Abstract
Akt promotes cell survival through phosphorylation. The physiological functions of cytoplasmic Akt have been well defined, but little is known about the nuclear counterpart. Employing a cell-free apoptotic assay and NGF-treated PC12 nuclear extracts, we purified Ebp1 as a factor, which contributes to inhibition of DNA fragmentation by CAD. Depletion of Ebp1 from nuclear extracts or knockdown of Ebp1 in PC12 cells abolishes the protective effects of nerve growth factor, whereas overexpression of Ebp1 prevents apoptosis. Ebp1 (S360A), which cannot be phosphorylated by PKC, barely binds Akt or inhibits DNA fragmentation, whereas Ebp1 S360D, which mimics phosphorylation, strongly binds Akt and suppresses apoptosis. Further, phosphorylated nuclear but not cytoplasmic Akt interacts with Ebp1 and enhances its antiapoptotic action independent of Akt kinase activity. Moreover, knocking down of Akt diminishes the antiapoptotic effect of Ebp1 in the nucleus. Thus, nuclear Akt might contribute to suppressing apoptosis through interaction with Ebp1.
Keywords: nuclear Akt, Ebp1, PKC, CAD, DNA fragmentation
Introduction
Nerve growth factor (NGF) regulates survival of several types of neurons by provoking a variety of signaling cascades including PI 3-kinase/Akt pathway, which plays an essential role in this process (Brunet et al, 2001). PI 3-kinase/Akt signaling blocks cell death both by impinging on the cytoplasmic apoptotic machinery and by mediating the expression of genes involved in cell death and survival (Brunet et al, 1999; Brazil et al, 2002). Although PI 3-kinase/Akt cascade is important for the NGF-dependent survival of sympathetic neurons (Kaplan and Miller, 2000), it is not the only required signaling pathway. Under certain culture conditions, PI 3-kinase/Akt appears to be dispensable for cell survival (Philpott et al, 1997; Tsui-Pierchala et al, 2000). Recently, it has been shown that PKC signaling is also involved in the survival of sympathetic neurons. Johnson and his collaborators demonstrated that inhibition of either PKC or PI 3-kinase alone caused only a modest attrition of neurons in the presence of NGF. In contrast, the simultaneous inhibition of both PKC and PI 3-kinase induced massive apoptotic death of NGF-treated sympathetic neurons (Pierchala et al, 2004). Thus, NGF promotes the survival of sympathetic neurons through the cooperative function of the PKC and PI 3-kinase pathways. However, the downstream effectors mediating this effect remain obscure.
NGF treatment rapidly increases the enzymatic activity of nuclear Akt, and PI 3-kinase inhibitor LY294002 pretreatment blocks Akt nuclear translocation (Borgatti et al, 2003). Moreover, Akt upstream kinase, PDK1, also localizes in both the cytoplasmic and nuclear compartments. Treatment with the nuclear export inhibitor, Leptomycin B (LMB), leads to constitutive nuclear localization of PDK1 (Lim et al, 2003). Presumably, Akt is phosphorylated and activated by PDK1 in the cytoplasm, which leads to its subsequent nuclear translocation. On the other hand, PDK1 may also directly phosphorylate Akt in the nucleus (Kikani et al, 2005). Recently, we demonstrated that nuclear PI 3-kinase and its upstream regulator PIKE mediate the antiapoptotic activity of NGF in isolated nuclei (Ahn et al, 2004). Nuclei from NGF-treated PC12 cells are resistant to DNA fragmentation initiated by the activated cell-free apoptosome, consisting of HEK293 cell cytosol supplemented with purified active caspase 3 (Liu et al, 1997). We showed that nuclear PI(3,4,5)P3 mimics the antiapoptotic action of NGF, while nuclear Akt appears necessary but not sufficient for this effect.
Ebp1, a ubiquitously expressed protein, localizes in both the nucleus and the cytoplasm and binds ErbB3 receptor in human serum-starved breast cancer cell lines (Yoo et al, 2000). Ebp1 is the human homolog of a previously identified cell cycle-regulated mouse protein p38-2G4 (Radomski and Jost, 1995). ErbB3/4 ligand Heregulin (HRG) treatment of serum-starved AU565 breast cancer cells results in dissociation of Ebp1 from ErbB-3 and translocation from the cytoplasm into the nucleus (Yoo et al, 2000). Ebp1 binds tumor suppressor retinoblastoma protein (Rb), leading to inhibition of the E2F1-regulated transcription (Xia et al, 2001; Zhang et al, 2003; Zhang and Hamburger, 2005). Recently, it has been shown that Ebp1 localizes to the nucleolus, and EBP1 overexpression inhibits proliferation of human fibroblasts. This effect is linked to its nucleolar localization (Squatrito et al, 2004).
Here, we report that PKC-dependent phosphorylation of Ebp1 stimulates binding to phosphorylated nuclear Akt in NGF-treated PC12 cells. The resulting complex interacts with CAD and inhibits its DNA fragmentation activity. NGF elicits Ebp1 and Akt complex formation in a PKC- and PI 3-kinase-dependent manner. Depletion of Ebp1 or disruption of its association with Akt abolishes its antiapoptotic activity. Therefore, a complex formed by active nuclear Akt and Ebp1 plays an integral role in the antiapoptotic actions of NGF in the nucleus.
Results
Ebp1 co-purifies with DNA fragmentation inhibitory activity
Nuclei from NGF-treated PC12 cells are resistant to DNA fragmentation initiated by the active cell-free apoptosome, consisting of purified His-DFF45/40 (DNA fragmentation factor) and active caspase 3 (Ahn et al, 2004), indicating that NGF activates nuclear pathways inhibiting CAD. To examine whether the nuclear fraction contains an activity that inhibits CAD, we preincubated various amounts of nuclear extracts from NGF-treated PC12 cells with the cell-free apoptotic solution and determined DNA cleavage with the control nuclei. In total, 3 μg of nuclear extract markedly suppresses DNA fragmentation (Figure 1A), suggesting the existence of CAD inhibitor(s) in NGF-treated nuclear extract. No inhibitory activity is detected with the same amount of cytosolic extract (data not shown). To identify the proteins accounting for the DNA fragmentation inhibitory activity, we employed classical biochemical purification columns using in vitro cell-free DNA fragmentation assay. NGF-treated PC12 cell nuclear extract is subjected to sequential column fractionations. The purification scheme is summarized in Figure 1B. Fraction #28 from Mono Q column displays potent inhibitory activity (Figure 1C). The fractions at about 300 mM NaCl are analyzed by SDS–PAGE and silver staining. Four proteins with molecular weights of 45, 50, 55, 65 kDa are present in this fraction with peak of inhibitory activity. Protein sequence analysis reveal their identities: Hexosaminidase A, β-3-glycosyltransferase, Ring finger Protein 149 and Ebp1 (Figure 1D). Among these proteins, only Ebp1 always co-purifies with CAD-inhibitory activity during the purification protocol (data not shown). Moreover, immunoblotting with anti-phospho-Ebp1 360 antibody reveals that Ebp1 distribution correlates with the inhibitory activities in the fractions, indicating that Ebp1 is responsible for DNA fragmentation inhibitory effect.
Figure 1.
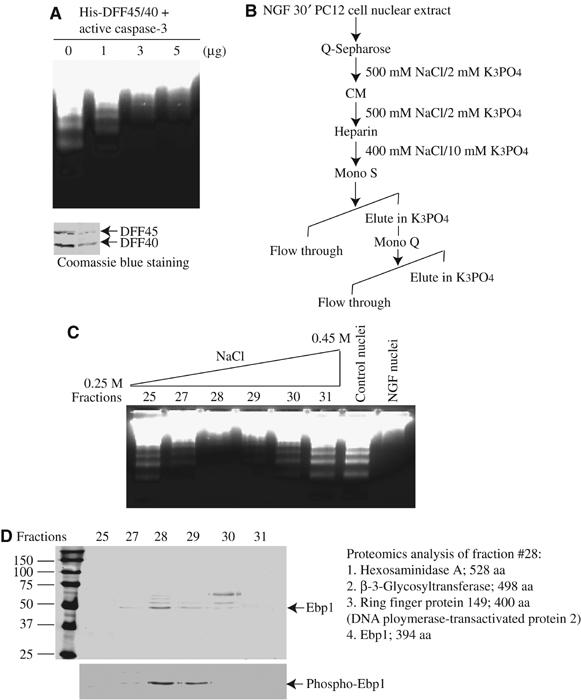
Purification of Ebp1 from NGF-treated PC12 cell nuclear extract. (A) DNA fragmentation assay. Various amount of nuclear extract was preincubated with active apoptosomes containing His-DFF45/40, pretreated with 100 ng active caspase-3, for 10 min at 4°C. The control nuclei from PC12 cells were added and incubated for another 40 min. The fragmented DNA was extracted and resolved on 2% agarose (upper panel). The purity of His-tagged DFF45/40 was verified by Coomassie blue staining (lower panel). (B) Purification chart. DNA fragmentation inhibitory activity was eluted from the columns with NaCl with the indicated concentrations. (C) Mono Q column purification of Ebp1. DNA fragmentation assay with various fractions from Mono Q column reveals that fraction #28 contains the inhibitory proteins. (D) Silver staining of purified proteins. The identity of each band from fraction #28 is described. The proteins were also analyzed with anti-phospho-Ebp1 S360 antibody. Ebp1 distribution in the fractions correlates with its inhibitory activity.
