Abstract
Background
Skeletal muscle cramps affect over a third of the ambulatory elderly population. Quinine is the established treatment, but there are safety concerns, and evidence for efficacy is conflicting. A recent meta-analysis established a small advantage for quinine, but identified the need for additional studies. N-of-1 trials compare two treatments, in a randomised, double-blind, multiple crossover study on a patient-by-patient basis. They have been used to compare treatments in osteoarthritis and may be suitable for determining the individual efficacy of quinine.
Aim
To establish efficacy and safety of quinine sulphate use for the treatment of leg-muscle cramp.
Design of study
Double-blind, randomised series of n-of-1 controlled trials of quinine versus placebo for muscle cramps.
Setting
New Zealand general practices.
Method
The participants were 13 general practice patients (six males; seven females; median age = 75 years) already prescribed quinine. Following a 2-week washout, each patient received three 4-week treatment blocks of quinine sulphate and matched placebo capsules with an individual, randomised crossover design. The main outcome measures were: patient diaries of cramp occurrence, duration and severity; capsule counts; and blood quinine levels in the final treatment block.
Results
Ten patients completed the trial. Three patients were identified for whom quinine was clearly beneficial (P<0.05), six showed non-significant benefit and one showed no benefit. All patients elected to continue quinine post-study.
Conclusion
Series of n-of-1 studies differentiated patients whom quinine had statistically significant effects; those with trend towards effectiveness; those for whom quinine was probably not effective. Ideally n-of-1 trial should be performed when a patient is commenced on quinine. More cycles in n-of-1 studies of quinine may address issues of statistical power.
Keywords: muscle cramp, placebos, quinine, randomized controlled trials
INTRODUCTION
Nocturnal leg cramps have been reported to affect over one-third of the ambulatory elderly population.1 Many treatment modalities have been utilised to prevent and relieve cramp symptoms, including alternative remedies (such as the use of magnets), stretching and manipulation of affected limbs, and drug therapy. The little research evidence available currently does not support effectiveness of folk remedies.2 Similarly there are insufficient data to determine the effectiveness of stretching.3 For over 50 years, the cornerstone drug therapy has been quinine salts. Other medicines have been tried, but quinine remains the most widely used and studied.4
While quinine is the established treatment, there are concerns about its safety and evidence for efficacy is conflicting.5 A meta-analysis of randomised, double-blind, crossover trials quantitatively assessed whether quinine was more efficacious than a placebo in providing relief for elderly people with nocturnal leg cramps.6 Five study outcomes were reported, but not all patient data was suitable for inclusion in each analysis.6 The need for additional studies to confirm efficacy and safety was identified.6 A more recent meta-analysis included three unpublished trials accessed from a drug regulatory agency that met the same eligibility criteria as the original meta-analysis.7 Pooling of both published and unpublished data gave a reduced estimate of quinine efficacy compared with the earlier meta-analysis. Using the relative risk from this pooled study of 0.43 applied to a 50% prevalence in patients aged over 65 years,8 gives a number needed to treat of 3.5 (95% confidence interval [CI] = 2.5 to 5.7).
How this fits in
Quinine is commonly used to treat nocturnal leg muscle cramps. A recent meta-analysis indicated that it can be efficacious but has potentially serious side-effects and drug interactions. Our study indicates that an n-of-1 trial can determine efficacy and safety of quinine use for individual patients with nocturnal cramps. Given patients’ reluctance to discontinue treatment when presented with evidence of limited or no efficacy, ideally n-of-1 trials should be instigated at the commencement of quinine treatment to avoid unnecessary long-term use in cases where efficacy is not demonstrated. Once patients are established on quinine for nocturnal leg cramps, there may be no point in conducting n-of-1 trials as patients are unlikely to want to stop taking the medication.
