Abstract
Background
Corticotropin-releasing hormone (CRH) is proposed to be involved in the regulation of the proliferative capacity of keratinocytes, based on its significant actions in the skin. These are mediated by CRH-R1α and represented by adenylate cyclase activation, Ca2+ influx, inhibition of cell proliferation and modifications in intracellular signal transduction by NF-κB.
Objectives
To define CRH action in the cell cycle we investigated its effects on the differentiation programme using the HaCaT keratinocytes model.
Methods
HaCaT keratinocytes were incubated with CRH in Dulbecco’s modified Eagles’s medium (containing 1·8 mmol L−1 calcium) or EpiLife (containing 0·06 mmol L−1 calcium) medium. Cell proliferation was assessed with the MTT assay. Flow cytometry was used for the measurement of DNA content, cell size and granularity and the expression of cytokeratin 14, cytokeratin 1 and involucrin. The electrophoretic mobility shift assay was used to determine DNA binding activity by AP-1 transcription factor. Expression of cytokeratin 1 was also assessed with immunofluorescence microscopy.
Results
CRH did produce inhibition of proliferation, which was dose-dependent; the shape of the inhibition curve was determined by the media calcium concentration. CRH action was pinpointed at inhibition of the G0/1 to the S phase transition of the cell cycle. CRH also increased AP-1 binding activity, cell granularity, cytokeratin 1 and involucrin expression, and inhibited cytokeratin 14 expression.
Conclusions
These results are consistent with CRH induction of the keratinocyte differentiation programme. Thus, the overall CRH cutaneous actions connote protective functions for the epidermis, that appear to include the triggering or acceleration of the differentiation programme.
Keywords: calcium, cell cycle, CRH, differentiation, keratinocyte
The pathogenesis of inflammatory and proliferative skin diseases is still far from being elucidated. Several locally produced cytokines are thought to regulate keratinocyte proliferation and are proposed to play a role in abnormal keratinocyte functions. Corticotropin-releasing hormone (CRH), a 41-amino acid peptide, coordinates central responses to stress through its interactions predominantly with the CRH receptor type 1 (CRH-R1).1,2 The skin, like other peripheral organs, produces CRH and related peptides and expresses the corresponding CRH-R1 and CRH-R2.3–7 In human skin cells, the phenotypic effects of CRH are mediated mostly by CRH-R1.6,7 Human skin keratinocytes, the main cell population of the epidermis, express CRHR1 but not CRH-R2 mRNA7–9 and, further, generate protein products that become fully functional cell surface receptors.4–7,10
HaCaT cells are the product of an experimental line derived from normal epidermal keratinocytes; they are immortal, maintain differentiation potential and are used as a model of keratinocyte function.11 They represent a convenient in vitro testing model, because of their homogeneity with lack of the donor-to-donor variability that always exists in primary cell lines derived from the skin. In HaCaT keratinocytes the CRH receptor expressed has been identified as the CRH-R1α isoform.8,10 CRH signal transduction in HaCaT keratinocytes involves stimulation of adenylate cyclase and of Ca2+ influx, predominantly mediated by activation of the voltage-activated calcium ion channels.4,12,13 In HaCaT cells, CRH inhibits cellular proliferation,6,10 modulates cytokine production,14 and modifies the nuclear factor of the κ light polypeptide gene enhancer in the B-cell (NF-κB) signal transduction system.15 Similar antiproliferative and immunomodulatory effects of CRH have been noted in normal human neonatal keratinocytes.6,7,16 Thus, CRH is emerging as an important regulator of local (skin) homeostasis.5–7,17,18
The differentiation programme of epidermal keratinocytes is tightly regulated by transcription factors mostly of the AP-1 family, and by intracellular calcium levels, with calcium levels being higher in the most differentiated upper layers.19–21 Differentiation is associated with epidermal layer-dependent production of specific cytokeratins. For example, the basal layer expresses cytokeratins 5 and 14 that decrease in the suprabasal layers where the cells express instead cytokeratins 1, 10 and involucrin.20 In the current work we investigated whether CRH affects the differentiation programme in HaCaT keratinocytes.6
Materials and methods
Chemicals
CRH (Molecular Research Laboratory, Herndon, VA, U.S.A), CRH-R1 agonist acetylcyclo(30–33)[D-Phe12, D-Glu20, Nle21, Glu30, D-His32, Lys33, D-Nle38]-CRH(4–41) (UCW 4938, California Peptide Research, Inc., Napa, CA, U.S.A.) and urocortin I (California Peptide Research) were dissolved in 0·1 mol L−1 acetic acid at the concentration of 2 μg μL−1 and stored at −70 °C until use. Carlson et al. described the biological properties of UCW 4938 peptide and its specificity for CRH-R1.22