Ebp1 is required for the antiapoptotic activity of NGF
Immunodepletion of Ebp1 from NGF-treated PC12 nuclear extract abolishes the capacity of NGF-treated nuclear extracts to inhibit Caspase-3/CAD-triggered DNA fragmentation, while mock depletion has no effect (Figure 2A, upper left panel lanes 3 and 4). Adding back purified GST-Ebp1, but not GST alone, restores the inhibitory activity (lanes 5 and 6). Moreover, immunodepletion with phospho-Ebp1 antibody but not preimmune antiserum or protein A/G beads markedly diminishes the inhibitory activity (Figure 2A, right panel). Both Ebp1 and phosphorylated Ebp1 are substantially removed by immunodepletion (Figure 2A, lower panels). The nuclei from PC12 cells, which were treated with or without NGF, act as positive and negative controls (lanes 1 and 2). Dose–response experiments reveal inhibition of DNA fragmentation following addition of purified GST-Ebp1; in contrast, GST control fails at the same concentration (Figure 2B). Thus, Ebp1 is required for the antiapoptotic activity of NGF in this cell-free assay. The amounts of recombinant Ebp1 necessary to inhibit DNA fragmentation are relatively high, suggesting that it might need phosphorylation or binding partners to reveal the inhibitory effect under physiological conditions.
Figure 2.
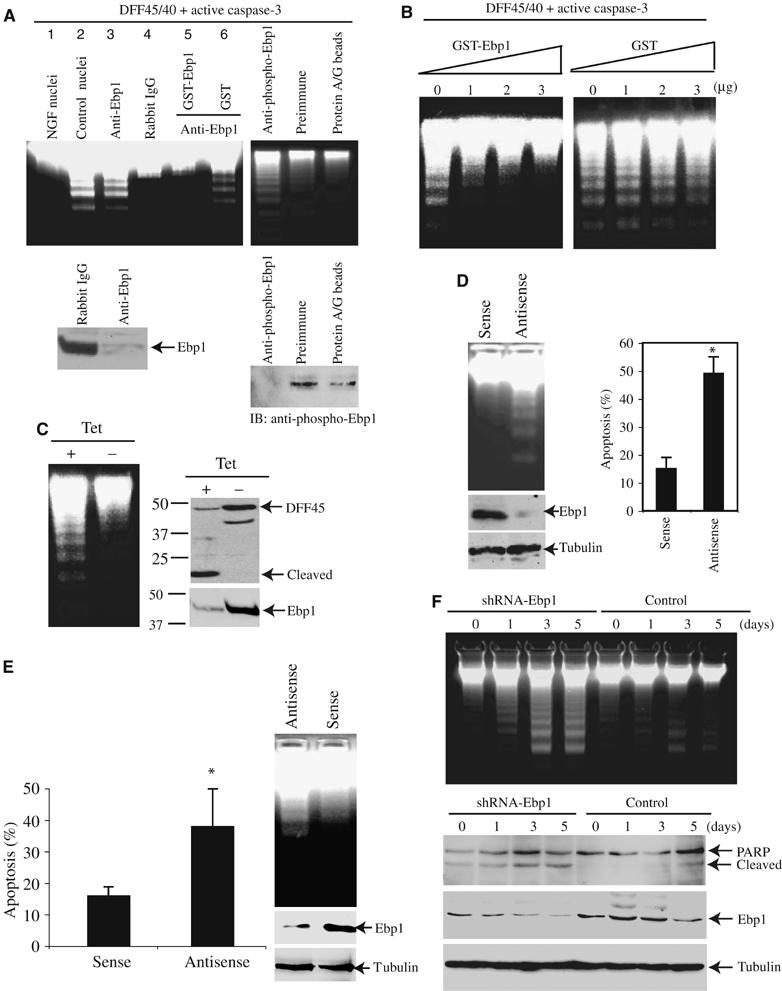
Ebp1 inhibits DNA fragmentation activity of CAD. (A) Immunodepletion of Ebp1 abolishes the inhibitory activity of nuclear extract. The immunodepleted supernatant was analyzed with DNA fragmentation assay. Compared to the control IgG, immunodepletion of Ebp1 diminishes the inhibitory activity (upper left panel, lanes 3 and 4). However, adding back 3 μg of GST-Ebp1 but not GST alone restores the inhibitory activity (upper left panel, lanes 5 and 6). The inhibitory activity is also abrogated by anti-phospho-Ebp1 antibody (upper right panel). Western blotting analysis of Ebp1 and its phosphorylated counterpart in the supernatant from control IgG, anti-Ebp1, anti-phospho-Ebp1 antibodies-depleted nuclear extract (lower panels). (B) Titration of the inhibitory activity of GST-Ebp1. DNA fragmentation reveals that 3 μg of GST-Ebp1 is sufficient to inhibit CAD, however, the same amount of GST alone fails. (C) Overexpression of Ebp1 prevents DNA fragmentation. PC12 cells were stably transfected with inducible form of Ebp1 and cultured in medium with or without tetracycline for 24 h, and followed by 250 nM staurosporine treatment for 24 h. Induction of Ebp1 prevents DNA fragmentation even in the absence of NGF (left panel). Compared with Ebp1-induced cells, DFF45 is markedly cleaved in uninduced cells (middle panel). The expression of induced myc-Ebp1 was verified (right panel). (D) Knocking down of Ebp1 enhances DNA fragmentation in PC12 cells. PC12 cells were treated with penetratin 1-conjugated sense or antisense oligonucleotides for 6 h. Apoptosis was introduced by addition of staurosporine in the presence of NGF. Knockdown of Ebp1 triggers DNA fragmentation compared to sense control. The protein expression of Ebp1 was clearly decreased by antisense oligonucleotide compared to sense. However, as a control, α-tubulin was not changed (left panels). Quantitative analysis of apoptotic rates with DAPI staining in Ebp1-depleted PC12 cells (right panel). (E) Knockdown of Ebp1 enhances apoptosis in primary cultured neurons. Hippocampal neurons were treated with penetratin 1-conjugated sense or antisense oligonucleotides. Apoptosis was introduced by addition of 300 μM glutamate for 16 h. Apoptotic percentage was determined from total 500 cells in different fields, and calculated as means (±s.d.) of three independent experiments (*P<0.005, Student's t-test) (left panel). The fragmented genomic DNA was extracted and analyzed on 2% agarose gel (right panel). (F) Ebp1 is required for preventing apoptosis in NGF-treated PC12 cells. PC12 cells were cultured in medium containing 50 ng/ml NGF for 0, 1, 3 and 5 days, respectively, and infected with control adenovirus or adenovirus expressing shRNA for 16 h, then induced apoptosis by NGF withdrawal for 24 h. Depletion of Ebp1 enhances DNA fragmentation. The DNA cleavage activities increase with the duration of NGF treatment (upper panel). PARP cleavage and Ebp1 knock down were monitored (lower panels).
To investigate whether Ebp1 could prevent DNA fragmentation in intact cells, we generated a stably transfected PC12 cell line with an inducible form of Ebp1. The pronounced DNA fragmentation elicited by staurosporine in uninduced control cells is markedly decreased in Ebp1-overexpressed cells (Figure 2C, left panel). The cleavage of DFF45, a well-characterized marker of apoptosis, couples to the DNA fragmentation activity (Figure 2C, right panel). Depletion of Ebp1 in PC12 cells by Penetratin 1-conjugated antisense oligonucleotide elicits evident fragmented DNA compared to sense control (Figure 2D, left panel), suggesting that Ebp1 plays a critical role in preventing DNA fragmentation in apoptosis. Quantitative analysis reveals that about 50% cells in apoptosis in antisense-treated samples compared with 15% in sense control. The expression level of Ebp1 is substantially decreased by antisense but not sense control (Figure 2D, lower left panels). We also extended these studies into primary culture of hippocampal neurons. We introduced Penetratin-conjugated antisense oligonucleotide into serum-starved neurons for 6 h. The neuronal preparations were treated with glutamate, and apoptosis was evaluated by DNA fragmentation and nuclear morphological alteration. Depleting Ebp1 with antisense oligonucleotide clearly enhances neuronal cell death compared to sense control (Figure 2E).
