INTRODUCTION
Chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) is a debilitating condition diagnosed in the presence of chronic pelvic pain and lower urinary tract symptoms.1 As prevalent as multiple sclerosis, CP/CPPS is the most common,2 yet most poorly understood “prostatitis syndrome”. With a quality of life similar to that following a myocardial infarction, angina, or Crohn’s disease,3 CP/CPPS is truly a devastating disease.
The diagnosis and treatment of CP/CPPS have remained elusive. The search for an “objective” diagnostic and therapeutic marker has proven futile. The lack of a diagnostic and therapeutic “handle” on CP/CPPS continues to fuel frustration in both the urologic and patient community. A new perception of CP/CPPS came following the 1995 NIH/NIDDK workshop which emphasized the importance of pain as the hallmark of CP/CPPS and questioned the role of the prostate in producing symptoms.4
The aim of the present review is to provide an evidence-based evaluation of the treatment of CP/CPPS.
EVIDENCE ACQUISITION AND SYNTHESIS
Search Strategy
An optimally sensitive strategy was developed for the purposes of the present review. The following databases were searched: MEDLINE (1966–2005), PubMed (1966–2005), EMBASE (1988–2005), CINAHL (1982–200), Healthstar (1975–2000), Current Contents (2000–2005), Web of Science (1980–2005), PsychInfo (1967–2005), Science Citation Indexes (1996–2005), and Cochrane Collaboration Reviews (1993–2005). The exploded Medical Subject Heading prostatitis and phrases chronic nonbacterial prostatitis, chronic abacterial prostatitis, chronic pelvic pain syndrome, and prostatodynia were combined with truncated keywords that described the type of publication, such as random, double–blind, random allocation, placebo, clinical trial, and comparative study and was limited to studies in humans. Additional studies were identified through a manual search of the bibliographies of retrieved articles, recent reviews, monographs, and proceedings from NIH/NIDDK meetings.
Inclusion Criteria
Clinical trials were included if they met all of the following six inclusion criteria (adapted from Jailwala et al5): study population defined and classified on the basis of the NIH-CPCRN criteria; administration of a pharmacologic intervention to more than 10 CP/CPPS patients for at least 2 weeks; inclusion of a control group that received placebo, sham intervention, or active pharmacologic or device therapy; use of a randomized, double–blind, parallel–group or crossover design study design; and of validated outcome measures of global status (NIH-CPSI) or individual symptoms (domain scores of the NIH-CPSI). For the purposes of the present analysis, only those studies which had successfully undergone rigorous peer review (i.e., published or in press in peer-reviewed journals), were included.
Data Extraction
The study characteristics, patient demographic information, enrollment criteria, therapy allocation, adverse effects, outcomes, and reasons for dropout were extracted independently by two reviewers. The main outcome was the efficacy of treatment for CP/CPPS compared with placebo, sham, or active control in improving NIH-CPSI total scores. Secondary outcomes were changes in the pain, urinary, and quality of life domains of the NIH-CPSI.
Qualitative Assessment
Using published studies for evaluating the quality of randomized, controlled trials in CFS,6 fibromyalgia,7 and IBS,8 we decided a priori on five criteria with which to assess the methodologic quality of each trial: clear inclusion criteria based on the NIH-CPCRN definition of CP/CPPS; baseline similarity of treatment and control group for symptom severity in parallel trials or absence of period or sequence effects in crossover trials; similar adherence in treatment and control groups; patient-rated validated outcomes (NIH-CPSI); and rigorous analysis, preferably by the intention–to–treat principle. Each criterion was rated as present or absent. Any trial that satisfied at least four of the five criteria was judged as high quality.
Quantitative Assessment
Because of small sample sizes; many different treatments, with few studies on each specific treatment; broad classes of medications, raising the question of whether drugs within these broad classes can be pooled; and considerable variation in the reporting of statistical details, such as exact P values and standard deviations, deriving a pooled estimates of the overall effect of any treatment was not possible with the exception of treatment with alpha-blockers. For alpha-blockers, pooled estimates from the RCTs were created using a random effects model.9 Each trial was otherwise classified simply as positive or negative in terms of efficacy.
We employed the vote–counting approach10 which relies on two outcomes: global improvement and improvement in specific CP/CPPS symptoms. Any trial that reported statistically significant improvement (P <0.05) in global status or individual symptoms was classified as positive. The absolute difference in frequency of global improvement between the intervention and control groups (along with the 95% CI) was calculated for each RCT that provided sufficient data. This difference is conceptually similar to absolute risk difference and allows the number needed to treat (NNT) for benefit to be calculated on the basis of global improvement.11 Because individual symptom outcomes could not be quantitatively summarized, they were simply described as positive (i.e., the treatment group had a significantly better outcome on that symptom compared to the control group) or negative for each trial that reported a particular outcome. The Student t–test was used to compare continuous variables, and the chi–square test to compare binary variables between certain study subgroups. The Mann-Whitney U-test was used to compare median sample sizes between positive and negative studies and between high and low quality studies as sample sizes were skewed. Heterogeneity was assessed using the Q and I2 statistic and visually with Galbraith plots. Publication bias was assessed with the Egger’s statistics and visually with funnel plots.12 The effects of publication bias were assessed using the metatrim method of Duval and Tweedie.13 All analyses were performed using Stata (Version 8, College Station, Texas).
