≈90%. There was no significant difference in the number of GCPs and Hs68 cells between treated and untreated groups (Fig. 1C). ZGA did cause a dose-dependent reduction in ptc1−/− cell number relative to untreated control, resulting in a 50% reduction at the highest dose. This moderate inhibition of ptc1−/− cell proliferation is less dramatic than that seen in PZp53MED cells. Overall, these data suggest a stronger dependence of MB cells on sterols than is seen with normal cell types.
We tested whether the inhibitory effects of two of the most potent SSPIs, ZGA and TPL, could be reversed by supplementing MB cells with exogenous cholesterol. These drugs inhibit the sterol-specific portion of the pathway and do not interfere with isoprenoid synthesis. Blocking isoprenoid synthesis is known to inhibit cell growth by preventing isoprenylation of important growth-regulatory proteins (28). If sterol supplementation can reverse the effects of ZGA and TPL, it would suggest that these drugs inhibit MB cell growth by blocking the synthesis of a critical downstream sterol product rather than by causing accumulation of a growth-inhibiting upstream intermediate. Supplementation with a water-soluble cholesterol (WSC) completely reversed the inhibitory effects of ZGA and TPL (Fig. 1D), indicating that cholesterol or a downstream derivative of cholesterol is the key SSP product required for MB cell proliferation.
An oxysterol derivative of cholesterol, 25-hydroxycholesterol (25-OHC), reversed the effect of ZGA more potently than unmodified cholesterol. 7β-hydroxycholesterol (7-OHC), a bile salt precursor, was unable to reverse inhibition by ZGA (Fig. 1E). The abilities of 25-OHC and other SSP products to reverse the antiproliferative effect of ZGA on PZp53MED cells (Fig. 3B) show that various sterols, but not all, are sufficient to restore MB cell proliferation in the presence of ZGA. Because some sterols do not restore proliferation, the rescue may be due to interaction of specific sterols with specific proteins rather than being due to nonspecific effects of general sterol concentration.
Fig. 3.
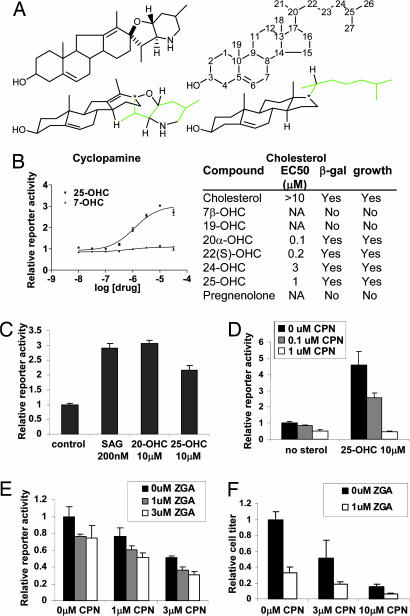
Sterols directly stimulate Smo activation. (A) Structural similarity between CPN and cholesterol. Standard and three-dimensional structures are shown. Notice the similarity of the four-ring core structure. The eight-carbon side chain is shown in green. Carbons of cholesterol are numbered to indicate the site of hydroxylation in the table in B. (B Left) 25-OHC, but not 7-OHC, can activate ptc1-lacZ expression in PZp53MED cells by 24 h. (B Right) Activities of other compounds tested and their abilities to rescue ptc1-lacZ expression (β-gal) and MB cell proliferation (growth) in the presence of ZGA are shown (NA, no activity). (C) 25-OHC and 20-OHC can activate ptc1-lacZ expression with the same maximal efficacy as the known Smo-binding agonist, SAG. (D) CPN can block activation of ptc1-lacZ reporter activity by 25-OHC. (E and F) Cooperativity of ZGA and CPN for inhibition of ptc1-lacZ expression after 48 h (E) and proliferation of PZp53MED cells after 72 h.
Sterols Are Required for Shh Signal Transduction in MB Cells.