To further determine whether Ebp1 is required for preventing DNA fragmentation, we incubated PC12 cells with NGF for various times, infected the cells with control adenovirus or adenovirus expressing shRNA of Ebp1, and induced apoptosis through NGF withdrawal. Compared with control adenovirus, knocking down of Ebp1 elicits strong DNA degradation. The amounts of fragmented DNA increase with the duration of NGF treatment, and the strongest DNA fragmentation occurs in cells treated with NGF for 3–5 days (Figure 2F, top panel). PARP cleavage correlates with DNA fragmentation activities. The expression level of Ebp1 is substantially decreased by its shRNA but not control. As a control, α-tubulin expression level is not changed (Figure 2F, lower panels). Collectively, these data support the notion that Ebp1 is required for the antiapoptotic actions.
Ebp1 interacts with active Akt
To determine potential partners for Ebp1 in protection of DNA from fragmentation, we performed co-immunoprecipitation experiments. Immunoblotting analysis of the coprecipitated proteins from NGF-treated nuclear extract reveals that Akt specifically associates with Ebp1 (Figure 3A). To examine whether Akt phosphorylation status plays any role in its binding to Ebp1, we cotransfected Flag-Ebp1 with various wild-type and mutant HA-Akt constructs. Co-immunoprecipitation demonstrates that the strongest interaction occurs between Ebp1 and constitutively active Akt (T308DS473D), a phosphorylation mimetic mutant, indicating that active Akt selectively binds to Ebp1. Interestingly, its kinase activity is not required for the binding (Figure 3B, top panel). EGF treatment of HEK293 cells, cotransfected with GST-Akt and Flag-Ebp1, substantially increases the association, suggesting that Akt phosphorylation promotes its interaction with Ebp1 (Figure 3C, top panel). Control experiments verify that both endogenous and transfected Akt are phosphorylated on S473 (Figure 3C).
Figure 3.
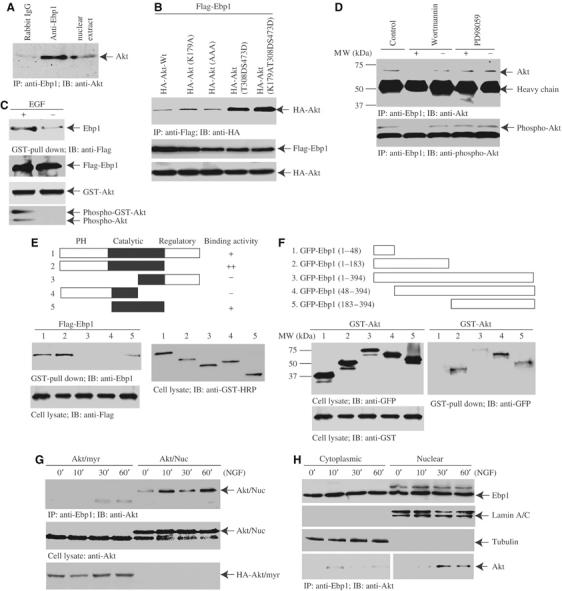
Ebp1 binds active nuclear Akt. (A) Ebp1 binds Akt in the nuclear extract. NGF-treated nuclear extract was incubated with agarose beads-conjugated Ebp1 antibody or control IgG for 2 h at 4°C. The co-precipitated proteins were analyzed with anti-Akt antibody. Nuclear Akt specifically binds Ebp1 but not control IgG. (B) Ebp1 selectively associates with phosphorylated Akt. HA-Akt constructs (kinase-dead K179A; dominant-negative K179AT308AS473A; active but kinase-deficient K179AT308DS473D) were cotransfected with Flag-Ebp1 into HEK293 cells. Co-immunoprecipitation reveals that the strongest interaction occurs to constitutively active Akt (T308DS473D) constructs (top panel). Equal levels of transfected HA- and Flag-constructs were expressed (middle and bottom panels). (C) EGF enhances the interaction between Akt and Ebp1. GST-Akt and Flag-Ebp1 were cotransfected into HEK293 cells, and stimulated with 50 ng/ml EGF for 20 min. The glutathione beads-bound proteins were analyzed with anti-Ebp1 antibody (top panel). Both transfected and endogenous Akt was phosphorylated (bottom panel). (D) Active Akt binds Ebp1. Akt stably transfected PC12 cells were preincubated with or without Wortmannin (20 nM) or PD98059 (20 μM), then treated with NGF for 30 min. The endogenous Ebp1 was immunoprecipitated with anti-Ebp1. Wortmannin but not PD98059 blocks the interaction between Akt and Ebp1 (upper panel). The phosphorylation status of Akt was verified by phospho-Akt-473 antibody (lower panel). (E) Ebp1 binds the N-terminal PH and catalytic domains of Akt. Diagram of Akt constructs is depicted (top panel). GST-Akt full-length and various truncates were cotransfected with Flag-Ebp1 into HEK293 cells. The full-length Akt and catalytic domain faintly interact with Ebp1, whereas C-terminal regulatory domain truncated fragment strongly binds Ebp1 (upper left panel). (F) Both N- and C-termini of Ebp1 bind to Akt. GST-Akt and various GFP-tagged Ebp1 fragments were cotransfected into HEK293 cells, followed by EGF stimulation. Both N-terminal 1–183 and C-terminal 183–394 fragments interact with Akt. Deletion of N-terminal 48 amino acids enhances Ebp1 to bind Akt. (G) Akt binds Ebp1 in an NGF-dependent manner. PC12 cells were infected with adenovirus expressing HA-tagged myristoylated-Akt or nuclear Akt, and treated with NGF for various times. Ebp1 clearly binds to nuclear Akt in a time-dependent manner; however, it just weakly interacts with plasma membrane Akt (upper panel). The expression of both plasma membrane and nuclear Akt was confirmed (lower panels). (H) NGF mediates endogenous Ebp1/Akt association in the cytoplasmic and nuclear fractions. PC12 cells were treated with NGF for various times, and the cytoplasmic and nuclear fractions were prepared. Ebp1 distributes in both fractions. Interestingly, a band with molecular weight at 48 kDa, recognized by Ebp1 antibody, selectively occurs in the nuclear fraction (top panel). The identity and purity of each fraction are confirmed by their specific markers (second and third panels). Co-immunoprecipitation reveals that NGF elicits cytoplasmic Akt and Ebp1 faint interaction at 10 min and decays thereafter. However, nuclear Akt and Ebp1 complex formation peaks at about 30 min (bottom panel).
To investigate further whether Ebp1 binds to Akt in cells, we conducted co-immunoprecipitation assay with Ebp1 antibody in Myc-NLS-Akt stably transfected PC12 cells, treated with NGF in the presence or absence of PI 3-kinase inhibitor Wortmannin or MEK1 inhibitor PD98059. NGF triggers demonstrable association between Ebp1 and Akt, and Wortmannin pretreatment disrupts the interaction. By contrast, PD98059 pretreatment fails to interrupt it (Figure 3D, upper panel). The phosphorylation status of Akt correlates with its interaction with Ebp1 (Figure 3D, lower panel). Equal amount of Ebp1 is immunoprecipitated (data not shown). Mapping experiment with GST-tagged Akt fragments reveals that Ebp1 evidently binds the N-terminal PH and catalytic domains of Akt (Figure 3E). Truncation assay with GFP-tagged Ebp1 fragments shows that both N- and C-termini of Ebp1 bind to Akt, and deletion of the N-terminal 48 residues increases Ebp1 binding to Akt (Figure 3F). Co-immunoprecipitation assay with Ebp1 antibody in PC12 cells, infected with adenovirus expressing Akt-NLS-Myc (Shiraishi et al, 2004) or myristoylated-Akt (Suhara et al, 2002), demonstrates that both plasma membrane and nuclear Akt interacts with Ebp1 in an NGF-dependent manner, but nuclear Akt binds much more Ebp1 than plasma membrane-bound Akt does (Figure 3G, upper panel). The expression of Myc-NLS-Akt and HA-myristoylated-Akt was verified (Figure 3G, lower panels). Subcellular fractionation and co-immunoprecipitation assay demonstrate that endogenous Ebp1 potently binds nuclear translocated Akt after 10–30 min of NGF stimulation, whereas cytoplasmic Ebp1 weakly associates with Akt at about 10 min (Figure 3H). Thus, NGF specifically regulates the interaction between Ebp1 and active Akt in the nucleus of PC12 cells.