RESULTS
A total of 107 CP/CPPS treatment trials were identified. Of these, 12 randomized controlled trials (RCTs) satisfied the inclusion criteria14-25. Selected study characteristics are presented in Table 1. The remaining 95 studies were excluded because they did not use the NIH-CPSI as a validated outcome measure (n = 23); did not address treatment of CP/CPPS and focused on chronic bacterial prostatitis (n = 46); did not use a randomized, double–blind, placebo–controlled, or controlled design (n = 9); included patients without a diagnosis of CP/CPPS (n = 7); were duplicate publications (n = 2); were presented in abstract form only (n = 5) or involved fewer than 10 patients (n = 3).
Table 1.
RCTs for the Treatment of Chronic Prostatitis/Chronic Pelvic Pain Syndrome (CP/CPPS)*
| Symptom Outcomes† | Effect | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study (Year) | Medication | N | Mean age | Duration in weeks | Pain | Urinary Symptoms | Quality of Life | Any | Overall (NNT) |
| Alpha-blockers, selective | |||||||||
| Mehik et al., (2003)14 | Alfuzosin | 70 | 49 | 24 | + | − | − | + | 2 |
| Cheah et al., (2003)15 | Terazosin | 100 | 35 | 14 | + | + | + | + | 5 |
| Alpha-blocker, subtype-1A-Selective | |||||||||
| Nickel et al., (2004)16 | Tamsulosin | 58 | 41 | 6 | + | + | + | + | 6 |
| Alexander et al., (2004)17 | Tamsulosin | 196 | 45 | 6 | − | − | − | − | 20 |
| 5-alpha reductase inhibitors | |||||||||
| Nickel et al., (2004)18 | Finasteride | 76 | 47 | 24 | − | − | − | − | 7 |
| Antibiotics | |||||||||
| Alexander et al., (2004)17 | Ciprofloxacin | 196 | 45 | 6 | − | − | − | − | 20 |
| Nickel et al., (2003)19 | Levofloxacin | 80 | 56 | 6 | − | − | − | − | 53 |
| Miscellaneous | |||||||||
| De Rose et al., (2004)20 | Mepartricin | 36 | 33 | 8 | + | − | + | + | N/A‡ |
| Nickel et al., (2005)21 | Pentosan polysulfate | 100 | 38 | 16 | − | − | + | + | 6 |
| Nickel et al., (2003)22 | Rofecoxib | 161 | 48 | 6 | + | + | + | + | 5 |
| Complementary and Alternative Medications | |||||||||
| Wang et al., (2004)23 | Chuanshenton g | 38 | NR | 1 | + | + | + | + | 3 |
| Lu et al., (2004)24 | Flavoxate | 45 | NR | 4 | + | + | + | + | N/A‡ |
| Shoskes et al., (1999)25 | Quercetin | 30 | 45 | 4 | + | − | + | + | 3 |
Abbreviations: NR – not reported,
+ means outcome significantly better than in control group, − means not significantly better,
N/A – not applicable, data for NNT calculation not provided
A single agent was evaluated in 11 of 12 trials, and a combination of two or more – in 1. The 12 trials spanned the years 1999 to 2005 and reported on 980 adult male patients. The patients’ mean age ranged from 33 to 47 years (median, 36 years). More than half of the trials (7 out of 12, 58%) were conducted in the United States, 2 trials (17%) were done in Europe, and 3 trials (25%) – in other countries. All studies were conducted in urological settings and half of them (50%) were done in multiple urological practices.
Patient Population
By definition, as per our inclusion criteria, all 12 of the studies (100%) used the 1999 International Prostatitis Collaborative Network diagnostic guidelines.26 All studies reported an adequate work–up to exclude organic genito-urinary disease, including history, physical examination, laboratory, and radiologic evaluation.
Study Design
All 12 studies employed parallel and none used a crossover design. Length of intervention ranged from one to 24 weeks, with a mean of 10 and a median of 6 weeks. Symptom severity, as assessed within each individual trial, was similar at baseline between the intervention and control groups in all of the trials. Treatment adherence and co-interventions, such as concurrent use of other medications to relieve symptoms during the intervention period, were reported in 1 study. Adequate concealment of treatment randomization was reported in 1 study.