To investigate why MB cell proliferation is especially dependent on sterols, we explored the effects of SSPIs on the Shh pathway. PZp53MED cell proliferation requires Shh target gene transcription; Shh pathway inhibitors block growth of this cell line (12). We found that MB cells treated with ZGA had reduced expression of the Shh target gene, gli1 (Fig. 2A). We tested the ability of SSPIs to reduce expression of the lacZ reporter present in MB cells and driven by the endogenous ptc1 promoter. Because ptc1 is a Shh target gene, β-gal production from the ptc1-lacZ allele is induced by Shh or allowed by loss of ptc1 function. ZGA inhibited PZp53MED cell lacZ expression in a dose-dependent manner, consistent with its effect on gli1 transcript levels (Fig. 2B). Maximum inhibition of lacZ expression by ZGA was similar to the maximum effect of known Shh pathway antagonist, cyclopamine (CPN) (29) (Fig. 2C). IC50s of both drugs for lacZ expression correlated with their IC50s for MB cell proliferation.
Fig. 2.
Sterol synthesis is required for Shh signaling in MB cells. All gene expression and reporter assays were conducted on confluent cells to ensure that any effect of SSPIs on proliferation did not affect results. Parallel plates were assessed for viable cell number by CellTiter 96 assay to ensure that no cytotoxicity occurred at the drug dosages used. (A) PZp53MED cells treated with 32 μM ZGA for 24 or 48 h show reduced expression of Shh target transcript gli1 relative to untreated controls. Gli1 transcript levels were determined by real-time PCR and normalized to β-actin control. (B) ZGA reduced activity of endogenous Shh-responsive ptc1-lacZ reporter gene in PZp53MED cells relative to untreated controls at 24 and 48 h. (C Upper) ZGA and CPN inhibit MB cell ptc1-lacZ reporter activity with similar maximal efficacy by 48 h. (C Lower) Correlation of IC50s for ptc1-lacZ inhibition (β-gal) and MB cell proliferation (growth) for various SSPIs are shown (NI, no inhibition). (D) WSC can reverse inhibition by 10 μM ZGA and 3 μM TPL of ptc1-lacZ reporter activity in PZp53MED cells (MB) and ptc1−/− fibroblasts (FB) by 48 h. Con, control. (E) 25-OHC, but not 7-OHC, can restore ptc1-lacZ reporter activity in MB cells treated with 10 μM ZGA compared with ethanol vehicle control (EtOH). Values are shown as ratio of reporter activity between ZGA-treated and untreated cells under each condition for 48 h. (F) Constitutive activation of Shh signal transduction by Gli1-transfection maintains proliferation of MB cells in the presence of 10 μM ZGA. Conversely, ZGA completely blocked proliferation in control CFP-transfected cells. Proliferation was assessed by measuring percentage of cells incorporating BrdU after 36 h.
We tested the ability of WSC to prevent reduction in ptc1-lacZ expression by ZGA and TPL in PZp53MED cells and in ptc1−/− fibroblasts. Ptc1−/− fibroblasts have constitutive Shh pathway-dependent transcription of lacZ because they are homozygous for the same ptc1-lacZ knockout allele found in PZp53MED cells. In the absence of exogenous sterols, ZGA and TPL inhibited lacZ expression in both cell types, indicating that sterols are required for Shh signal transduction in MB cells and fibroblasts (Fig. 2D). Inhibition of Shh target gene transcription by SSPIs may explain why ZGA had a modest antiproliferative effect on ptc1−/− cells (Fig. 1C); the proliferation of these cells can be inhibited by Shh pathway antagonists (29). Supplementation with WSC restored lacZ expression to control levels, showing that inhibition of Shh signal transduction by SSPIs is a sterol-specific effect.
25-OHC, which potently restored MB cell proliferation in the presence of ZGA, also completely restored lacZ expression in ZGA-treated PZp53MED cells. Conversely, 7-OHC, which did not reverse the antiproliferative effect of ZGA, did not restore lacZ expression (Fig. 2E). Only sterols that restore MB cell growth also restore Shh pathway transcriptional activity.