Dulbecco’s modified Eagles’s medium (DMEM), fetal calf serum (FCS), 0·25% trypsin/1 mmol L−1 ethylenediamine tetraacetic acid solution, phosphate buffered saline (PBS) and solution containing penicillin G, streptomycin and amphotericin B were purchased from Gibco, Invitrogen Life Technnologies (Carlsbad, CA, U.S.A). EpiLife medium and EpiLife® Defined Growth Supplement containing purified bovine serum albumin, purified 5 μg mL−1 bovine transferrin, 0·18 μg mL−1 hydrocortisone, recombinant human insulin-like growth factor type-1 (rhIGF-1), prostaglandin E2 (PGE2), 0·2 ng mL−1 recombinant human epidermal growth factor (rhEGF) and antibiotics were purchased from Cascade Biologics, Inc. (Portland, OR, U.S.A.). Bovine serum albumin, hydrochloric acid, iodide propidium, isopropanol, MTT, nocodazole, ribonuclease IA, saponin, sodium azide and Triton-X were purchased from Sigma (St Louis, MO, U.S.A.). Mouse monoclonal antibodies against human cytokeratin 14, cytokeratin 1, involucrin and sheep antimouse fluorescein–isothiocyanate (FITC)-conjugated antibody were purchased from Novocastra Laboratories Ltd (Newcastle upon Tyne, U.K.) and mouse purified IgG3 and IgG1 from Caltag Laboratories (Burlingame, CA, U.S.A.). Rabbit polyclonal antibody against human cytokeratin 1 was purchased from Babco (Richmond, CA, U.S.A.) and FITC-conjugated goat antirabbit antibody from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, U.S.A.). Vectashield mounting medium was purchased from Vector Laboratories, Inc. (Burlingame, CA, U.S.A.). AP-1 oligonucleotide probe and T4 polynucleotide kinase were purchased from Promega (Madison, WI, U.S.A.). [γ-32P]deoxy-ATP was purchased from Amersham Biosciences (Piscataway, NJ, U.S.A.).
Cell culture
HaCaT keratinocytes were grown in DMEM medium supplemented with 10% FCS and 1% of penicillin G, streptomycin and amphotericin B solution as described previously.10 Two types of media were used to measure the cell proliferation and differentiation: (i) DMEM containing high calcium concentration (1·8 mmol L−1 calcium) supplemented with or without 10% FCS, and (ii) Epilife medium with low calcium concentration (0·06 mmol L−1) with or without EpiLife® Defined Growth Supplement. Cells were seeded at a density described below and grown for 24 h in the supplemented media. Thereafter media were changed to supplement-free before adding CRH, the modified CRH peptide UCW 4938 or urocortin I every 12 h for 24–48 h at the concentrations listed in the figures.
Cell proliferation assay
Keratinocytes were seeded at a density of 5000 cells per well into 96-well plates in medium supplemented with growth factors. After 24 h media were changed to supplement-free Epi-Life medium containing graded concentrations of CRH or urocortin. The cells were incubated for 48 h and the media containing fresh peptides were changed every 12 h. Thereafter, 20 μL MTT (5 mg mL−1 in PBS) was added and the plates were incubated at 37 °C for 4 h in the presence of 5% CO2. At the end of the incubation period, media were discarded and 100 μL of acid (0·1 mol L−1 hydrochloric acid) isopropanol was added before measuring optical density at 570 nm with a plate reader.
Cell cycle analysis
Cell DNA content was measured by flow cytometry. Keratinocytes were seeded at a density of 500 000 cells into 10-cm Petri dishes in medium supplemented with growth factors. After 24 h media were changed to serum-free DMEM medium or supplement-free EpiLife medium with or without CRH. Separate preliminary experiments were performed to determine the minimal nocodazole concentration that holds the cells in G2/M phase. Cells were treated with CRH, nocodazole (0·5 μg mL−1) or a combination of both for 24 h in supplement-free medium, and incubated as indicated. Media containing fresh peptide were changed every 12 h. After incubation the cells were trypsinized, washed with PBS and fixed with 70% ice-cold ethanol. Ethanol was removed by centrifugation and the pellets were washed twice with PBS before adding 0·5 mL of a solution of propidium iodide (50 μg mL−1) and ribonuclease IA (0·1 mg mL−1) in Ca2+-and Mg2+-free PBS. Samples were shaken for 30 min in 37 °C and analysed with a FACS Calibur cytometer (Beckton Dickinson, San Diego, CA, U.S.A.). The DNA content was estimated with the ModFit 2·0 program (Verity Software House, Topsham, ME, U.S.A.). Events were gated in the forward scatter/side scatter window to exclude debris, and in 10 area/width FL2 windows to exclude doublets. The results are presented as mean ± SEM from three cultures and P-values were determined with Student’s t-test.