Quinine produces a range of reversible dose-related adverse effects known as cinchonism, usually arising at plasma quinine levels of 5–10 mg/l.5 These levels may occur in people taking quinine for muscle cramps. Symptoms include tinnitus, headache, nausea, and disturbed vision. Vomiting, vertigo, abdominal pain, diarrhoea, fever, pruritis and rashes may also occur at higher plasma levels.9 Rare, serious hypersensitivity reactions resulting in pancytopenia, disseminated intravascular coagulation and death have been reported. Quinine is toxic in overdose and may cause seizures, arrhythmias and blindness.10 Quinine salts also have potential interactions with a number of drugs including anticoagulants, digoxin, quinidine and phenothiazines.
In all 107 patients in the initial meta-analysis, only one patient experienced severe adverse effects, including thrombocytopenia, which resolved 3 days after discontinuing treatment.6 Other studies either reported occasional or no minor adverse events.11,12 When the unpublished trials were considered, quinine was associated with a higher overall incidence of side-effects, especially tinnitus, compared with placebo.7 This indicated a publication bias and strengthens the case for caution before physicians initiate quinine therapy for cramps.
In view of quinine's many potential adverse effects, an n-of-1 trial was suggested for individual patients.6 Standard comparative statistical procedures are usually used to analyse results. An n-of-1 trial provides the safeguards of a conventional randomised controlled trial transferred to a single patient study, with the three critical components being ‘randomisation, blinding of patient and physician to treatment assignment, and defining and quantifying the outcomes'.13 The main principle of an n-of-1 trial is that the patient serves as their own control when the efficacy of two treatments, or active drug and placebo, are compared:
‘In an n-of-1 randomised controlled trial a patient undergoes pairs of treatment periods (one period of each pair with the active drug and one with matched placebo, assigned at random); both the patient and the clinician are blind to allocation, and treatment targets are monitored’.14
Randomisation of treatments either throughout the whole trial or in blocks, is necessary to prevent systematic biases related to treatment order and to maintain a double-blind study design.13 At least three treatment blocks are recommended.14
N-of-1 trials are recommended for chronic stable conditions with reversible symptoms and require quantifiable treatment effects.14 Study endpoints must also be clearly identified.15 Study length should be determined by balancing participant safety and convenience with the amount of data required to produce a definite clinical or statistical outcome. It was considered important that patients commencing treatment for muscle cramps have a therapeutic trial period of at least 4 weeks duration, with close monitoring of benefits and risks. The aims of this study were to establish the efficacy and safety of quinine sulphate use in patients on chronic treatment for skeletal muscle cramp and to evaluate the suitability of n-of-1 trials to establish treatment efficacy in individuals on chronic therapy.
METHOD
The study was conducted in general practices in the Otago region, New Zealand. Patients prescribed quinine sulphate were identified using a regional general practice database16 and suitability was confirmed by their GP. Letters explaining the study and inviting participation were mailed to potential participants.
An interview confirmed eligibility (active muscle cramps, current quinine treatment, ability to complete daily diary entry) and absence of exclusion criteria (electrolyte disturbance, hepatic or renal dysfunction, serious illness, medication interacting with quinine). Participants were further excluded for less than four cramps over a 2-week period, an incomplete diary record, or measurable plasma quinine in the quinine-free run-in period. Rescue physiotherapy stretches were taught to all participants.
The individual studies ran for 14 weeks. Following a 2-week run-in period, quinine sulphate and matched placebo capsules were compared in three 4-week treatment blocks (each block consisting of 2 weeks active drug and 2 weeks placebo in random order). Patients were randomly assigned to one of eight possible treatment sequences of active drug and placebo in a crossover design. Participants received the same quinine dose as their most recent prescription. Both the patients and the researcher interacting with them and conducting the analyses were blinded to when patients were taking the active drug or the placebo. A sealed copy of the randomisation code form detailing the individual's randomisation sequence was held at the medical practice. Forms could be opened by the GP in an emergency to determine which study medication was dispensed at a particular time. A master copy of randomisation codes was also held by the research supervisor, so that the double-blind design of the study would not be compromised.