Protein kinase C phosphorylates Ebp1
Ebp1 is a target for phosphorylation by PKC in vitro and in vivo, and its C-terminus has been suggested to harbor the phosphorylation site (Lessor and Hamburger, 2001). To determine the PKC phosphorylation residue on Ebp1, we performed an in vitro PKC assay in the presence of [γ-32P]ATP with various purified Ebp1 recombinant proteins (Figure 4A). Both full-length Ebp1 and a C-terminal fragment (292–394) are clearly phosphorylated; by contrast, N-terminal fragments (1–136) and (133–336) are not phosphorylated. Previous in vitro PKC phosphorylation study with Ebp1 peptide suggests that threonine 366 might be a putative phosphorylate site (Lessor and Hamburger, 2001). Surprisingly, Ebp1 T366A mutant is also phosphorylated, indicating that it is not the phosphorylate site by PKC (Figure 4B, upper panel). Kinase assay with a variety of Ebp1 mutants suggests that S360 and S361 residues are responsible for the phosphorylation (Figure 4C, upper panel). Phosphorylation assay with full-length Ebp1 S360A and Ebp1 S361A mutants reveals that serine 360 is the PKC phosphorylation site (Figure 4D, lower panel). Nevertheless, Ebp1 is not significantly phosphorylated by active Akt, suggesting that Ebp1 might not be a physiological substrate of Akt (data not shown).
Figure 4.
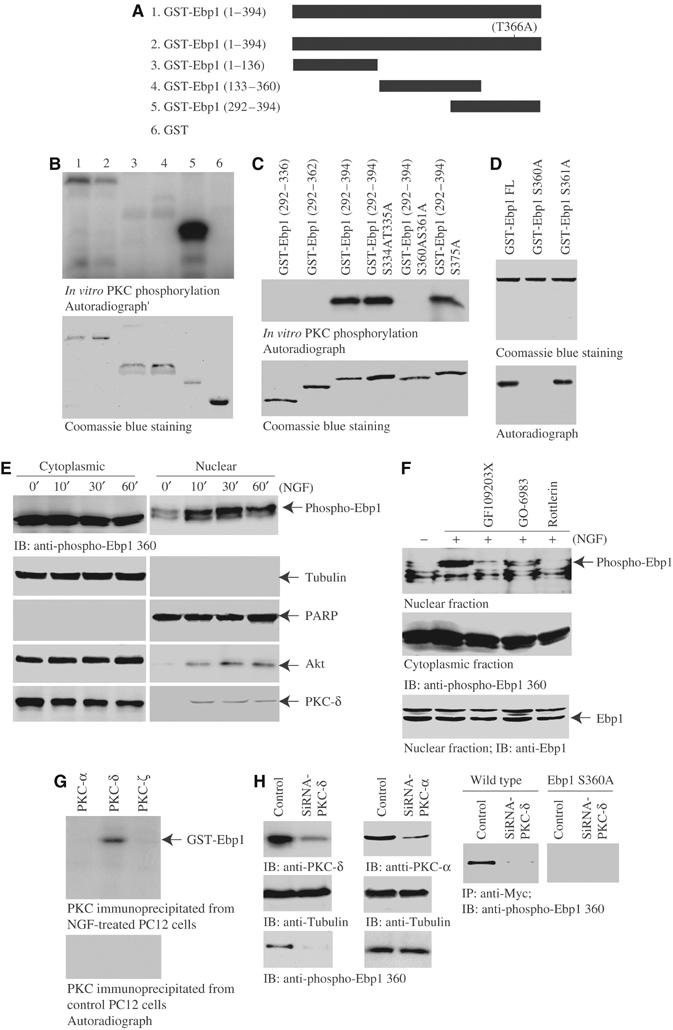
PKC phosphorylates Ebp1 on serine 360. (A) Diagram of various GST-Ebp1 fragments. (B) In vitro PKC assay. In total, 2 μg of purified GST-Ebp1 fusion proteins was incubated with active PKC in the presence of γ-32P-ATP. Both wild-type Ebp1 and T366A mutant were strongly phosphorylated. Interestingly, fragment 292–394 was strongly phosphorylated (upper panel). The identity of purified recombinant proteins were verified by Coomassie blue staining (lower panel). (C) In vitro PKC assay with C-terminal fragments of Ebp1. S360 and 361 might be the phosphorylation sites (upper panel). Coomassie blue staining of GST-Ebp1 fragments (lower panel). (D) In vitro PKC assay with Ebp1 mutants. S360A mutation disrupts PKC phosphorylation (lower panel). Coomassie blue staining of GST-Ebp1 mutants (upper panel). (E) PKC phosphorylates Ebp1 in cells. PC12 cells were treated with NGF for various times, and the cytoplasmic and nuclear fractions were prepared. NGF stimulation does not significantly alter the cytoplasmic Ebp1 phosphorylation. By contrast, NGF markedly provokes nuclear Ebp1 phosphorylation at 30 min. The purity and identity of each fraction was confirmed by immunoblotting with anti-tubulin and anti-PARP antibodies (second and third panels). NGF provokes both Akt and PKC-δ nuclear translocation (fourth and bottom panels). (F) PKC inhibitors block NGF-elicited Ebp1 phosphorylation in the nucleus. PC12 cells were pretreated with various PKC inhibitors for 30 min, followed by NGF for 30 min, and the cytoplasmic and nuclear fractions were prepared. NGF-provoked nuclear Ebp1 phosphorylation was inhibited by 10 μM GF109203X and 60 nM Go6983, and completely abrogated by 6 μM Rotterlin. By contrast, cytoplasmic Ebp1 phosphorylation is not diminished. The total Ebp1 in the nuclear fraction is verified by immunoblotting (bottom panel). (G) In vitro kinase assay with PKC isoform proteins. GST-Ebp1 (1 μg) and [γ-32P]ATP were incubated with various PKC isoforms at 30°C for 1 h. PKC isoforms were immunoprecipitated from NGF-treated or control PC12 cells. Compared to control, NGF-treated PKC-δ but not -α or -ζ selectively phosphorylates Ebp1. (H) Knocking down of PKC-δ abolishes Ebp1 phosphorylation. PC12 cells were transfected with PKC-δ and -α siRNA, respectively. In 48 h, the cells were stimulated with NGF. Both PKC isoforms were substantially removed upon transfection of their siRNA (left top panel). Depletion of PKC-δ but not PKC-α blocks the phosphorylation of nuclear Ebp1 (left bottom panels). Myc-tagged wild-type Ebp1 and S360A stably transfected PC12 cells were transfected with PKC-δ siRNA. In 48 h, the cells were stimulated with NGF, Ebp1 was immunoprecipitated from nuclear fraction with anti-Myc antibody. Potent Ebp1 phosphorylation was revealed in wild-type Myc-Ebp1 stably transfected cells, which was completely diminished in PKC-δ-knocked down cells. By contrast, no phosphorylation was detected in Myc-Ebp1S360A-transfected cells (right panels).
To explore whether Ebp1 S360 can also be phosphorylated by PKC in intact cells, we examined the subcellular fractionated Ebp1 phosphorylation status with its specific anti-phospho-Ebp1 S360 antibody. NGF elicits Ebp1 phosphorylation at 10 min in nuclear fraction and peaks at 30 min. However, cytoplasmic Ebp1 is markedly phosphorylated, and NGF does not significantly increase its phosphorylation level (Figure 4E, top panel). The identity and purity of each fraction were verified by their specific markers (Figure 4E, second and third panels). Both Akt and PKC-δ translocate into the nucleus upon NGF stimulation (Figure 4E, second and third panels). To assess which isoform of PKC contributes to phosphorylate Ebp1 in intact cells, we pretreated PC12 cells with a variety of PKC inhibitors, followed by NGF stimulation for 30 min. NGF provokes Ebp1 phosphorylation in the nuclear fraction, which is inhibited by GF109203X and Go 6983, and blocked by Rottlerin, indicating that PKC-δ is implicated in phosphorylating Ebp1 in the nucleus. By contrast, these inhibitors reveal negligible effect on cytoplasmic Ebp1 phosphorylation (Figure 4F). In vitro kinase assay with various PKC isoforms verifies that PKC-δ from NGF-treated PC12 cells selectively phosphorylates Ebp1, and no activity is detected from control cells (Figure 4G). Knocking down of PKC isoforms with their specific siRNA in control PC12 cells, wild-type Ebp1 or Ebp1S360A stably transfected PC12 cells further confirms that PKC-δ but not PKC-α is responsible for phosphorylating Ebp1 (Figure 4H). Other PKC isoform siRNAs fail to inhibit Ebp1 phosphorylation (data not shown). Collectively, these findings demonstrate that Ebp1 can be phosphorylated by PKC upon NGF stimulation.