To determine whether Shh pathway inhibition is the primary mechanism by which SSPIs affect MB growth, we tested whether constitutive activation of Shh target gene transcription by overproduction of Gli1 could render MB cells insensitive to the antiproliferative effects of SSPIs. Sterol depletion inhibits the Shh pathway at the level of Smo (19), and thus, the Gli1 transcription factor, a pathway activator downstream of Smo, can activate Shh pathway target genes even in the absence of sterols. Although ZGA significantly reduced proliferation of control MB cells, it did not reduce proliferation in Gli1-transfected cells (Fig. 2F). Thus, Shh pathway inhibition is the primary mechanism by which SSPIs inhibit MB proliferation. These data underscore the idea that the antiproliferative effect of SSPIs is specific to Shh pathway-dependent cells rather than being a general effect on rapidly dividing cells. Indeed, Gli1-expressing cells exhibit a 3-fold increase in proliferation relative to control cells yet are insensitive to ZGA by virtue of constitutive Shh pathway activity.
Sterols Directly Activate the Shh Signaling Pathway Through Smo.
Cooper et al. (19) showed that sterols are necessary for Smo activity. Sterols might indirectly allow Smo to be activated in response to a non-sterol signal. Alternatively, sterols could be the actual signal that stimulates Smo activity. Precedent for a supporting role of sterols in signal transduction comes from observations that sterol depletion can disrupt interactions between signaling proteins which normally occur at lipid and sterol-rich membrane microdomains (30). Likewise, there is precedent for sterols as direct signaling messengers. Sterols directly bind to SCAP to prevent activation of sterol regulatory element-binding proteins (31), and oxysterols bind to and activate the nuclear liver X receptor (LXR) transcription factor (32).
To distinguish between these possibilities, we tested whether sterols are sufficient to stimulate Smo activation above baseline levels. 25-OHC increased PZp53MED cell ptc1-lacZ expression in a dose-dependent manner, causing a >3-fold increase in maximal reporter activity. 7-OHC did not affect ptc1-lacZ expression, consistent with its inability to restore Shh pathway activity in the presence of ZGA (Fig. 3B). Effects of other sterols are also shown. Sterols activate lacZ expression as strongly as the known Smo agonist SAG (8), which activates Smo more than any other known molecule (Fig. 3C). Because PZp53MED cells do not express Ptc (12), 25-OHC must activate the pathway downstream of Ptc. The ability of 25-OHC to activate lacZ expression can be blocked by CPN, which binds to and inhibits Smo (Fig. 3D), indicating that 25-OHC activates the pathway upstream of or at the level of Smo. 25-OHC so strongly stimulates Smo activity that it is likely to be acting, like SAG, directly upon Smo.
Interestingly, modest concentrations of CPN (0.1 μM) that normally inhibit Shh signal transduction can be overcome by high sterol concentrations to activate target genes (Fig. 3D). This finding suggests a possible competition between sterols and CPN for regulation of Smo activity. Because sterol concentration can modulate Smo activity, decreased sterol concentrations caused by SSPIs might enhance the activity of CPN by reducing competition from endogenous sterols. Indeed, CPN and ZGA had an additive negative effect on Shh target gene expression and MB cell proliferation, suggesting a potential role for these agents in combination therapy for Shh pathway-driven tumors (Fig. 3 E and F).
Discussion
Cholesterol or a Distal Derivative Is Required for Shh Signal Transduction.
Impairment of sterol synthesis has previously been shown to block Shh signal transduction. Here we show that certain sterols are potent triggers of Shh target gene expression and proliferation in MB cells. Our pharmacologic study of SSP inhibition shows that cholesterol or a cholesterol derivative, such as an oxysterol, is required for Shh signaling in MB cells. Conversely, steroid synthesis is not required for Shh signaling. Aminoglutethimide, which prevents conversion of cholesterol to steroid precursors but does not affect sterol synthesis, did not inhibit Shh target gene transcription. Supplementation with exogenous pregnenolone, the earliest steroid precursor, also does not rescue Shh target gene transcription in the presence of SSPIs, further demonstrating that steroids are not the critical SSP product.