During home visits by the researcher every 2 weeks, treatment and diary compliance were checked, any symptoms (leg cramps and/or side effects) were reported, and quinine/placebo capsules and diaries were exchanged. Any reported adverse events or deterioration of a concurrent condition were referred to the participant's GP for advice regarding continued participation.
During the third treatment block, two blood samples were taken for spectrofluorometric measurement of plasma quinine levels to confirm medication adherence.17
Primary outcome measures were daily symptom diaries, quinine sulphate/placebo capsule returns and plasma quinine levels. Secondary measures were patient reports from the post-trial interview about whether they would continue taking quinine when presented with their individual findings, plus their use and perceived benefit of the stretching exercises.
Data were analysed using descriptive statistics, 90% CIs of mean treatment differences and a two-sided paired t-test (degrees of freedom = 2) for each subject. Data from the second week of each treatment period was used to estimate efficacy to avoid any carryover or rebound effects.18 Individual results were reported back to participants and their GPs. Participants were asked the importance of continuing quinine treatment and their preference for regular or symptomatic dosing.
RESULTS
From the 18 patients identified as suitable by their GP who volunteered to participate, 13 met trial entry criteria. Ten people completed the trial. The reasons for the three withdrawals were that one patient died from an unrelated cardiovascular event, one required unrelated urgent surgery, and one withdrew because of intolerable cramps in the run-in period.
Although all recruited patients met the inclusion criterion of four or more episodes of cramp during the 2-week run-in period, two participants reported infrequent cramps during their placebo phase below the minimum required for study entry.
Table 1 outlines the total number of cramps and number of days of cramps for each participant. Quinine significantly reduced the number of cramps and days with cramp in three participants (2, 4 and 5). Six other participants reported reduction in cramps on quinine that were not statistically significant. One participant did not show any reduction in cramps (Figure 1).
Table 1.
Results summary of participants who completed study.
| Total number of cramps | Total days with crampa | ||||||
|---|---|---|---|---|---|---|---|
| Participantb | Quinine dose (mg) | Quinine | Placebo | P-valuec | Quinine | Placebo | P-valuec |
| 2 | 300 | 7 | 84 | 0.035 | 3 | 14 | 0.004 |
| 3 | 200 | 2 | 4 | 0.184 | 2 | 4 | 0.184 |
| 4 | 200 | 9 | 37 | 0.03 | 6 | 16 | 0.038 |
| 5 | 200 | 7 | 34 | 0.016 | 5 | 18 | 0.006 |
| 6 | 200 | 1 | 1 | – | 1 | 1 | – |
| 7 | 200 | 19 | 44 | 0.13 | 12 | 15 | 0.478 |
| 9 | 200 | 6 | 11 | 0.3 | 5 | 6 | 0.74 |
| 10 | 200 | 12 | 46 | 0.262 | 6 | 12 | 0.184 |
| 11 | 300 | 49 | 58 | 0.449 | 18 | 20 | 0.184 |
| 12 | 200 | 4 | 10 | 0.184 | 4 | 10 | 0.184 |
Out of 21.
Participants 1, 8 & 13 did not complete study (one due to unrelated death, one due to unrelated urgent hospitalisation and one withdrawal due to intolerable cramps in run-in period).
Paired student's t-test.
Figure 1.
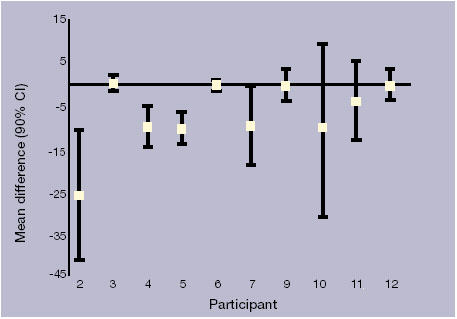
Changes in numbers of cramps with quinine treatment compared with placebo.
Compliance with study protocols, diary keeping, and being available for study visits was good. Few quinine/placebo capsules were returned and diaries were conscientiously kept. Plasma quinine levels 12 hours post-dose in block 3 also indicated that participants were compliant with the study medication regime.