Ebp1 phosphorylation by PKC is required for its association with active nuclear Akt and prevents DNA fragmentation
To investigate whether the association between Ebp1 and active Akt is regulated by PKC, we transfected HEK293 cells with HA-Akt (K179A), a kinase-dead mutant, and stimulated the transfected cells with EGF for 10 min (Figure 5A). In the absence of EGF stimulation, clear association occurs between Akt and GST-Ebp1 S360D, while no interaction is detected between GST-Ebp1 wild type or S360A and Akt. By contrast, EGF treatment provokes evident binding by Akt to both wild-type Ebp1 and S360D. Notably, Ebp1 S360A displays weak interaction with active Akt, demonstrating that Ebp1 phosphorylation by PKC plays a critical role in mediating its association with active Akt. We observed the similar binding activity for wild-type Akt as well (data not shown), suggesting that Akt kinase activity is not required for its interaction with Ebp1. Immunofluorescent staining on NGF-treated PC12 cells reveals the Ebp1 resides in both the cytoplasm and the nucleus, and colocalizes with the nuclear Akt. In transfected HEK293 cells, all GFP-Ebp1 proteins reside in the cytoplasm. Interestingly, wild type and S360A specifically occur in the nucleolus, whereas S360D evenly distributes in the nucleus (Figure 5B). Moreover, immunofluorescent staining and subcellular fractionation assays show that expression of mutant versions of Ebp1 (either S360D or S360A) do not alter Akt nuclear translocation. On the other hand, expression of Myc-NLS-Akt CA or KD does not enhance Ebp1 nuclear translocation either (data not shown), suggesting that nuclear translocation of either Ebp1 or Akt is not impacted by each other.
Figure 5.
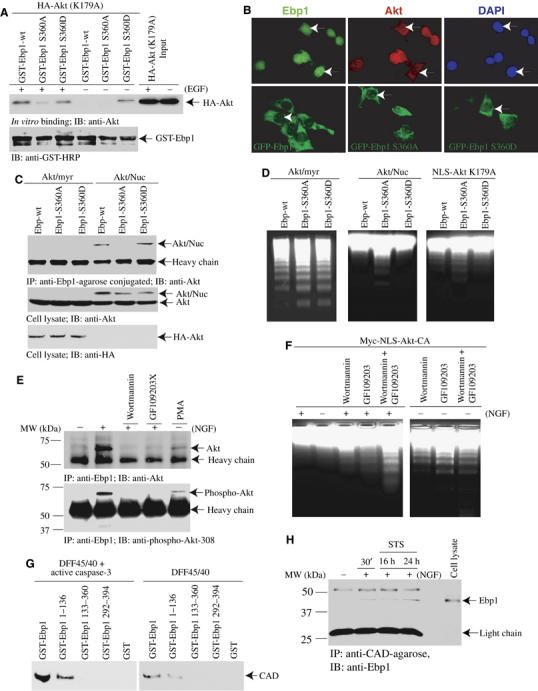
PKC and PI 3-kinase cooperatively mediate Ebp1 and nuclear Akt interaction. (A) In vitro binding assay. Glutathione beads-conjugated GST-Ebp1 wt, S360A and S360D were incubated with lysate of 293 cells, transfected with HA-Akt (K179A) and stimulated with or without EGF. In the absence of EGF, S360D selectively binds to Akt; in contrast, neither wt nor S360A binds. After EGF treatment, both wt and S360D interact with Akt; however, S360A does not associate with Akt. (B) Ebp1 colocalizes with Akt in the nucleus. PC12 cells were treated with NGF for 45 min, then stained with anti-Ebp1 and anti-Akt antibodies, respectively. Both Ebp1 and Akt colocalize in the nucleus (white arrows, upper panel). HEK293 cells were transfected with GFP-Ebp1 constructs. All constructs reside in the cytoplasm. However, both GFP-Ebp1 wild-type and S360A localize in the nucleolus, whereas S360D uniformly distribute in the nucleus (white arrows, lower panel). (C) Nuclear but not plasma membrane Akt selectively binds to Ebp1 wild type and S360D mutant in PC12 cells. Ebp1 cell lines were infected with adenovirus expressing myristoylated-Akt or nuclear Akt, respectively. Nuclear Akt-NLS binds to S360D mutant stronger than Ebp1 wild type; however, it does not interact with S360A mutant. By contrast, plasma membrane HA-tagged Akt-myr displays negligible interaction with Ebp1 (upper panel). The expression of infected Akt constructs in PC12 cells was verified (lower panels). (D) Nuclear but not plasma membrane Akt prevents DNA fragmentation. Ebp1 cell lines were infected with Akt-myr or nuclear Akt adenovirus, respectively. The nuclei were isolated and analyzed in the active apoptosome. DNA fragmentation occurs in the nuclei from Akt/myr-infected cells regardless of Ebp1 wild type or mutants; in contrast, nuclear Akt-infected wild-type Ebp1 and S360D strongly antagonize DNA fragmentation, while S360A fails, and this effect seems independent of Akt kinase activity. The experiments were repeated three times, and similar DNA fragmentation patterns were observed. (E) Inhibition of PI 3-kinase or PKC abolishes Akt and Ebp1 interaction. Myc-NLS-Akt-CA stably transfected PC12 cells were pretreated with wortmannin (20 nM), GF109203X (10 μM) or PMA (10 μM) for 30 min, respectively, then stimulated with NGF. Compared to control, NGF stimulation yields Akt/Ebp1 interaction, which is disrupted by PI 3-kinase or PKC inhibitor. Interestingly, PMA also elicits the association (upper panel). The precipitated Akt phosphorylation status correlates with its binding effects (lower panel). (F) PI 3-kinase and PKC signaling cooperatively regulate NGF's antiapoptotic effect. Myc-NLS-Akt-CA cells were pretreated with 20 nM wortmannin, 10 μM GF109203X or wortmannin+GF109203X, then incubated with or without NGF. Apoptosis was initiated by 250 nM staurosporine for 24 h. DNA fragmentation reveals when both inhibitors were employed, but much lesser extent DNA fragmentation occurs while wortmannin or GF109203X alone was used. The faint DNA cleavage in control condition was completely inhibited by NGF. (G) Ebp1 and its N-terminal 1–136 truncate strongly bind to active CAD. Various purified GST-Ebp1 fragments were incubated with His-DFF45/40, pretreated with or without active caspase-3. The proteins associated with glutathione beads were analyzed with anti-CAD antibody. (H) NGF provokes Ebp1 to associate with CAD. PC12 cells were pretreated with NGF, followed by staurosporine incubation for 16 or 24 h. CAD was immunoprecipitated with agarose-conjugated beads. Ebp1 binds to CAD upon NGF treatment. Staurosporine does not alter Ebp1 to bind active CAD.
We also extend this study into Ebp1 stably transfected PC12 cells. Myc-Ebp1 wild-type, S360A and S360D cells were infected with Myristoylated-Akt or Akt-NLS-Myc adenovirus, respectively. Co-immunoprecipitation assay reveals that Akt-NLS-Myc tightly binds to Ebp1 S360D, followed by wild-type Ebp1. By contrast, no interaction is observed with Ebp1 S360A, supporting that the interaction between Ebp1 and nuclear active Akt is PKC dependent. However, the association between Ebp1 and plasma membrane Akt-myr is negligible (Figure 5C), consistent with NGF-provoked interaction between Ebp1 and nuclear Akt, but not plasma membrane located Akt-myr (Figure 3). DNA fragmentation assay with the isolated nuclei shows DNA cleavage in all the samples from myristoylated-Akt-expressing cells. By contrast, DNA fragmentation is substantially inhibited in Ebp1 wild-type and S360D cells upon nuclear Akt infection. However, DNA cleavage is observed in Ebp1 S360A cells, and this effect seems independent of Akt kinase activity (Figure 5D). Thus, these findings suggest that nuclear but not plasma membrane Akt is responsible for preventing DNA fragmentation. Further, PKC phosphorylation regulates assembly of the complex that is critical for this protective effect.