Electrophoretic mobility shift assay
Keratinocytes were seeded at a density of 500 000 cells into 10-cm Petri dishes in DMEM medium supplemented with FCS. After 24 h the medium was changed to serum-free DMEM and cells were incubated with or without CRH for 60 min. Then nucleus extracts were prepared as previously described23 and used for an electrophoretic mobility shift assay (EMSA). Double-stranded AP-1 oligonucleotide probe (5′-CGC TTG ATG AGT CAG CCG GAA-3′) was end-labelled with [γ-32P]deoxy-ATP using T4 polynucleotide kinase and incubated with 5 μg of nuclear extract. Protein-DNA complexes were separated on 5% polyacrylamide gel. To determine binding specificity, a 50 × excess of unlabelled oligonucleotide was used. Radioactivity was quantified with a Packard Cyclone phosphorimager, and analysed with Optiquant (PerkinElmer Life Sciences Inc., Boston, MA, U.S.A.) and Adobe Photoshop (San Jose, CA, U.S.A.) software.11
Flow cytometric analysis of expression of cytokeratin 14, cytokeratin 1, involucrin, cell size and granularity
Keratinocytes were seeded at a density of 500 000 cells into 10-cm Petri dishes in medium supplemented with growth factors. After 24 h media were changed to serum-free DMEM medium or supplement-free EpiLife medium with or without CRH. Cells, control or treated with CRH peptide, were fixed with cold 2% paraformaldehyde in PBS for 1 h, washed in PBS and the resulting pellets (200 000 cells per sample) suspended in 100 μL of a permeabilizing solution containing saponin 0·25%, 0·1% bovine serum albumin, 0·1% sodium azide in PBS and one of the primary antibodies [mouse anti-human cytokeratin 14 (IgG3, 0·25 μg), mouse antihuman cytokeratin 1 (IgG1, 0·07 μg), mouse antihuman involucrin (IgG1, 0·2 μg)]. The amount of antibody added had been defined in preliminary titration experiments. Unstained cells and cells stained with isotype control antibody (mouse purified IgG3, IgG1) were used as controls. After 12 h of incubation the cells were washed twice with PBS and resuspended in 100 μL of a permeabilizing solution that contained as secondary antibody sheep antimouse FITC-conjugated antibody (1 : 50). After 3 h the cells were washed with PBS, resuspended in 400 μL of PBS, and the fluorescence read with a FACS Calibur flow cytometer. The FL-1 signal from 10 000 events from the side scatter/forward scatter window after debris exclusion was collected and recorded. Forward scatter histograms (relative to cell size) and side histograms (relative to cell granularity) were generated and the mean signal intensity recorded. FL-1 signal values are presented as dMFI (difference between mean fluorescence intensities of samples stained with specific and with isotype control antibodies). Scatter signal values are presented as MFI (mean fluorescence intensity). Signal intensities were analysed with Cell Quest (BD Biosciences, San Diego, CA, U.S.A.) and graphical representations of the FL-1 signal were prepared with WinMdi 2·8 (shareware from Joseph Trotter, The Scripps Research Institute, La Jolla, CA, U.S.A.).
Immunofluorescence microscopy analysis of cytokeratin 1 expression
Cells were seeded in eight-well Laboratory-Tek II chamber slides (25 000 cells per chamber, Nalge Nunc, Inc., Naperville, IL, U.S.A.) and after 24 h treated with or without CRH in supplement-free Epilife medium for 48 h. The cells were then fixed in 4% paraformaldehyde (in PBS) for 10 min, permeabilized with 0·1% Triton X-100 (in PBS) for 5 min, blocked with 1% bovine serum albumin (in PBS) for 30 min and stained with polyclonal primary antibody against 13 cytokeratin 1 followed by secondary FITC-conjugated antirabbit antibody for 1 h in PBS containing 1% bovine serum albumin. The slides were washed extensively between staining with PBS, and mounted with Vectashield mounting medium. In control stains (background) the primary antibody was omitted. The slides were examined with a NIKON Eclipse TE300 microscope (Nikon, Melville, NY, U.S.A.).