Inhibition of Shh signal transduction by SSP blockade is likely due to reduced synthesis of a key product rather than to accumulation of an inhibitory intermediate for three reasons. First, SSPIs that block Shh signaling inhibit the SSP at multiple distinct points, and each SSPI causes accumulation of distinct intermediates (24–26). It is unlikely that these agents cause accumulation of a common intermediate that inhibits the Shh pathway. Second, an inhibitory upstream intermediate is inconsistent with blockage of Shh signaling by cyclodextrin, which binds and sequesters hydrophobic molecules. Any inhibitory upstream lipid or sterol intermediate should also be depleted by cyclodextrin. Third, Shh signaling can be restored by adding exogenous sterols in the presence of ZGA or TPL, so it is the lack of cholesterol or a cholesterol derivative that matters, not the presence of an inhibitory intermediate.
TPL, the most distal SSPI that blocks Shh signal transduction, prevents synthesis of lathosterol and 7-dehydrocholesterol (provitamin D3) as well as cholesterol. Although our data do not rule out lathosterol, 7-dehydrocholesterol, or their derivatives as regulators of Shh signaling, several lines of evidence make this unlikely. First, Smith–Lemli–Opitz syndrome, which results from a defect in 7-dehydrocholesterol reductase, produces holoprosencephaly and impairment of Shh signaling. This defect leads to accumulation rather than deficiency of lathosterol and 7-dehydrocholesterol (15). Second, AY-9944, a specific inhibitor of 7-dehydrocholesterol reductase, also causes holoprosencephaly in mouse models and can block induction of Shh-responsive genes in neural tube explants (30). Third, we rescued Shh signaling during SSP blockade with cholesterol and oxysterols. These sterols are “beyond” lathosterol and 7-dehydrocholesterol in the SSP.
Therefore, cholesterol and/or its derivatives are required for Shh signal transduction. We found that certain oxysterols are sufficient to restore Shh signaling when sterol synthesis is blocked. Specific oxysterols restore Shh signaling more potently than cholesterol, suggesting that an oxysterol, rather than cholesterol, is the critical SSP product required for Shh signaling. In this scenario, cholesterol would restore Shh signal transduction in the presence of SSPIs through biosynthetic conversion to an oxysterol product. Given the potent ability of oxysterols to stimulate Smo activity, one of them may be an endogenous signal that turns on Smo in response to Shh. Ptc, which is related to the intestinal Niemann–Pick C1-like1 cholesterol transporter and the resistance-nodulation-cell division family of pump proteins (9, 10), may control Smo activity by restricting its contact with activating sterols (Fig. 4A).
Fig. 4.
Model for sterol regulation of Smo activity. (A) Ptc, which is structurally related to transporter and pump proteins, may inhibit Smo by pumping activating sterols away from Smo. In the presence of Shh or in the genetic absence of Ptc, sterols would be free to directly promote Smo activity. (B) Sterols may activate Smo by direct binding. In the absence of sterols, Smo remains in an inactive conformation. When sterols are present, they may bind to Smo, causing an activating conformational change. CPN may compete with sterols for binding to Smo by virtue of the structural similarity of CPN and sterols. Binding of CPN to Smo would stabilize the inactive conformation.
Sterols Can Directly Stimulate Smo Activity.
There are several ways in which sterols might activate Smo. The first is through an impact on membrane properties, causing an activating conformational change in Smo. The constitutively active oncogenic form of Smo (SmoM2 or SmoA1), which results from a single point mutation in the seventh transmembrane domain and retains activity in the presence of Ptc, might be a protein trapped in an active conformation, as suggested in ref. 29. SmoM2 is able to signal under conditions of sterol depletion (19), suggesting that a constantly active conformation of Smo eliminates the need for a conformational change induced by sterols. The specific sterol content of membranes influences ligand-binding and activity of certain seven-transmembrane proteins, including the oxytocin and cholecystokinin receptors (33), and Smo may be regulated by sterols in a similar fashion.