To further investigate whether the interaction between Ebp1 and Akt is regulated by PKC, we employed Myc-NLS-Akt-CA stably transfected PC12 cells, in which Myc-Akt is specifically expressed in the nucleus (Ahn et al, 2004). NGF treatment elicits Ebp1 binding to Akt (lane 2), while PI 3-kinase inhibitor Wortmannin or PKC inhibitor GF109203X pretreatment diminishes the binding; by contrast, PMA stimulates Ebp1 binding to Akt even in the absence of NGF, suggesting that both PI 3-kinase and PKC signalings coordinately contribute to mediate Akt and Ebp1 association (Figure 5E, upper panel). The co-precipitated Akt is also phosphorylated (lower panel). Collectively, these experiments demonstrate that the association between active nuclear Akt and Ebp1 requires PKC-dependent phosphorylation of Ebp1. Apoptosis analysis with staurosporine demonstrates that DNA degradation is abolished after NGF treatment. Notably, either the PI 3-kinase inhibitor wortmannin or the PKC inhibitor GF109203X pretreatment diminishes NGF-provoked protective activity. The strongest DNA cleavage occurs when both inhibitors are employed together. Nevertheless, in the absence of NGF, pharmacological agents trigger DNA fragmentation much more potently than the counterpart with NGF (Figure 5F). Thus, these findings support that PI 3-kinase and PKC activities are required for formation of a nuclear Akt/Ebp1 complex integral to suppression of DNA fragmentation in the nucleus.
In vitro binding assay with various purified GST-Ebp1 proteins reveals that full-length Ebp1 and an N-terminal fragment (1–136) but not C-terminal truncates strongly bind to active CAD, released from ICAD/DFF45 by caspase-3 cleavage. Nevertheless, weak association is also observed between Ebp1 and the inactive His-DFF45/40 complex (Figure 5G), suggesting that Ebp1 through its N-terminus directly binds and inhibits active CAD. To assess whether the complex of Ebp1/CAD exist in intact cells, we treated PC12 cells with NGF and performed a co-immunoprecipitation assay with CAD antibody-conjugated beads. NGF treatment provokes Ebp1 to bind CAD. Staurosporine treatment does not alter their association, suggesting that CAD is not required to release from its inhibitory ICAD (Figure 5H). This finding is consistent with the results that Ebp1 binding to CAD does not require ICAD cleavage (Figure 5G).
Nuclear Akt/Ebp1 complex inhibits DNA fragmentation activity of CAD
To explore whether active Akt regulates Ebp1's inhibitory activity against active CAD in the cell-free apoptosome, we preincubated purified GST-Ebp1 full-length and various truncates with phosphorylated and unphosphorylated Akt, respectively. DNA fragmentation assay reveals that both full-length and N-terminal fragment (1–136) possess obvious inhibitory activity in the presence of active but not inactive Akt; by contrast, C-terminal fragments or GST alone display no activity regardless of the phosphorylation status of Akt (Figure 6A, left panels). Phosphorylated but kinase-dead Akt reveals same activity as active wild-type Akt does (data not shown), underscoring that Akt kinase activity is not required for enhancing Ebp1's antiapoptotic activity. Dose–response experiment demonstrates that active Akt alone has no activity, for which both phosphorylated Akt and Ebp1 (0.75 μg of each) are required (Figure 6B). Thus, phosphorylated Akt substantially enhances Ebp1's inhibitory effect through their interaction, and this action is independent of its kinase activity. In the presence of active Akt, Ebp1 S360D suppresses CAD activity with the strongest effect, followed by wild-type Ebp1. By contrast, Ebp1 S360A fails to block DNA fragmentation, suggesting that Ebp1 phosphorylation by PKC is essential for its inhibitory activity (Figure 6C).
Figure 6.
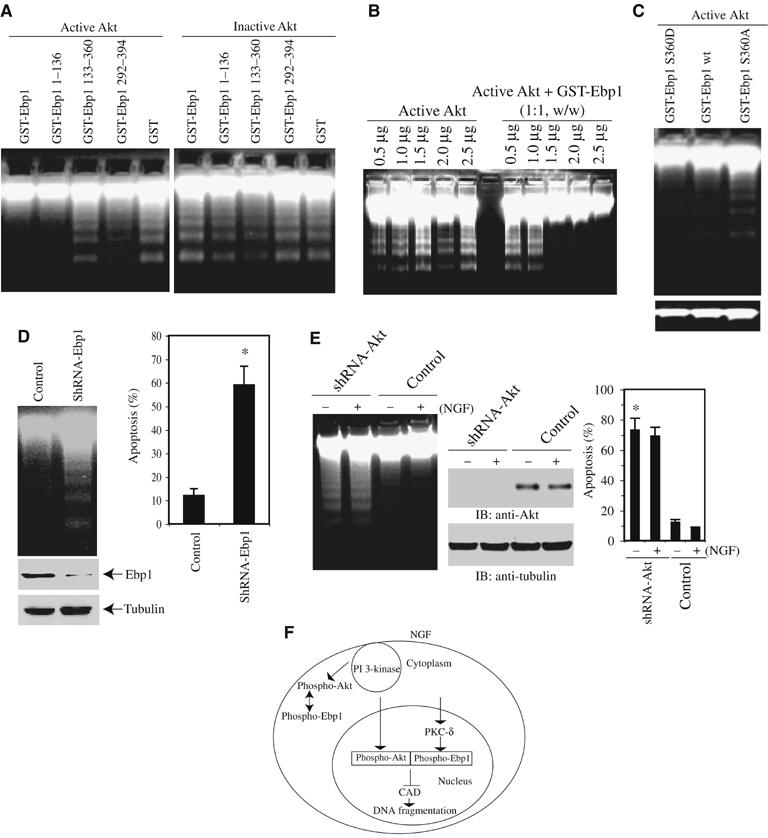
Akt/Ebp1 complex inhibits DNA fragmentation activity of CAD. (A) Akt/Ebp1 complex potently inhibits active CAD. Various GST-recombinant proteins (1 μg) were treated with purified active Akt or inactive Akt for 30 min. The mixture was introduced to the active CAD in the cell-free apoptosome. Full-length Ebp1 and its N-terminal 1–136 truncate inhibit DNA fragmentation elicited by CAD (top panel) by contrast, none of Ebp1 proteins is able to prevent DNA cleavage in the presence of inactive Akt. (B) Active Akt is required for the inhibitory effect of Ebp1. 1.5 μg of 1:1 ratio of active Akt and Ebp1 mixture evidently suppresses DNA fragmentation, and Akt alone fails even at higher concentration. (C) Ebp1 S360D displays inhibitory effect on DNA fragmentation. GST-recombinant Ebp1 proteins (0.5 μg) were incubated with same amount of active Akt. GST-Ebp1 S360D demonstrates the strongest inhibitory activity, followed by wild-type GST-Ebp1. In contrast, GST-Ebp1 S360A fails to prevent DNA fragmentation (upper panel). Equal amount of extracted DNA was loaded in each lane (lower panel). (D) Knocking down of Ebp1 enhances DNA fragmentation. Myc-NLS-Akt-CA cells were infected with adenovirus expressing shRNA of Ebp1, and treated with staurosporine for 24 h. Ebp1 depletion yields substantial DNA cleavage compared with control (upper left panel). Quantitative analysis of apoptotic rates in Ebp1-depleted cells (right panel). (E) Depletion of Akt abolishes the antiapoptotic effect of Ebp1. Ebp1 stably transfected cells were infected with control adenovirus or adenovirus expressing shRNA-Akt for 36 h, followed by NGF stimulation. The isolated nuclei were analyzed in active CAD apoptotic solution (left panel). Quantitative analysis of apoptotic rates in Akt-depleted cells (right panel). Apoptotic percentage was determined from total 500 cells in different fields, and calculated as means (±s.d.) of three independent experiments (*P<0.005, Student's t-test). Morphological changes in the nuclear chromatin of cells undergoing apoptosis were detected by staining with 4,6-diamidino-2-phenylindole (DAPI). Only the nuclei possessing multiple condensed and aggregated chromatin were counted. (F) A model for NGF-mediated antiapoptotic action by nuclear Akt and PKC-δ-phosphorylated Ebp1 complex.