Outcome Assessment
Both global as well as individual symptom improvement was reported in all studies. A standardized symptom questionnaire – the National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI) – was used all 12 trials and was reportedly validated in 1 trial.27
Statistical Analysis and Methodologic Quality
An intention–to–treat analysis was used in 6 trials.16-19,21,22 The dropout rate for trials ranged from 3% to 21%. Out of a maximum quality score of 5, the mean study quality was 4.6 and the median was 5. Eight of 12 trials satisfied all five quality criteria.14-19,21,22 Mean quality scores did not differ significantly between positive and negative trials.
Alpha-blockers
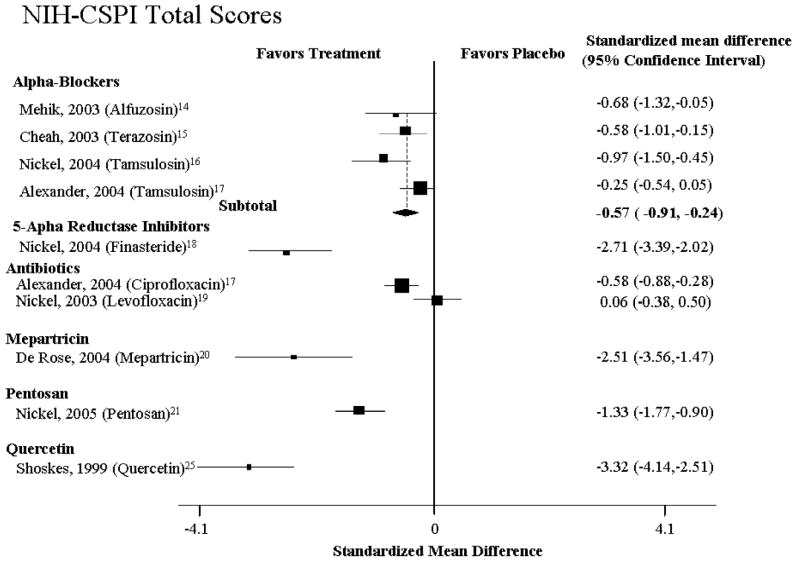
Four studies examined the efficacy of alpha-blockers (Table 1, Fig. 1 and 2).14-17 Overall, there was evidence for modest efficacy among these trials (Fig. 1 and 2), with a pooled relative risk for improvement of 0.57 (95% CI: 0.24-0.91, n=4 studies, Q= 10.70, p=0.10, I2 =43.9%). The absolute risk difference was 0.18 (95% CI: 0.005-0.35), translating to a NNT of 6 (95% CI: 2.8-200). There was no evidence of publication bias (P = 0.88). While there was a 42% increase in the likelihood of improvement, the amount of improvement was modest, with an effect size of improvement on the NIH–CPSI of 0.78 (95% CI: 0.3-1.2) (Table 1, Fig. 1 and 2).
Figure 1. Relative Risk of Overall Improvement.
Figure 2. Relative Risk of Pain Improvement.
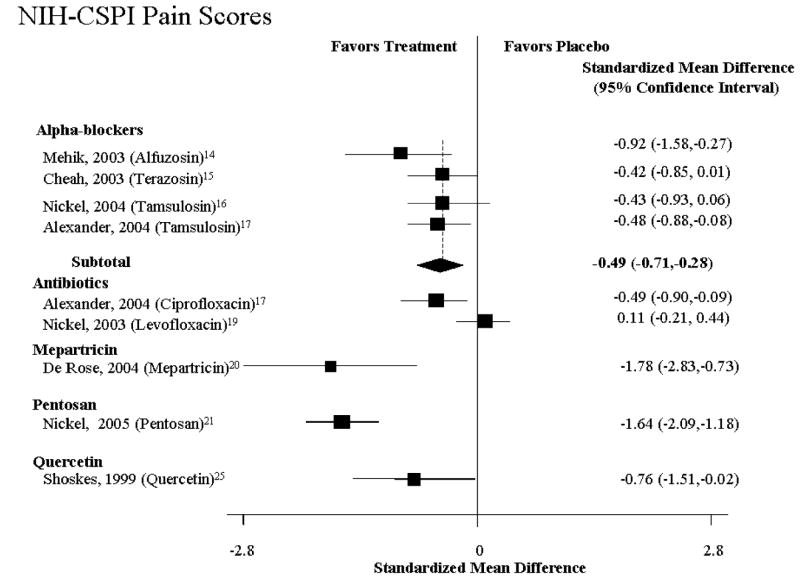
In one of the studies, Mehik et al.14 randomized 70 patients to alfuzosin 5 mg twice daily (19 patients) or placebo (21 patients) for 6 months with a follow-up of another 6 months after medication was discontinued (Table 1, Fig. 1 and 2). The 30 men who declined randomization received standard treatment (hot Sitz baths plus anti-inflammatories). Improvement was defined as a 33% decrease in NIH-CPSI total and domain scores as compared to baseline. Compared to placebo, 6 months of alfuzosin treatment resulted in significant symptom and pain reduction (change in total NIH–CPSI score from baseline: −9.9 with alfuzosin vs. −3.8 with placebo; P = 0.01; change in NIH–CPSI pain score from baseline: −5.1 with alfuzosin vs. −1.1 with placebo; P = 0.01). Twelve months after baseline and 6 months after the active treatment phase, the pain score in the placebo group (+1.6) was higher than the score in the alfuzosin group (−1.8) (P = 0.02). At this point in time, patients in the standard treatment group experienced the greatest benefit as evidenced by the greatest reduction of pain scores (−1.7).