Alternatively, sterols may bind directly to Smo, causing an activating conformational change (Fig. 4B). The possibility that certain sterols might be Smo ligands is suggested by the structural similarity between one Smo ligand, CPN, and cholesterol (Fig. 3A). In this scenario, CPN and sterols may bind to Smo by virtue of their well conserved four-ring core structure but induce distinct inhibitory or activating conformational changes because of differing side chain structures. The structures of Shh pathway-activating versus nonactivating oxysterols suggest a direct binding event because the site of hydroxylation on the cholesterol backbone correlates with oxysterol activity. 7- and 19-OHC, which are hydroxylated on the four-ring core of cholesterol that is conserved in CPN, are not active. Perhaps hydroxyl groups in these positions prevent a specific protein-binding interaction. In contrast, oxysterols hydroxylated on the flexible cholesterol side chain, which is not conserved in CPN, are able to activate Smo. The most potent sterols activate the Shh pathway to the same maximal extent as known Smo-binding agonist SAG (Fig. 3C), which has greater maximal activity than even Shh in other cell types (8), suggesting that sterols may also be direct Smo agonists.
Third, sterols could regulate the subcellular localization of Smo or Smo-interacting proteins, affecting their activity. Sterols bind to the sterol-sensing, domain-containing protein SCAP to regulate cellular trafficking and activation of sterol regulatory element-binding proteins (34). This mechanism is intriguing in light of recent findings that localization of Smo to the primary cilium is required for activity (35). Finally, sterols could cause activation of Smo through a yet unidentified component of the Shh pathway.
Sterol Synthesis as a Therapeutic Target in MB.
An adequate supply of sterols and lipids is required for cell proliferation in general, but it appears that the antiproliferative mechanism of SSPIs on MB cells is different. Similar concentrations of drug failed to block the proliferation of other cell types. Reduction in Shh pathway activity is the primary mechanism by which SSPIs inhibit MB cell proliferation.
The role of SSPIs as therapeutic agents for human cancer has primarily focused on statins (28). The activity of these agents is mainly due to inhibition of isoprenoid synthesis, blocking isoprenylation of key growth-regulatory proteins like Ras and Rho. Statin levels needed for anti-tumor activity correlate with high levels required for blocking isoprenylation rather than with lower levels needed for inhibition of sterol synthesis. In vitro, the antiproliferative effect of statins can be overcome by addition of exogenous isoprenoid intermediates specific to isoprenylation pathways and not involved in sterol synthesis (36).
Our data suggest possible applications of SSPIs in MB and other Shh pathway-derived tumors. Even inhibitors of early steps in sterol synthesis, such as statins, could be useful in MB both through their established effects on protein isoprenylation and concurrent inhibition of Shh signaling by blocking sterol synthesis. SSPIs and CPN combine to more effectively block Shh target gene transcription and MB cell proliferation. Therefore, concurrent blockade of sterol synthesis may enhance effects of Smo antagonists, which are currently under clinical development for treatment of Shh pathway-dependent tumors (37).
Materials and Methods
Compounds.
Compounds were purchased from Sigma-Aldrich except as noted. 19-OHC was purchased from Steraloids (Newport, RI); CPN was purchased from Toronto Research Chemicals (Downsview, ON, Canada); simvastatin was purchased from Calbiochem; SAG was kindly provided by James Chen (Stanford); and TPL was provided by Yvonne Lange (Rush-Presbyterian-St. Luke’s Medical Center, Chicago).
Cell Culture and Proliferation Assays.