To establish whether the nuclear Akt/Ebp1 complex is indispensable for mediating the antiapoptotic effect of NGF in intact cells, we depleted Ebp1 with adenovirus expressing its shRNA. Removal of Ebp1 substantially enhances staurosporine-triggered DNA fragmentation in myc-NLS-Akt-CA PC12 cells, demonstrating that Ebp1 plays a critical role in mediating nuclear Akt's antiapoptotic action. Endogenous Ebp1 levels have been decreased by shRNA-Ebp1, whereas α-tubulin control remains the same (Figure 6D, left panels). Quantitative analysis of apoptosis couples to DNA fragmentation activity (Figure 6D, right panel). Knocking down of Akt with adenovirus expressing shRNA of Akt increases DNA fragmentation in the nuclei isolated from Ebp1 stably transfected cells regardless of NGF treatment. In contrast, no fragmented DNA was detected in NGF-treated control adenovirus-infected sample (Figure 6E, left panel). Quantitative analysis of apoptosis correlates with DNA fragmentation activity. Endogenous Akt is completely eliminated by shRNA-Akt, whereas α-tubulin control remains the same (Figure 6E, middle and right panels). Thus, these observations suggest that the active Akt/Ebp1 complex inhibits DNA fragmentation activity of CAD, and the nuclear active Akt/Ebp1 complex is essential for mediating the antiapoptotic action of NGF. In summary, these experiments demonstrate that nuclear Akt mediates the antiapoptotic actions of NGF by antagonizing DNA fragmentation activity of CAD through active nuclear Akt/Ebp1 complex.
Discussion
Our discovery that active nuclear Akt/Ebp1 complex mediates the antiapoptotic actions of NGF demonstrates that nuclear Akt contributes to suppression of apoptosis in the final stage. Surprisingly, this effect appears to be independent of its kinase activity. Biochemical purification experiments and titration assays with Ebp1 indicate that Ebp1 directly inhibits active CAD. Further, active Akt enhances the inhibitory activity of Ebp1 against DNA fragmentation through a nuclear active Akt/Ebp1 complex. Moreover, active Akt interacts with Ebp1 in PC12 cells upon NGF treatment, which is coordinately regulated by both PI 3-kinase and PKC signal cascades. This finding is consistent with previous result that the simultaneous inhibition of both PKC and PI 3-kinase induces the apoptotic death of NGF-maintained sympathetic neurons (Pierchala et al, 2004).
NGF promotes survival of serum-deprived PC12 cells and sympathetic neurons through a proposed protein phosphorylation-driven pathway (Batistatou and Greene, 1991). Mounting evidence shows that nuclear Akt is implicated in antagonizing apoptosis by phosphorylating transcription factors in the nucleus. However, Ebp1 does not contain the well-conserved Akt phosphorylation motif. Akt kinase assay reveals that phosphorylation on purified GST-Ebp1 protein is negligible compared to the positive control GSK3 (data not shown), suggesting that Ebp1 might not be a physiological substrate for Akt. In addition, our binding assay reveals that Akt kinase activity is not necessary for its association with Ebp1 (Figures 3B and 5A). Furthermore, Akt kinase activity seems unnecessary for upregulating the antiapoptotic action of Ebp1, because wild-type NLS-Akt and kinase-dead K179A substantially elevated both wild-type and S360D, but not S360A Ebp1's antiapoptotic effects (Figure 5D), underscoring the critical nature of the Akt/Ebp1 interaction for antagonizing DNA fragmentation. However, we cannot rule out other possibilities. For example, overexpressed Ebp1 represses numerous transcription factors, which might regulate apoptotic gene expression, leading to decrease of apoptosis (Zhang and Hamburger, 2005). Kinase-deficient nuclear Akt could stimulate the survival signal through suppressing proapoptotic gene expression or enhancing antiapoptotic gene transcription through Ebp1 or other unknown factors. Recently, it has been reported that nuclear targeting of Akt enhances its kinase activity and promotes survival of cardiomyocytes (Shiraishi et al, 2004). These results demonstrate that nuclear Akt selectively initiates antiapoptotic machinery to prevent programmed cell death independent of cytoplasmic events. The identification of a nuclear Ebp1/active Akt complex provides a molecular mechanism accounting for this effect.
Overexpression of Ebp1 not only blocks DNA fragmentation but also suppresses caspase-3 substrate ICAD apoptotic degradation (Figure 2C). Moreover, knocking down of Ebp1 stimulate PARP cleavage (Figure 2F). These findings indicate that Ebp1 is implicated in inhibiting apoptosis at multiple levels. Probably, the Ebp1/phospho-Akt complex suffocates the upstream apoptotic machinery like caspase-3 activation as well. The purified recombinant GST-Ebp1 protein alone is also able to antagonize DNA fragmentation in vitro (Figure 2B), however, it requires a large amount of Ebp1. PKC phosphorylation enhances its inhibitory effect. In addition, in the presence of purified phosphorylated Akt, the necessary amount of Ebp1 to inhibit DNA fragmentation is substantially decreased (Figure 6B and C). These observations suggest that Ebp1 might require both phosphorylation by PKC and nuclear Akt to antagonize DNA fragmentation under physiological conditions.
Ebp1 is basally phosphorylated by PKC in AU565 breast cancer cells and this phosphorylation is enhanced by HEG treatment (Lessor and Hamburger, 2001). Our data show that Ebp1 can be phosphorylated by PKC on serine 360 in vitro and in cells (Figure 4). However, it is the N-terminus of 1–136 amino acids of Ebp1 that directly binds to CAD, preventing DNA fragmentation. Therefore, the phosphorylation in the C-terminus of Ebp1 might alter its conformation, leading to interaction with both active Akt and CAD through the N-terminus of Ebp1. Employing site-directed mutagenesis, we demonstrate that PKC phosphorylation on Ebp1 is required for its association with active nuclear Akt and prevention of DNA fragmentation, supporting the concept that PI 3-kinase and PKC signaling cascades cooperatively mediate the antiapoptotic action of NGF in the nucleus (Figure 6F).
Recently, we showed that nuclei from NGF-treated PC12 cells are resistant to DNA fragmentation initiated by the activated cell-free apoptosome, and nuclear PI 3-kinase and its upstream regulator PIKE mediate the antiapoptotic activity of NGF in the isolated nuclei (Ahn et al, 2004). Here, we show that Ebp1, a nucleolar protein, directly interacts with nuclear translocated Akt in a PKC-dependent manner. NGF treatment provokes formation of an Ebp1/Akt complex in the nucleus of PC12 cells. PI 3-kinase inhibitor but not MEK1 inhibitor abolished the interaction between Ebp1 and Akt, concomitantly, Akt was phosphorylated in the immunocomplex precipitated by anti-Ebp1 antibody, indicating that Akt phosphorylation is required for their interaction (Figure 3D). Nevertheless, we did not co-purify Ebp1 and Akt in a same fraction (Figure 1). This complex might be disrupted during multiple-step fractionation. Our co-immunoprecipitation with NGF-treated nuclear extract demonstrates that this complex exists in the nucleus of PC12 cells. Most recently, we show that nuclear Akt phosphorylates Acinus, a caspase-3-cleaved nuclear protein triggering chromatin condensation, and prevents its degradation by caspase-3, inhibiting chromatin condensation (Hu et al, 2005). Presumably, NGF promotes cell survival by simultaneously activating the parallel protective pathways including B23 (Ahn et al, 2005). Our finding that Ebp1 selectively associates with active Akt resulting in inhibition of DFF40/CAD and prevention of apoptosis might disclose a novel role of Ebp1 in human cancers and neuronal survival.
Materials and methods
Cells and reagents
PC12 cells were maintained in medium A (DMEM with 10% fetal bovine serum (FBS), 5% horse serum and 100 U of penicillin–streptomycin) at 37°C with 5% CO2 atmosphere in a humidified incubator. The PIKE and Myc-NLS-Akt stably transfected PC12 cells (Tet-off cell line) were cultured in medium B (DMEM, 10% horse serum, 5% FBS, 100 μg/ml G418, 100 μg/ml hygromycin B, 2 μg/ml tetracycline and 100 U of penicillin–streptomycin). NGF was from Roche. Penetratin 1 was from Oncor, Inc. Phospho-Akt-473 or 308, Akt and lamin A/C antibodies were from Cell Signaling. Anti-Ebp1, anti-CAD, anti-DFF45 antibodies and active (phosphorylated) and inactive (unphosphorylated) Akt proteins were from Upstate Biotechnology, Inc. All the chemicals not included above were purchased from Sigma. GFP-Ebp1 constructs are kindly supplied by Dr Giulio Draetta (European Institute of Oncology, Milan, Italy). The siRNA sequences for PKC-α: TCCTTGTCCAAGGAGGCTG; PKC-δ: CCATGAGTTTATCGCCACC and PKC-ζ: GTGAGAGACATGTGTCGTC.