Statistical analysis
Data is presented as mean ± SEM and analysed with Student’s t-test (for two groups) or one-way analysis of variance with appropriate posthoc tests (for more than two groups) using Excel (Microsoft) and Prism 4·00 (GraphPad Software, San Diego, CA, U.S.A.). Statistically significant differences are denoted with asterisks: *P < 0·05, **P < 0·005.
Results
Corticotropin-releasing hormone receptor type 1 agonists inhibit proliferation of HaCaT keratinocytes
CRH was previously shown to inhibit growth of HaCaT keratinocytes cultured in DMEM (medium high in calcium) at only nmol L−1 concentrations; such an effect was not seen at μmol L−1 CRH concentrations (bell-shape response curve).10 In cells cultured in supplement-free low-calcium Epilife medium we compared the effect of the agonist UCW 4938, which selectively activates CRH-R1, with that of urocortin I (peptide that is equipotent at CRH-R1 and CRH-R2). Treatment of HaCaT keratinocytes for 48 h with those peptides resulted in significant inhibition of cell proliferation in a dose-dependent manner (Fig. 1; P < 0·005). UCW 4938 was significantly more potent than urocortin I, with the EC50 for UCW 4938 agonist being 3·3 × 10−10 mol L−1 and that for urocortin I 1·4 × 10–8 mol L−1.
Fig 1.

Corticotropin-releasing hormone receptor type 1 selective agonist (continuous curve, square points) and urocortin I (dotted curve, circle points) attenuate proliferation of HaCaT cells. HaCaT cells were stimulated for 48 h in supplement-free low-calcium EpiLife medium with peptides at graded concentrations. Cell viability was estimated with a MTT test. The results are presented as mean ± SEM (n = 16). Fitting of the dose–response curve was performed with the Prism 4·0 software (GraphPad, San Diego, CA, U.S.A).
Corticotropin-releasing hormone arrests keratinocytes at the G0/1 phase of the cell cycle
To define the mechanism for the proliferation inhibitory effect we performed flow cytometry analyses of DNA content in the cells cultured in high- and low-calcium media. HaCaT keratinocytes were stimulated for 24 h in serum-free DMEM medium (containing a relatively high concentration of calcium) (Fig. 2). While the majority of control cells remained in the G0/1 phase (72%), 17% of cells were in S phase and 11% in G2/M phase; treatment with nocodazole resulted in arrest of the cell cycle at the G2/M phase. Treatment with CRH resulted in the accumulation of cells in the G0/1 phase in both the presence and the absence of nocodazole. These results are presented in Figure 2(A). A similar effect was seen when HaCaT keratinocytes were cultured in low-calcium EpiLife medium. As shown in the insert in Figure 2(B), treatment with CRH resulted in a significant increase of cell accumulation in the G0/1 phase (Fig. 2B). Thus, CRH-induced inhibition of HaCaT cells growth is exerted at the same phase of the cell cycle in media high or low in calcium concentrations, e.g. arrest at G0/1.
Fig 2.

Corticotropin-releasing hormone (CRH) increases accumulation of cells in the G0/1 phase of the cell cycle. Cells were treated with 1 nmol L−1 CRH for 24 h in serum-free high-calcium Dulbecco’s modified Eagles’s medium (A) or with 100 nmol L−1 CRH for 48 h in supplement-free low-calcium Epilife medium (B). The DNA content was measured by flow cytometry. Data are presented as mean ± SEM (n = 3). Significant differences are denoted with asterisks.
Electrophoretic mobility shift assay analysis of AP-1 binding activity
As CRH is known as a potent inducer of Ca2+ influx in HaCaT keratinocytes,4,12,13 we tested its effects on the transcriptional coordinator of calcium signal AP-1 by determining AP-1 binding activity. HaCaT keratinocytes were incubated for 1 h in serum-free DMEM medium, with or without 100 nmol L−1 CRH. As shown in Figure 3, CRH caused a dramatic increase in AP-1 binding activity. Although the specificity of DNA binding was confirmed by a competition assay, the actual composition of CRH-stimulated AP-1 complexes is still under investigation.
Fig 3.