PZp53MED cells were kindly provided by Philip Beachy (Johns Hopkins University, Baltimore) and were grown in DMEM with 10% FBS. Cell titer assays were conducted by using the CellTiter 96 nonradioactive proliferation assay (Promega). PZp53MED cells were seeded at 1,500 cells per well in 96-well plates in DMEM with 0.5% FBS. Compounds were diluted in DMEM with 0.5% FBS and added to cells after 3 h. Cells were assayed 72 h later according to the manufacturer’s protocol.
BrdU-incorporation assays were conducted by seeding PZp53MED cells at 6,000 cells per well of a 24-well plate on glass coverslips. Twenty-four hours later, each well was cotransfected with 1 μg of pECFP-N2 (Clontech) or a CMV promoter-driven Gli1-expression vector along with 0.5 μg of pEYFP-N3 (Clontech). After another 24 h, cells were switched to DMEM with 0.5% FBS with or without 10 μM ZGA. Cells were cultured for an additional 36 h, and BrdU (10 μM) was added for the final 6 h. Cells were washed, fixed in 4% paraformaldehyde, permeabilized and blocked with 0.2% Triton X-100 and 10% normal goat serum, treated with DNase for 1 h, and incubated with biotinylated anti-BrdU antibody overnight at 4°C. Anti-BrdU was detected with Cy3-conjugated streptavidin. The percentage of cells expressing YFP that were also BrdU-positive was calculated for each sample.
Reporter Assays.
PZp53MED cells or ptc1−/− fibroblasts were seeded at 15,000 cells per well in 96-well plates. The next day, at confluence, cells were washed twice, and drugs were added at indicated concentrations in DMEM with 0.5% FBS. Cells were incubated for 24 or 48 h as noted, washed with PBS, and assayed for β-gal activity by using Galacto-Light assay (Applied Biosystems) according to manufacturer’s protocol. Reporter assays were normalized for lysate protein concentration or viable cell number by using CellTiter 96.
Real-Time PCR.
PZp53MED cells were grown to confluence. Cells were washed and incubated in DMEM with 0.5% FBS with or without 32 μM ZGA for 24 or 48 h. RNA was isolated by using TRIzol (GIBCO). cDNA was synthesized by using oligo(dT) primers. Gene expression assays for mouse gli1 and β-actin were purchased from Applied Biosystems and used according to manufacturer’s protocol. Relative gli1 levels were normalized to a β-actin control.
Acknowledgments
We thank Drs. James Chen, Rajat Rohatgi, and Joel Hyman for discussion and comments on the manuscript. R.B.C. thanks the Stanford Medical Scientist Training Program for the training grant. This work was supported by National Institutes of Health Grant R01 CA088060 (to M.P.S.). M.P.S. is an Investigator of the Howard Hughes Medical Institute.
Abbreviations
- Shh
Sonic hedgehog
- GCP
granule cell precursor
- ZGA
zaragozic acid A
- TPL
triparanol
- WSC
water-soluble cholesterol
- MB
medulloblastoma
- SSP
sterol synthetic pathway
- SSPI
SSP inhibitor
- 25-, 7-, and 19-OHC
25-, 7β-, and 19-hydroxycholesterol
- CPN
cyclopamine.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Goussia A. C., Burner J. M., Kyritsis A. P., Agnantis N. J., Fuller G. N. Anticancer Res. 2000;20:65–73. [PubMed] [Google Scholar]