Purification of Ebp1 from PC12 cell nuclear extract
All the purification steps were carried out at 4°C unless otherwise indicated. Mono Q and S columns were carried out using an automatic fast protein liquid chromatography (FPLC) station (Pharmacia).
Q-Sepharose chromatography. The nuclear extract from PC12 cells were applied to a column (5 × 16 cm) of Q-Sepharose equilibrated with buffer A. After washing with six bed volumes of the same buffer, the column was eluted using step gradient with buffer A containing 20 mm, 200 mM, 400 mM, respectively. The flow rate was kept at 50 ml/h.
CM-Sepharose chromatography. The active fractions from Q-Sepharose column were combined, desalted, and applied to a column (2.5 × 14 cm) of CM-Sepharose.
Heparin Sepharose chromatography. The active pool from previous step was applied to a Heparin Sepharose column (1.5 × 8 cm).
Mono S 5/5 chromatography. The active fractions from Heparin column were pooled, and desalted, and loaded onto a Mono S 5/5. The proteins were then eluted using linear gradient made with increasing concentration of NaCl from 50 mM to 1 M.
Subcellular fractionation and co-immunoprecipitation
The cytosolic and nuclear fractions from transfected or infected cells were prepared according to the manufacturer's protocol (Pierce, Nuclear and Cytoplasmic Extraction Reagent). The cytosolic and nuclear Ebp1 from NGF-treated PC12 cells was immunoprecipitated by anti-Ebp1 antibody. The co-precipitated proteins were analyzed with anti-Akt antibody.
Generation and characterization of phosph-Ebp1 S360 anti-sera
Rabbit polyclonal anti-phospho-Ebp1 S360 antibody was generated by Covance Research Products Inc. The peptide LQS-PO4-SASRKTQKKK was used as antigens to immunize rabbits. The antisera were purified by nonphosphorylated peptide crosslinked affinity chromatography. The antisera were eluted with 100 mM glycine (pH 2.5), and dialyzed against 40% glycerol/PBS.
DNA fragmentation assay
DNA fragmentation assays were carried out as described (Liu et al, 1997). See also Supplementary methods. The total amount of extracted DNA from each sample was normalized, and equal amount of DNA was loaded in each lane. A representative of DNA fragmentation assay from multiple repetitions is presented. Similar results were obtained each time.
Supplementary Material
Supplementary methods
Acknowledgments
This work was supported by grants from National Institute of Health (RO1, NS045627) and American Cancer Society (#RSG-04-077-01) to K Ye (RO1 CA76047) to A Hamburger. We are indebted to Drs Mark Sussman and Kenneth Walsh for Akt adenovirus.
References
- Ahn JY, Liu X, Cheng D, Peng J, Chan PK, Wade PA, Ye K (2005) Nucleophosmin/B23, a nuclear PI(3,4,5)P(3) receptor, mediates the antiapoptotic actions of NGF by inhibiting CAD. Mol Cell 18: 435–445 [DOI] [PubMed] [Google Scholar]
- Ahn JY, Rong R, Liu X, Ye K (2004) PIKE/nuclear PI 3-kinase signaling mediates the antiapoptotic actions of NGF in the nucleus. EMBO J 23: 3995–4006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batistatou A, Greene LA (1991) Aurintricarboxylic acid rescues PC12 cells and sympathetic neurons from cell death caused by nerve growth factor deprivation: correlation with suppression of endonuclease activity. J Cell Biol 115: 461–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgatti P, Martelli AM, Tabellini G, Bellacosa A, Capitani S, Neri LM (2003) Threonine 308 phosphorylated form of Akt translocates to the nucleus of PC12 cells under nerve growth factor stimulation and associates with the nuclear matrix protein nucleolin. J Cell Physiol 196: 79–88 [DOI] [PubMed] [Google Scholar]
- Brazil DP, Park J, Hemmings BA (2002) PKB binding proteins. Getting in on the Akt. Cell 111: 293–303 [DOI] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME (1999) Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96: 857–868 [DOI] [PubMed] [Google Scholar]
- Brunet A, Datta SR, Greenberg ME (2001) Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol 11: 297–305 [DOI] [PubMed] [Google Scholar]
- Hu Y, Yao J, Liu Z, Liu X, Fu H, Ye K (2005) Akt phosphorylates acinus and inhibits its proteolytic cleavage, preventing chromatin condensation. EMBO J 24: 3543–3554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DR, Miller FD (2000) Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol 10: 381–391 [DOI] [PubMed] [Google Scholar]
- Kikani CK, Dong LQ, Liu F (2005) ‘New'-clear functions of PDK1: beyond a master kinase? J Cell Biochem 96: 1157–1162 [DOI] [PubMed] [Google Scholar]
- Lessor TJ, Hamburger AW (2001) Regulation of the ErbB3 binding protein Ebp1 by protein kinase C. Mol Cell Endocrinol 175: 185–191 [DOI] [PubMed] [Google Scholar]
- Lim MA, Kikani CK, Wick MJ, Dong LQ (2003) Nuclear translocation of 3′-phosphoinositide-dependent protein kinase 1 (PDK-1): a potential regulatory mechanism for PDK-1 function. Proc Natl Acad Sci USA 100: 14006–14011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zou H, Slaughter C, Wang X (1997) DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell 89: 175–184 [DOI] [PubMed] [Google Scholar]
- Philpott KL, McCarthy MJ, Klippel A, Rubin LL (1997) Activated phosphatidylinositol 3-kinase and Akt kinase promote survival of superior cervical neurons. J Cell Biol 139: 809–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierchala BA, Ahrens RC, Paden AJ, Johnson EM Jr (2004) Nerve growth factor promotes the survival of sympathetic neurons through the cooperative function of the protein kinase C and phosphatidylinositol 3-kinase pathways. J Biol Chem 279: 27986–27993 [DOI] [PubMed] [Google Scholar]
- Radomski N, Jost E (1995) Molecular cloning of a murine cDNA encoding a novel protein, p38-2G4, which varies with the cell cycle. Exp Cell Res 220: 434–445 [DOI] [PubMed] [Google Scholar]
- Shiraishi I, Melendez J, Ahn Y, Skavdahl M, Murphy E, Welch S, Schaefer E, Walsh K, Rosenzweig A, Torella D, Nurzynska D, Kajstura J, Leri A, Anversa P, Sussman MA (2004) Nuclear targeting of Akt enhances kinase activity and survival of cardiomyocytes. Circ Res 94: 884–891 [DOI] [PubMed] [Google Scholar]
- Squatrito M, Mancino M, Donzelli M, Areces LB, Draetta GF (2004) EBP1 is a nucleolar growth-regulating protein that is part of pre-ribosomal ribonucleoprotein complexes. Oncogene 23: 4454–4465 [DOI] [PubMed] [Google Scholar]
- Suhara T, Kim HS, Kirshenbaum LA, Walsh K (2002) Suppression of Akt signaling induces Fas ligand expression: involvement of caspase and Jun kinase activation in Akt-mediated Fas ligand regulation. Mol Cell Biol 22: 680–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui-Pierchala BA, Putcha GV, Johnson EM Jr (2000) Phosphatidylinositol 3-kinase is required for the trophic, but not the survival-promoting, actions of NGF on sympathetic neurons. J Neurosci 20: 7228–7237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X, Cheng A, Lessor T, Zhang Y, Hamburger AW (2001) Ebp1, an ErbB-3 binding protein, interacts with Rb and affects Rb transcriptional regulation. J Cell Physiol 187: 209–217 [DOI] [PubMed] [Google Scholar]
- Yoo JY, Wang XW, Rishi AK, Lessor T, Xia XM, Gustafson TA, Hamburger AW (2000) Interaction of the PA2G4 (EBP1) protein with ErbB-3 and regulation of this binding by heregulin. Br J Cancer 82: 683–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Hamburger AW (2005) Specificity and heregulin regulation of Ebp1 (ErbB3 binding protein 1) mediated repression of androgen receptor signalling. Br J Cancer 92: 140–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Woodford N, Xia X, Hamburger AW (2003) Repression of E2F1-mediated transcription by the ErbB3 binding protein Ebp1 involves histone deacetylases. Nucleic Acids Res 31: 2168–2177 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary methods