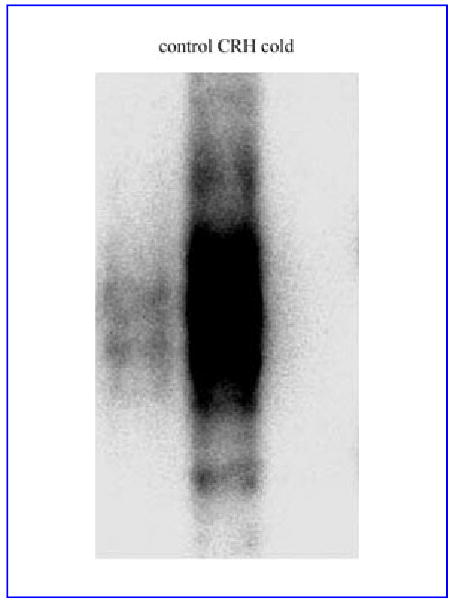
Corticotropin-releasing hormone (CRH) increases AP-1 binding activity in HaCaT cells. HaCaT cells were stimulated with 100 nmol L−1 CRH for 60 min in serum-free Dulbecco’s modified Eagles’s medium. Nuclear extracts were subjected to electrophoretic mobility shift assay analysis using a 32P-labelled AP-1 oligonucleotide probe (5′-CGC TTG ATG AGT CAG CCG GAA-3′). Cold represents nuclear extracts preincubated with 50 × excess of cold oligonucleotide.
Corticotropin-releasing hormone modifies cytokeratin 14, cytokeratin 1, involucrin, granularity and size of HaCaT keratinocytes
The effect of CRH on keratinocyte differentiation was tested in both serum-free DMEM (high Ca2+ levels) and in supplement-free Epilife medium (low Ca2+ levels) (Fig. 4 and Table 1). Flow cytometric analysis of cells incubated in high-calcium DMEM medium and exposed to different concentrations of CRH peptide showed that CRH decreases significantly the expression of cytokeratin 14, and further that the 1 nmol L−1 concentration was more potent than 100 nmol L−1 (Fig. 4A, B). These same ligand concentrations had no effect on either involucrin or cytokeratin 1 expression (not shown). In addition, CRH significantly increased the mean values of side scatter (representative of cell granularity) (Fig. 4A). There was no effect on forward scatter (representative of cell size) (not shown). The effect of CRH in cells cultured in low-calcium EpiLife medium is shown in Figure 4(C, D). CRH significantly stimulated the expression of cytokeratin 1 and involucrin (Fig. 4C, D), but had no effect on cytokeratin 14 (not shown). CRH also increased side scatter (Fig. 4C), but did not affect forward scatter (not shown). Table 1 summarizes the results of flow cytometry experiments.
Fig 4.

Corticotropin-releasing hormone (CRH) triggers an early differentiation programme in HaCaT keratinocytes. HaCaT cells were stimulated with for 24 h in serum-free high-calcium Dulbecco’s modified Eagles’s medium (A, B) or for 48 h in supplement-free low-calcium EpiLife medium (C, D). Tables in A and C list flow cytometry analyses in relative values. Results are expressed as the mean ± SEM. Histograms in B are representative of cells treated with CRH or untreated (controls) and stained with the antibody against cytokeratin 14. In D the lower panel shows immunofluorescence microscopy (IF) analysis of cytokeratin 1 expression in cells treated with CRH and untreated (controls). The upper panel shows representative light microscopy (LM) images. The CRH-induced increase in cell numbers that express high levels of cytokeratin 1 is in agreement with the results of quantitative flow cytometry analysis showing increased mean fluorescence intensity of the whole cell population.
Table 1.
Effects of CRH on differentiation parameters in HaCaT keratinocytes grown in high calcium (1·8 mm, DMEM medium) or low calcium (0·06 mm L−1, EpiLife medium)
| Parameter | High Calcium | Low Calcium |
|---|---|---|
| DNA content | G0/1 inhibition | G0/1 inhibition |
| Cytokeratin 14 expression | Inhibition | – |
| Cytokeratin 1 expression | – | Stimulation |
| Involucrin expression | – | Stimulation |
| Granularity (side scatter) | Stimulation | Stimulation |
Discussion
CRH has multiple effects on epidermal functions4,6,7,10 that require a unifying concept to explain its actions. To that end, we have investigated the effect of CRH-R1 agonists on the proliferation and differentiation of HaCaT keratinocytes cultured in vitro. We found that in Epilife medium containing 0·06 mmol L−1 of calcium, CRH-R1 agonists inhibit cell proliferation; the dose–response curve was sigmoid, with modified CRH peptide agonist acetylcyclo(30–33)[D-Phe12, D-Glu20, Nle21, Glu30, D-His32, Lys33, D-Nle38]-CRH(4–41) being more potent than urocortin I. CRH-induced growth inhibition was further characterized by the consistent detection of arrest at the G0/1 phase. Most importantly, the inhibition of cell proliferation was accompanied by induction of the keratinocyte differentiation programme. Human keratinocytes express functional CRH receptors4,6,7,10 that mediate increases in cyclic adenosine monophosphate production,10 cytosolic calcium flux,4,12,13 inhibition of cell proliferation,10,16 a pattern shift in the expression of cell surface adhesion molecules16 and production of cytokines.14 HaCaT cells cultured in standard high-calcium (1·8 mmol L−1) DMEM medium showed significant inhibition of proliferation by CRH at low concentrations (nmol L−1 range), but not at high concentrations, in the μmol L−1 range (bell-shaped curve).6,10 We also show that the antiproliferative CRH-R1 effect is activated by both selective UCW 4938 and nonselective urocortin I (former urocortin) agonists in media containing 0·06 mmol L−1 calcium. Concordantly, the kinetic profile of this action represents a typical sigmoid response curve with higher potency for the selective CRH-R1 agonist. Thus, the tested effects mediated by CRH-R1 are dependent on both extra-cellular calcium concentration and the type of agonist and its dose.