- 2.Raffel C., Jenkins R. B., Frederick L., Hebrink D., Alderete B., Fults D. W., James C. D. Cancer Res. 1997;57:842–845. [PubMed] [Google Scholar]
- 3.Johnson R. L., Rothman A. L., Xie J., Goodrich L. V., Bare J. W., Bonifas J. M., Quinn A. G., Myers R. M., Cox D. R., Epstein E. H., Jr, Scott M. P. Science. 1996;272:1668–1671. doi: 10.1126/science.272.5268.1668. [DOI] [PubMed] [Google Scholar]
- 4.Fuse N., Maiti T., Wang B., Porter J. A., Hall T. M., Leahy D. J., Beachy P. A. Proc. Natl. Acad. Sci. USA. 1999;96:10992–10999. doi: 10.1073/pnas.96.20.10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalderon D. Cell. 2000;103:371–374. doi: 10.1016/s0092-8674(00)00129-x. [DOI] [PubMed] [Google Scholar]
- 6.Goodrich L. V., Johnson R. L., Milenkovic L., McMahon J., Scott M. P. Genes Dev. 1996;10:301–312. doi: 10.1101/gad.10.3.301. [DOI] [PubMed] [Google Scholar]
- 7.Marigo V., Johnson R. L., Vorkamp A., Tabin C. J. Dev. Biol. 1996;180:273–283. doi: 10.1006/dbio.1996.0300. [DOI] [PubMed] [Google Scholar]
- 8.Chen J. K., Taipale J., Young K. E., Maiti T., Beachy P. A. Proc. Natl. Acad. Sci. USA. 2002;99:14071–14076. doi: 10.1073/pnas.182542899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taipale J., Cooper M. K., Maiti T., Beachy P. A. Nature. 2002;418:892–896. doi: 10.1038/nature00989. [DOI] [PubMed] [Google Scholar]
- 10.Davies J. P., Levy B., Ioannou Y. A. Genomics. 2000;65:137–145. doi: 10.1006/geno.2000.6151. [DOI] [PubMed] [Google Scholar]
- 11.Goodrich L. V., Johnson R. L., Milenkovic L., Higgins K. M., Scott M. P. Science. 1997;277:1109–1113. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- 12.Berman D. M., Karhadkar S. S., Hallahan A. R., Pritchard J. I., Eberhart C. G., Watkins D. N., Chen J. K., Cooper M. K., Taipale J., Olson J. M., Beachy P. A. Science. 2002;297:1559–1561. doi: 10.1126/science.1073733. [DOI] [PubMed] [Google Scholar]
- 13.Romer J. T., Kimura H., Magdaleno S., Sasai K., Fuller C., Baines H., Connelly M., Stewart C. F., Gould S., Rubin L. L., Curran T. Cancer Cell. 2004;6:229–240. doi: 10.1016/j.ccr.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 14.Porter J. A., Young K. E., Beachy P. A. Science. 1996;274:255–259. doi: 10.1126/science.274.5285.255. [DOI] [PubMed] [Google Scholar]
- 15.Wassif C. A., Maslen C., Kachilele-Linjewile S., Lin D., Linck L. M., Connor W. E., Steiner R. D., Porter F. D. Am. J. Hum. Genet. 1998;63:55–62. doi: 10.1086/301936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiang C., Litingtung Y., Lee E., Young K. E., Corden J. L., Westphal H., Beachy P. A. Nature. 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- 17.Waterham H. R., Koster J., Romeijin G. J., Hennekam R. C., Vreken P., Andersson H. C., FitzPatrick D. R., Kelley R. I., Wanders R. J. Am. J. Hum. Genet. 2001;69:685–694. doi: 10.1086/323473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooper M. K., Porter J. A., Young K. E., Beachy P. A. Science. 1998;280:1603–1607. doi: 10.1126/science.280.5369.1603. [DOI] [PubMed] [Google Scholar]
- 19.Cooper M. K., Wassif C. A., Krakowiak P. A., Taipale J., Gong R., Kelley R. I., Porter F. D., Beachy P. A. Nat. Genet. 2003;33:508–513. doi: 10.1038/ng1134. [DOI] [PubMed] [Google Scholar]