Cheah et al15 demonstrated treatment benefit for alpha-blocker-naïve CP/CPPS patients with a NIH-CPSI quality of life (QoL) score of 4 or greater (mostly dissatisfied). A total of 86 CP/CPPS patients were randomized over 14 weeks to either terazosin (with dose escalation from 1–5 mg/day) or placebo (Table 1, Fig. 1 and 2). Patients reporting an NIH-CPSI QoL score of 0-2 (“delighted” to “mostly satisfied”) at 14 weeks as compared to a baseline score of 4-6 (“mostly dissatisfied” to “terrible”) at baseline were considered responders. Terazosin significantly improved QoL and significantly reduced pain at 14 weeks compared with placebo (NIH–CPSI QoL score 0–2: 24/43 (56%) with terazosin vs. 14/43 (33%) with placebo, P = 0.03; reduction of NIH–CPSI pain score >50% from baseline: 26/43 (60%) with terazosin vs. 16/43 (37%) with placebo; P = 0.03). No significant differences were found between terazosin and placebo in “objective parameters” (change in peak flow rate: 15.4–18.7 mL/second with terazosin vs. 18.1–19.7 mL/second with placebo; P value not reported; change in residual volume: 24.8–17.1 mL with terazosin vs. 20.6–16.0 mL with placebo; P value not reported).
Two studies examined the efficacy of tamsulosin, an alpha-1A subtype-selective blocker. Following a two-week placebo run-in, Nickel et al.16 randomized 58 men (younger than 55) to 0.4 mg tamsulosin or placebo (Table 1, Fig. 1 and 2). Overall, tamsulosin significantly improved symptoms compared with placebo after 45 days (treatment effect −3.6, [95% CI −7.0 to −0.3]; P = 0.04 in favor of tamsulosin). Subgroup analyses demonstrated clinically and statistically significant response in patients with severe symptoms (75th percentile, treatment effect, −8.3 points; P <0.01) and lack thereof in those with mild symptoms (25th percentile, −1.6 points; P = 0.53). Alexander et al17 randomized 169 heavily-retreated men in a double-blind RCT comparing 6 weeks of therapy with ciprofloxacin, tamsulosin, both drugs, or placebo (Table 1, Fig. 1 and 2). At baseline, patients had severe symptoms (total NIH-CPSI score of at least 15) and a mean duration of symptoms of 6.2 years. At 6 weeks, there was no significant difference in NIH-CPSI total score for tamsulosin compared with placebo (slope estimate in the longitudinal regression model, − 4.4 [CI, −5.8 to −2.9] vs. −4.8 [CI, −6.2 to −3.3]; P >0.2).
Antibiotics
Two large-scale studies reported on the efficacy of antibiotics. Nickel et al.19 randomized 80 CP/CPPS patients with no demonstrable infection localized to the prostate to levofloxacin (45) or placebo (35) for 6 weeks (Table 1, Fig. 1 and 2). Based on the NIH-CPSI, both groups experienced progressive symptom improvement, but the difference was not statistically or clinically significant at the end of treatment (6 weeks) or follow-up visits (12 weeks). At both timepoints, the levofloxacin group experienced a mean total NIH-CPSI decrease of 5.4 and 5.6 points from baseline compared to a decrease of 2.9 and 3.1 points for the placebo group (treatment effect at 6 weeks 3.2; P > 0.05). Based on a 6-point NIH-CPSI score decrease and compared to placebo, more patients responded in the levofloxacin group; the difference was statistically significant only at 3 weeks (P = 0.02).
Similar to the findings for tamsulosin (see above), Alexander et al.17 found no significant difference in the decrease in NIH-CPSI total score from baseline to 6 weeks for ciprofloxacin compared with placebo (slope estimate in the longitudinal regression model, −5.4 [95% CI, −6.8 to −3.9] vs. −3.9 [CI, −5.3 to −2.4]; P = 0.15). The difference in the slope estimate between these 2 groups was −1.5 (CI, −3.5 to 0.5).