Growth inhibition per se was, however, independent of extracellular calcium levels as both high- and low-calcium media resulted in CRH-induced G0/1 arrest. The mechanism of this arrest was defined by synchronizing the cells in the G2/M phase of the cell cycle with the mitotic spindle inhibitor nocodazole. This inhibitor causes the cell population to gradually accumulate in G2/M phase thus decreasing the proportion of cells in G0/1 phase. Concomitant treatment of these cells with factors that cause G0/1 arrest causes fewer cells to accumulate in G2/M and more cells to remain in G0/1 phase,24 precisely what was observed with CRH treatment. The maximal growth inhibitory activity for both CRH-R1 agonist and urocortin I was observed at 100 nmol L−1, albeit with the tested peptides differing in potency. This is consistent with the differences in potencies reported in other assay systems,22,25,26 and with the exclusive selectivity for CRH-R1 exhibited by the agonist acetyl-cyclo(30–33)[D-Phe12, D-Glu20, Nle21, Glu30, D-His32, Lys33, D-Nle38]-CRH(4–41).22
The observed keratinocyte arrest in G0/1 phase induced by CRH was accompanied by the triggering of the differentiation programme. While this was observed with both calcium environments, there were also culture medium-specific effects. Thus, in high-calcium media CRH inhibited the expression of cytokeratin 14 (characteristic of the basal layer) and increased cell granularity, and was without effect on cytokeratin 1 and involucrin. In contrast, in the low-calcium medium CRH stimulated cytokeratin 1 and involucrin expression and increased cell granularity (Table 1). These correspond to similar changes in the skin, where decreases in cytokeratin 14 and increases in cytokeratin 1 and involucrin are characteristic of cells intermediately located between the basal and spinous layers.20 Increased cellular granularity reflects keratohyalin aggregate formation or secretory function.27,28 Thus, the protein expression data are consistent with coupling of induction of the differentiation programme to the entering of cells into G0. Likewise cellular granularity may be also ascribed to the differentiation programme or to specific CRH stimulation of cytokine production and secretion. CRH stimulation of AP-1 binding activity in HaCaT keratinocytes suggests that these transcription factors may be also involved in the pathway. In fact proteins of the AP-1 family of transcription factors are actively engaged in the regulation of cell proliferation and keratinocyte differentiation.19 Further characterization of CRH effects on normal skin cells is the subject of current research in our laboratory.
The difference in the responses of CRH in high- vs. low-calcium media are likely to be due to interactions between the intracellular transduction pathways mediating the signalling for CRH and for calcium receptors. In the keratinocyte calcium receptor signal transduction involves phospholipase C-γ1 activation with the formation of inositol 1,4,5-triphosphate and subsequent activation of protein kinase C-α -dependent pathways.20 Inositol 1,4,5-phosphate may also directly activate membrane calcium channels.20 In contrast, CRH activates adenylate cyclase (highest efficacy at 10−7 mol L−1), and it also stimulates intracellular Ca2+ influx through voltage-activated Ca2+ ion channels (highest efficacy at 10−10 mol L−1).10,12,13 A particular property of the Ca2+ influx effect into HaCaT cells is its qualitative dependency on the ligand, e.g. CRH induces Ca2+ flux into the cytoplasm, while urocortin induces Ca2+ flux into the nucleus with remarkable oscillatory characteristics.13 It is possible that urocortin action may be mediated through activation of an intracellular signal transduction pathway unique to HaCaT keratinocytes. The interactions between these pathways and their potential coupling to the differential expression of CRH-R1 isoforms8 merits further study.