- 20.Lewis P. M., Dunn M. P., McMahon J. A., Logan M., Martin J. F., St. Jacques B., McMahon A. P. Cell. 2001;105:599–612. doi: 10.1016/s0092-8674(01)00369-5. [DOI] [PubMed] [Google Scholar]
- 21.Brown M. S., Goldstein J. L. Curr. Top. Cell. Regul. 1985;26:3–15. doi: 10.1016/b978-0-12-152826-3.50008-5. [DOI] [PubMed] [Google Scholar]
- 22.Goodrum J. F. J. Neurochem. 1991;56:2082–2086. doi: 10.1111/j.1471-4159.1991.tb03469.x. [DOI] [PubMed] [Google Scholar]
- 23.Berger G. M., Marais A. D., Seftel H. C., Baker S. G., Mendelsohn D., Welsh N. H., Joffe B. I. Cardiovasc. Drugs Ther. 1989;3:219–227. doi: 10.1007/BF01883868. [DOI] [PubMed] [Google Scholar]
- 24.Bergstrom J. D., Kurtz M. M., Rew D. J., Amend A. M., Karkas J. D., Bostedor R. G., Bansal V. S., Dufresne C., VanMiddlesworth F. L., Hensens O. D., et al. Proc. Natl. Acad. Sci. USA. 1993;90:80–84. doi: 10.1073/pnas.90.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engelhardt D., Mann K., Hormann R., Braun S., Karl H. J. Klin. Wochenschr. 1983;61:373–375. doi: 10.1007/BF01485030. [DOI] [PubMed] [Google Scholar]
- 26.Malhotra H. C., Nes W. R. J. Biol. Chem. 1971;246:4934–4937. [PubMed] [Google Scholar]
- 27.Cohen M. P. Proc. Soc. Exp. Biol. Med; 1968. pp. 1086–1090. [DOI] [PubMed] [Google Scholar]
- 28.Swanson K. M., Hohl R. J. Curr. Cancer Drug Targets. 2006;6:15–37. doi: 10.2174/156800906775471743. [DOI] [PubMed] [Google Scholar]
- 29.Taipale J., Chen J. K., Cooper M. K., Wang B., Mann R. K., Milenkovic L. M., Scott M. P., Beachy P. A. Nature. 2000;406:1005–1009. doi: 10.1038/35023008. [DOI] [PubMed] [Google Scholar]
- 30.Incardona J. P., Gaffield W., Kapur R. P., Roelink H. Development (Cambridge, U.K.) 1998;125:3553–3562. doi: 10.1242/dev.125.18.3553. [DOI] [PubMed] [Google Scholar]
- 31.Radhakrishnan A., Sun L. P., Kwon H. J., Brown M. S., Goldstein J. L. Mol. Cell. 2004;15:259–268. doi: 10.1016/j.molcel.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 32.Michael L. F., Schkeryantz J. M., Burris T. P. Mini Rev. Med. Chem. 2005;5:729–740. doi: 10.2174/1389557054553767. [DOI] [PubMed] [Google Scholar]
- 33.Gimpl G., Burger K., Fahrenholz F. Biochemistry. 1997;36:10959–10974. doi: 10.1021/bi963138w. [DOI] [PubMed] [Google Scholar]
- 34.Yang T., Espenshade P. J., Wright M. E., Yabe D., Gong Y., Aebersold R., Goldstein J. L., Brown M. S. Cell. 2002;110:489–500. doi: 10.1016/s0092-8674(02)00872-3. [DOI] [PubMed] [Google Scholar]
- 35.Corbit K. C., Aanstad P., Singla V., Norman A. R., Stainier D. Y., Reiter J. F. Nature. 2005;437:1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- 36.Crick D. C., Andres D. A., Danesi R., Macchia M., Waechter C. J. J. Neurochem. 1998;70:2397–2405. doi: 10.1046/j.1471-4159.1998.70062397.x. [DOI] [PubMed] [Google Scholar]
- 37.Williams J. A., Guicherit O. M., Zaharian B. I., Xu Y., Chai L., Wichterle H., Kon C., Gatchalian C., Porter J. A., Rubin L. L., Wang F. Y. Proc. Natl. Acad. Sci. USA. 2003;100:4616–4621. doi: 10.1073/pnas.0732813100. [DOI] [PMC free article] [PubMed] [Google Scholar]