Miscellaneous
In one recent study, 100 men with CP/CPPS were randomized to 300 mg PPS or placebo 3 times daily for 16-weeks (Table 1, Fig. 1 and 2).21 Based on clinical global improvement (CGI) assessment, significantly more PPS-treated patients experienced moderate to marked improvement (18 or 37% vs. 8 or 18%, P = 0.04). The mean CGI scores between the PPS and placebo groups, however, failed to reach statistical significance (1.0 vs. 0.6, P = 0.107). Likewise, for the total domain-specific NIH-CPSI scores the difference between the two groups was not statistically significant (−5.9 or 22% vs. −3.2 or 12%, P = 0.068). At 16 weeks, NIH-CPSI QoL scores were significantly better in the PPS as compared to the placebo group (−2.0 or 22% vs. −1.0 or 12%, P = 0.031). At this time, the mechanism by which PPS improves symptom is unclear.
One RCT evaluated the efficacy of rofecoxib in nonbacterial prostatitis patients. 161 CPPS patients were randomized over 6 weeks to either 25mg or 50 mg rofecoxib or placebo. The difference between patients treated with either 25 mg or 50 mg rofecoxib and placebo failed to reach statistical significance. 63% of the CPPS patients randomized to 50 mg rofecoxib reported greater than 25% improvement in NIH-CPSI total scores as compared with 40% of the placebo group (P<0.05). In terms of pain improvement, 79% of the 50mg group reported no or mild pain (vs. 59% on placebo), and 56% reported significant improvement in the quality of life (vs. 27% in the placebo group, P<0.005).
One study examined the efficacy of 40 mg of mepartricin, an estrogen-lowering agent, versus placebo in 26 CPPS patients. Following 60 days of treatment, there was a statistically significant decrease in total NIH-CPSI, pain and quality of life scores and 17-beta-estradiol levels. Compared to baseline, the changes in urinary scores and the serum levels of luteinizing, follicle-stimulating hormone, and testosterone at the end of the study failed to reach statistical significance.
DISCUSSION
Our analysis aimed to answer a basic research question: In subjects with CP/CPPS, can experimental interventions reduce the risk of pain, voiding dysfunction and quality of life impairment? Results from RCTs indicate that there is no universally effective treatment, which can provide significant lasting benefit for this chronic pelvic pain syndrome (Fig. 1 and 2). Compared with placebo, sham, or active control, none of the medications was consistently beneficial in improving NIH-CPSI total scores or patient–reported GRA. Likewise, no medication proved consistently superior to placebo in alleviating pain or urinary symptoms or improving quality of life as reflected in the lack of subdomain changes of the NIH-CPSI (Fig. 1 and 2).
What are the take-home messages from our analysis? Urologists treating patients with CP/CPPS have long known that research evidence is not the only factor that affects clinical decision making. Urologists are no strangers to the complex evolution of conventional wisdom about the relation of clinicians to research evidence. Our knowledge and treatment recommendations for CP/CPPS have emerged from the era of “optimism” through that of “innocence lost and regained” and the era of “industrialization” into the era of “information technology and systems engineering,” as insightfully described by Naylor.28 In this respect, results from RCTs are the starting point for treatment recommendations for CP/CPPS.
The situation is quite similar to that in the hypertension community several years ago as dissected by Naylor.28 Briefly, at that time, the Fifth and Sixth Joint National Committee on the Detection, Evaluation, and Treatment of High Blood Pressure (JNC V and VI) endorsed diuretics and beta-blockers as first-line, and alpha-blockers – as second-line antihypertensive therapy.29,30 However, physiological inferences and trial evidence supported a contrarian view in the hypertension community that “attaining a target blood pressure with tolerable adverse effects was ultimately more important than choice of agent”.28 As Naylor notices, “alpha-blockers were astutely marketed as preferable for patients with diabetes, and their salutary effects on prostatism offered an attractive secondary effect in treating older men”.28