In summary, using the HaCaT keratinocytes model we were able to evaluate the regulated coupling of CRH-R1 activity with its phenotypic effects, as well as cross-talk between different signal transduction and transcriptional pathways activated by CRH or CRH-related peptides. We thus found that activation of CRH-R1 triggers both G0/1 arrest and early differentiation indicating that the previously observed CRH effect of inhibiting keratinocyte proliferation does not represent a random event; instead, this is a key component for the triggering of sequential differentiation events.22
Acknowledgments
Flow cytometry data were collected on a FACS Calibur Cytometer in the Molecular Resource Center at the University of Tennessee, Memphis. We greatly appreciate Dr Jacek Witkowski’s expert review of the flow cytometric data. We also thank Ms Christine Crawford for skilful secretarial assistance. This work was supported by National Institutes of Health grant AR047079 (AS), by Polish Science Committee grant 4P05A 046 19 (AM, BZ) and by a Johnson and Johnson Skin Research Center Training Grant for the year 2003 awarded by Johnson & Johnson Consumer Products Worldwide, Skillman, NJ, U.S.A. (BZ).
Footnotes
Conflicts of interest: None declared
References
- 1.Chrousos GP, Gold PW. The concepts of stress and stress system disorders. JAMA. 1992;267:1244–52. [PubMed] [Google Scholar]
- 2.Perrin MH, Vale WW. Corticotropin releasing factor receptors and their ligand family. Ann NY Acad Sci. 1999;885:312–28. doi: 10.1111/j.1749-6632.1999.tb08687.x. [DOI] [PubMed] [Google Scholar]
- 3.Kono M, Nagata H, Umemura S, et al. In situ expression of corticotropin-releasing hormone (CRH) and proopiomelanocortin (POMC) genes in human skin. FASEB J. 2001;15:2297–9. doi: 10.1096/fj.01-0254fje. [DOI] [PubMed] [Google Scholar]
- 4.Slominski A, Botchkarev V, Choudhry M, et al. Cutaneous expression of CRH and CRH-R: is there a ‘skin stress system?’. Ann NY Acad Sci. 1999;885:287–311. doi: 10.1111/j.1749-6632.1999.tb08686.x. [DOI] [PubMed] [Google Scholar]
- 5.Slominski A, Wortsman J, Luger T, et al. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80:979–1020. doi: 10.1152/physrev.2000.80.3.979. [DOI] [PubMed] [Google Scholar]
- 6.Slominski AT, Wortsman J, Pisarchik A, et al. Cutaneous expression of corticotropin-releasing hormone (CRH), urocortin, and CRH receptors. FASEB J. 2001;15:1678–93. doi: 10.1096/fj.00-0850rev. [DOI] [PubMed] [Google Scholar]
- 7.Slominski A, Wortsman J, Linton E et al. The skin as a model for the immunodulatory effects of corticotropin-releasing hormone. In: Mind Over Matter – Regulation of Peripheral Inflammation by the CNS (Schäfer M, Stein C, eds). In: Progress in Inflammation Research (Parnham MJ, series ed), 1st edn. Basel: Birkhäuser-Verlag, 2003; 149–76.