In this context, attaining optimal symptom control in CP/CPPS is ultimately more important than the choice of agent. The lessons learned from the recent RCTs with alpha-blocker illustrate this point best. Three14-16 of the four trials RCTs showed statistically significant benefits and modest clinical efficacy. Cheah’s study reported on newly diagnosed, alpha-blocker-naïve, primary care, 20 to 50-year-old Asians.15 The subjects in the Mehik14, Nickel16 and Alexander17 trials were heavily-pretreated, tertiary-referral, older Finnish and North American. Moreover, the clinical benefit from long-term terazosin therapy was attributed to pharmacologic action above and beyond the bladder neck.31 Duration of treatment is also particularly important. Patients on long-term alpha-blocker therapy benefited from treatment as shown in the recent open-label long-term follow-up phase of the Cheah et al. study.32
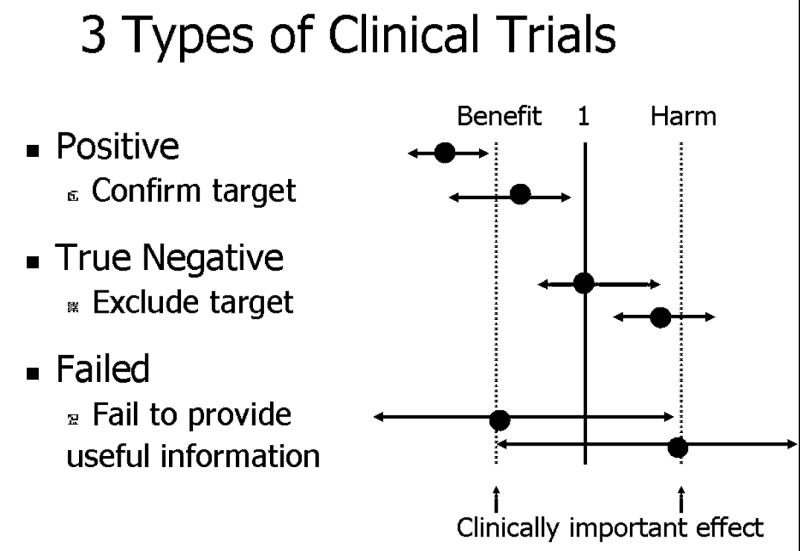
The one negative trial that failed to show benefit from alpha-blocker therapy enrolled men with long-standing and refractory symptoms, more likely to be referred to tertiary medical centers, and therefore enrolled in clinical trials. As noted by Alexander,33 these subjects might be end-stage prostatitis patients who failed treatment with the study drugs, which are commonly prescribed in the community setting. In this context, while the Cheah,15 Nickel16 and Mehik14 trials fall in the category I or II trials (Figure 3), the Alexander trial17 falls in the category III (true negative trial). The main challenge, however, remains defining the clinical question and the patient population in the lack of an objective diagnostic or therapeutic marker.
Figure 3. Types of Clinical Trials Based on Outcomes.
Center line: Neutral relative risk/benefit; Relative risk = 1; Dotted lines show magnitude of clinically important effect. First trial: clinically beneficial effect and both ends of the confidence limit of the study are to the right of the benefit line; Second trial: statistically significant benefit, but may not be as clinically important; Third trial – neutral; Forth trial – harmful. Last two are failed trials and fail to provide useful information since confidence limits too large (indicating inadequate power or other reasons).
Several caveats are in place here. First, results from any trial in CP/CPPS need to be considered in light of the variability of symptoms, regression to the mean, the duration and size of the placebo effect.34
Second, in the absence of a validated disease biomarker, conclusions regarding therapeutic efficacy based on a known mechanism of action in other conditions, should be discouraged. For example, alpha-blockers have been extensively used in CP/CPPS on the assumption that irritative symptoms in CP/CPPS share a common mechanism with BPH. Our analysis shows that patients benefit from alpha-blockers even without urodynamic35 or subjective36 evidence of alpha-adrenergic blockade.
Third, identification of patient subsets that are more likely to respond to specific treatments is problematic. One possibility is to employ severity stratification based on the NIH-CPSI score.34 However, even in this scenario, evidence suggests that naïve and heavily-pretreated patients respond differently. Therefore, future studies should enroll and evaluate patients based both on severity and previous treatment status.
CONCLUSION
Current evidence from RCTs for the treatment of CP/CPPS shows that there is no effective treatment for this chronic pelvic pain syndrome. Future RCTs must take into consideration variability of symptoms and regression to the mean, using an appropriate sample size and optimal duration and follow-up of participants. A diagnostic and therapeutic biomarker for CP/CPPS is urgently needed.
Acknowledgments
This work was supported by NIH/NIDDK grant R01 DK 065990 to J.D.D. and by grant P50 DK 65298 to M.R.F.
References
- 1.Schaeffer AJ, Datta NS, Fowler JE, Jr, Krieger JN, Litwin MS, Nadler RB, Nickel JC, Pontari MA, Shoskes DA, Zeitlin SI, et al. Overview summary statement. Diagnosis and management of chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) Urology. 2002;60:1–4. doi: 10.1016/s0090-4295(02)01979-9. [DOI] [PubMed] [Google Scholar]
- 2.Collins MM, Stafford RS, O’Leary MP, Barry MJ. How common is prostatitis? A national survey of physician visits. J Urol. 1998;159:1224–8. [PubMed] [Google Scholar]
- 3.McNaughton Collins M, Pontari MA, O’Leary MP, Calhoun EA, Santanna J, Landis JR, Kusek JW, Litwin MS. Quality of life is impaired in men with chronic prostatitis: the Chronic Prostatitis Collaborative Research Network. J Gen Intern Med. 2001;16:656–62. doi: 10.1111/j.1525-1497.2001.01223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krieger JN, Nyberg L, Jr, Nickel JC. NIH consensus definition and classification of prostatitis. Jama. 1999;282:236–7. doi: 10.1001/jama.282.3.236. [DOI] [PubMed] [Google Scholar]
- 5.Jailwala J, Imperiale TF, Kroenke K. Pharmacologic treatment of the irritable bowel syndrome: a systematic review of randomized, controlled trials. Ann Intern Med. 2000;133:136–47. doi: 10.7326/0003-4819-133-2-200007180-00013. [DOI] [PubMed] [Google Scholar]
- 6.Whiting P, Bagnall AM, Sowden AJ, Cornell JE, Mulrow CD, Ramirez G. Interventions for the treatment and management of chronic fatigue syndrome: a systematic review. Jama. 2001;286:1360–8. doi: 10.1001/jama.286.11.1360. [DOI] [PubMed] [Google Scholar]
- 7.Goldenberg DL, Burckhardt C, Crofford L. Management of fibromyalgia syndrome. Jama. 2004;292:2388–95. doi: 10.1001/jama.292.19.2388. [DOI] [PubMed] [Google Scholar]
- 8.Jailwala J, Imperiale TF, Kroenke K. Pharmacologic treatment of the irritable bowel syndrome: a systematic review of randomized, controlled trials. Ann Intern Med. 2000;133:136–47. doi: 10.7326/0003-4819-133-2-200007180-00013. [DOI] [PubMed] [Google Scholar]
- 9.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 10.Light RJ PD: Summing Up the Science of Reviewing Research. Cambridge, MA, Harvard University Press, 1984.
- 11.Guyatt GH, Sackett DL, Cook DJ. Users’ guides to the medical literature. II. How to use an article about therapy or prevention. A. Are the results of the study valid? Evidence-Based Medicine Working Group. Jama. 1993;270:2598–601. doi: 10.1001/jama.270.21.2598. [DOI] [PubMed] [Google Scholar]
- 12.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 14.Mehik A, Alas P, Nickel JC, Sarpola A, Helstrom PJ. Alfuzosin treatment for chronic prostatitis/chronic pelvic pain syndrome: a prospective, randomized, double-blind, placebo-controlled, pilot study. Urology. 2003;62:425–9. doi: 10.1016/s0090-4295(03)00466-7. [DOI] [PubMed] [Google Scholar]
- 15.Cheah PY, Liong ML, Yuen KH, Teh CL, Khor T, Yang JR, Yap HW, Krieger JN. Terazosin therapy for chronic prostatitis/chronic pelvic pain syndrome: a randomized, placebo controlled trial. J Urol. 2003;169:592–6. doi: 10.1097/01.ju.0000042927.45683.6c. [DOI] [PubMed] [Google Scholar]
- 16.Nickel JC, Narayan P, McKay J, Doyle C. Treatment of chronic prostatitis/chronic pelvic pain syndrome with tamsulosin: a randomized double blind trial. J Urol. 2004;171:1594–7. doi: 10.1097/01.ju.0000117811.40279.19. [DOI] [PubMed] [Google Scholar]
- 17.Alexander RB, Propert KJ, Schaeffer AJ, Landis JR, Nickel JC, O’Leary MP, Pontari MA, McNaughton-Collins M, Shoskes DA, Comiter CV, et al. Ciprofloxacin or tamsulosin in men with chronic prostatitis/chronic pelvic pain syndrome: a randomized, double-blind trial. Ann Intern Med. 2004;141:581–9. doi: 10.7326/0003-4819-141-8-200410190-00005. [DOI] [PubMed] [Google Scholar]
- 18.Nickel JC, Downey J, Pontari MA, Shoskes DA, Zeitlin SI. A randomized placebo-controlled multicentre study to evaluate the safety and efficacy of finasteride for male chronic pelvic pain syndrome (category IIIA chronic nonbacterial prostatitis) BJU Int. 2004;93:991–5. doi: 10.1111/j.1464-410X.2003.04766.x. [DOI] [PubMed] [Google Scholar]
- 19.Nickel JC, Downey J, Clark J, Casey RW, Pommerville PJ, Barkin J, Steinhoff G, Brock G, Patrick AB, Flax S, et al. Levofloxacin for chronic prostatitis/chronic pelvic pain syndrome in men: a randomized placebo-controlled multicenter trial. Urology. 2003;62:614–7. doi: 10.1016/s0090-4295(03)00583-1. [DOI] [PubMed] [Google Scholar]