- 8.Pisarchik A, Slominski AT. Alternative splicing of CRH-R1 receptors in human and mouse skin: identification of new variants and their differential expression. FASEB J. 2001;15:2754–6. doi: 10.1096/fj.01-0487fje. [DOI] [PubMed] [Google Scholar]
- 9.Slominski A, Ermak G, Hwang J, et al. Proopiomelanocortin, corticotropin releasing hormone and corticotropin releasing hormone receptor genes are expressed in human skin. FEBS Lett. 1995;374:113–16. doi: 10.1016/0014-5793(95)01090-2. [DOI] [PubMed] [Google Scholar]
- 10.Slominski AT, Roloff B, Zbytek B, et al. Corticotropin releasing hormone and related peptides can act as bioregulatory factors in human keratinocytes. In Vitro Cell Dev Biol. 2000;36A:211–16. doi: 10.1290/1071-2690(2000)036<0211:CRHARP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 11.Boukamp P, Petrussevska RT, Breitkreutz D, et al. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–71. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fazal N, Slominski A, Choudhry MA, et al. Effect of CRF and related peptides on calcium signalling in human and rodent melanoma cells. FEBS Lett. 1998;435:187–90. doi: 10.1016/s0014-5793(98)01067-9. [DOI] [PubMed] [Google Scholar]
- 13.Wiesner B, Roloff B, Fechner K, et al. Intracellular calcium measurements of single human skin cells after stimulation with corticotropin-releasing factor and urocortin using confocal laser scanning microscopy. J Cell Sci. 2003;116:1261–8. doi: 10.1242/jcs.00301. [DOI] [PubMed] [Google Scholar]
- 14.Zbytek B, Mysliwski A, Slominski A, et al. Corticotropin-releasing hormone affects cytokine production in human HaCaT keratinocytes. Life Sci. 2002;70:1013–21. doi: 10.1016/s0024-3205(01)01476-x. [DOI] [PubMed] [Google Scholar]
- 15.Zbytek B, Pfeffer L, Slominski A. CRH inhibits NF-κB pathway in human HaCaT keratinocytes. J Invest Dermatol. 2003;121:1496–9. doi: 10.1111/j.1523-1747.2003.12612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quevedo ME, Slominski A, Pinto W, et al. Pleiotropic effects of corticotropin releasing hormone on normal human skin keratinocytes. In Vitro Cell Dev Biol Anim. 2001;37:50–4. doi: 10.1290/1071-2690(2001)037<0050:peocrh>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 17.Slominski A, Pisarchik A, Tobin DJ, Mazurkiewicz JE, Wortsman J. Differential expression of cutaneous corticotropin releasing hormone system. Endocrinology. 2004;145:941–50. doi: 10.1210/en.2003-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr Rev. 2000;21:457–87. doi: 10.1210/edrv.21.5.0410. [DOI] [PubMed] [Google Scholar]
- 19.Angel P, Szabowski A, Schorpp-Kistner M. Function and regulation of AP-1 subunits in skin physiology and pathology. Oncogene. 2001;20:2413–23. doi: 10.1038/sj.onc.1204380. [DOI] [PubMed] [Google Scholar]
- 20.Bikle DD, Ng D, Tu CL, et al. Calcium-and vitamin D-regulated keratinocyte differentiation. Mol Cell Endocrinol. 2001;177:161–71. doi: 10.1016/s0303-7207(01)00452-x. [DOI] [PubMed] [Google Scholar]
- 21.Menon GK, Grayson S, Elias PM. Ionic calcium reservoirs in mammalian epidermis: ultrastructural localization by ion-capture cytochemistry. J Invest Dermatol. 1985;84:508–12. doi: 10.1111/1523-1747.ep12273485. [DOI] [PubMed] [Google Scholar]
- 22.Carlson KT, Navy SS, Wei ET, et al. Inhibition of mouse melanoma cell proliferation by corticotropin releasing hormone and its analogs. Anticancer Res. 2001;21:1173–80. [PubMed] [Google Scholar]
- 23.Yang CH, Murti A, Pfeffer SR, et al. IFN α/β promotes cell survival by activating NF-κB. Proc Natl Acad Sci USA. 2000;97:13631–6. doi: 10.1073/pnas.250477397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wieder R. Selection of methods for measuring proliferation. In: Cell Growth, Differentiation and Senescence, a Practical Approach (Studzinski GP, ed.), 1st edn. Oxford: Oxford University Press, 1999; 1–30.
- 25.Chalmers DT, Lovenberg TW, Grigoriadis DE, et al. Corticotropin releasing factor receptors: from molecular biology to drug design. Trends Pharmacol Sci. 1996;17:166–72. doi: 10.1016/0165-6147(96)81594-x. [DOI] [PubMed] [Google Scholar]
- 26.Wei ET, Thomas HA, Christian HC, et al. D-amino acid-substituted analogs of corticotropin-releasing hormone and urocortin with selective agonist activity at CRH1 and CRH2 beta-receptors. Peptides. 1998;19:1183–90. doi: 10.1016/s0196-9781(98)00085-0. [DOI] [PubMed] [Google Scholar]
- 27.Fletcher MP, Seligmann BE. Monitoring human neutrophil granule secretion by flow cytometry: secretion and membrane potential changes assessed by light scatter and a fluorescent probe of membrane potential. J Leukoc Biol. 1985;37:431–47. doi: 10.1002/jlb.37.4.431. [DOI] [PubMed] [Google Scholar]
- 28.Resing KA, Dale BA. Proteins of keratohyalin. In: Physiology, Biochemistry, and Molecular Biology of the Skin (Goldsmith LA, ed.), 2nd edn, Vol. 1. Oxford: Oxford University Press, 1991; 148–68.