- 20.De Rose AF, Gallo F, Giglio M, Carmignani G. Role of mepartricin in category III chronic nonbacterial prostatitis/chronic pelvic pain syndrome: a randomized prospective placebo-controlled trial. Urology. 2004;63:13–6. doi: 10.1016/j.urology.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Nickel JC, Forrest JB, Tomera K, Hernandez-Graulau J, Moon TD, Schaeffer AJ, Krieger JN, Zeitlin SI, Evans RJ, Lama DJ, et al. Pentosan Polysulfate Sodium Therapy For Men With Chronic Pelvic Pain Syndrome: A Multicenter, Randomized, Placebo Controlled Study. J Urol. 2005;173:1252–1255. doi: 10.1097/01.ju.0000159198.83103.01. [DOI] [PubMed] [Google Scholar]
- 22.Nickel JC, Pontari M, Moon T, Gittelman M, Malek G, Farrington J, Pearson J, Krupa D, Bach M, Drisko J. A randomized, placebo controlled, multicenter study to evaluate the safety and efficacy of rofecoxib in the treatment of chronic nonbacterial prostatitis. J Urol. 2003;169:1401–5. doi: 10.1097/01.ju.0000054983.45096.16. [DOI] [PubMed] [Google Scholar]
- 23.Wang W, He H, Hu W, Zhang X, Wang Y. Efficacy and safety of intraprostatic injection of chuanshentong for chronic abacterial prostatitis/chronic pelvic pain syndrome. Zhonghua Nan Ke Xue. 2004;10:182–4. [PubMed] [Google Scholar]
- 24.Lu M, Zhao ST, Wang SM, Shi BK, Fan YD, Wang JZ. Alpha-blockers and bioflavonoids in men with chronic nonbacterial prostatitis (NIH-IIIa): a prospective, placebo-controlled trial. Zhonghua Liu Xing Bing Xue Za Zhi. 2004;25:169–72. [PubMed] [Google Scholar]
- 25.Shoskes DA, Zeitlin SI, Shahed A, Rajfer J. Quercetin in men with category III chronic prostatitis: a preliminary prospective, double-blind, placebo-controlled trial. Urology. 1999;54:960–3. doi: 10.1016/s0090-4295(99)00358-1. [DOI] [PubMed] [Google Scholar]
- 26.Nickel JC, Nyberg LM, Hennenfent M. Research guidelines for chronic prostatitis: consensus report from the first National Institutes of Health International Prostatitis Collaborative Network. Urology. 1999;54:229–33. doi: 10.1016/s0090-4295(99)00205-8. [DOI] [PubMed] [Google Scholar]
- 27.Litwin MS, McNaughton-Collins M, Fowler FJ, Jr, Nickel JC, Calhoun EA, Pontari MA, Alexander RB, Farrar JT, O’Leary MP. The National Institutes of Health chronic prostatitis symptom index: development and validation of a new outcome measure. Chronic Prostatitis Collaborative Research Network. J Urol. 1999;162:369–75. doi: 10.1016/s0022-5347(05)68562-x. [DOI] [PubMed] [Google Scholar]
- 28.Naylor CD. The complex world of prescribing behavior. Jama. 2004;291:104–6. doi: 10.1001/jama.291.1.104. [DOI] [PubMed] [Google Scholar]
- 29.The fifth report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure (JNC V) Arch Intern Med. 1993;153:154–83. [PubMed] [Google Scholar]
- 30.The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. . Arch Intern Med. 1997;157:2413–46. doi: 10.1001/archinte.157.21.2413. [DOI] [PubMed] [Google Scholar]
- 31.Cheah PY, Liong ML, Yuen KH, Teh CL, Khor T, Yang JR, Yap HW, Krieger JN. Reply by the authors. Urology. 2005;66:232–3. [Google Scholar]
- 32.Cheah PY, Liong ML, Yuen KH, Teh CL, Khor T, Yang JR, Yap HW, Krieger JN. Initial, long-term, and durable responses to terazosin, placebo, or other therapies for chronic prostatitis/chronic pelvic pain syndrome. Urology. 2004;64:881–6. doi: 10.1016/j.urology.2004.06.041. [DOI] [PubMed] [Google Scholar]
- 33.Alexander R. Treatment of chronic prostatitis. Nature Clinical Practice Urology. 2004;1:2–4. doi: 10.1038/ncpuro0003. [DOI] [PubMed] [Google Scholar]
- 34.Propert KJ, Alexander RB, Nickel JC, Kusek JW, Litwin MS, Landis JR, Nyberg LM, Schaeffer AJ. Design of a multicenter randomized clinical trial for chronic prostatitis/chronic pelvic pain syndrome. Urology. 2002;59:870–6. doi: 10.1016/s0090-4295(02)01601-1. [DOI] [PubMed] [Google Scholar]
- 35.Mayo ME, Ross SO, Krieger JN. Few patients with “chronic prostatitis” have significant bladder outlet obstruction. Urology. 1998;52:417–21. doi: 10.1016/s0090-4295(98)00202-7. [DOI] [PubMed] [Google Scholar]
- 36.Mehik A, Alas P, Nickel JC, Sarpola A, Helstrom PJ. Alfuzosin treatment for chronic prostatitis/chronic pelvic pain syndrome: a prospective, randomized, double-blind, placebo-controlled, pilot study. Urology. 2003;62:425–9. doi: 10.1016/s0090-4295(03)00466-7. [DOI] [PubMed] [Google Scholar]