There was no correlation (R2 = 0.004) between the steady state plasma quinine level and the number of cramps reported in any participant. Adverse effects were infrequent and no differences in the incidence or type of reported symptoms (P>0.05) were found between placebo and quinine. When the results were presented to each participant, no-one was persuaded to cease quinine treatment. Stretches for acute cramp relief were used by 7/10 patients, all of whom reported benefit.
DISCUSSION
Summary of main findings
The n-of-1 design is an attractive technique to define efficacy on an individual basis. It identified three patients for whom quinine was clearly beneficial, six who showed non-significant benefit and one who showed no benefit.
Strengths and weaknesses of the study
A strength of this study was the rigorous attention paid to blinding, randomisation and compliance testing. A limitation of the trial was the variability or small number of muscle cramp symptoms in the group showing non-significant benefit. This meant that a definitive result was not obtained, possibly related to insufficient power. For participants 7 and 10 a statistically significant result may have been found if more cycles had been undertaken. Also we only analysed the last 10 days of treatment to avoid crossover effects. A crossover effect could underestimate a finding and this may have affected our results. There is also an issue about whether or not a single patient trial has the power to show no effect.
Relationship of our study to existing literature
While not observed in this study, the documented adverse effects of quinine are serious,4 and individuals should not commence chronic therapy without evaluation of its efficacy and safety.
Because most patients treated with regular quinine tend to be elderly, they frequently have other pathologies and are receiving poly-pharmacy. The potential for drug interactions strengthens the need for caution before initiating quinine therapy.
It has been suggested that the main role of n-of-1 trials in clinical practice is to cancel useless treatment rather than advocate drug treatment.19 This effect has been shown in several studies where use of a safer, equally effective treatment has been advocated. When results were reported back, we recommended that quinine be continued in participants who showed benefit and ceased in those who did not. Several options were available to the other participants. They could have ceased treatment because statistical significance was not reached, completed another block to see if this clarified the trend, or continued treatment, believing quinine to be effective.
Unlike previously reported studies20,21 all participants chose to continue quinine treatment with these preferences expressed at the post-study interview. It is unknown whether the supervising GPs would have had greater success than the researcher at withdrawing quinine from those with no benefit, although we suspect not.
The firmly held belief of some participants that quinine was effective for them, despite their results, was not an expected finding. Actions to be taken if quinine was not effective were not discussed prior to commencement and participants may have assumed that their n-of-1 study was a ‘success’ only if the treatment ‘worked’. The researchers believe a strongly negative study was also successful if it resulted in cessation of unnecessary treatment.
Implications for clinical practice
Results from our series of n-of-1 studies divided the participants into three groups: those for whom quinine had statistically significant effects in reducing leg cramps, those who showed a trend towards effectiveness, and those for whom quinine was probably not effective. Because no evidence exists to evaluate which patients will benefit from quinine treatment, an n-of-1 trial is the best option for GPs and patients who wish to effectively treat frequent leg cramps without committing a patient to unnecessary long-term medication. More than three cycles may be needed to convince both GPs and patients of effectiveness/lack of effectiveness due to the issues of ‘potentially’ low statistical power.
Our findings suggest that once patients are receiving quinine they may be reluctant to stop treatment. We recommend that possible study outcomes be discussed during the recruitment process of n-of-1 trials and that participants be willing to consider treatment changes after the study, should their doctor recommend them. The decision to commence a patient on quinine treatment needs to be made judiciously knowing that the act of prescribing is committing the patient to indefinite medication. Some people will get limited or no benefit from quinine but risk rare, but serious, harm.
Once quinine has been prescribed, the performance of an n-of-1 trial is probably not warranted, given that patients are unlikely to stop taking their medication even if presented with evidence of limited or no efficacy.
Supplementary Material
Acknowledgments
Thanks to Dr Ruth Ferguson for her supervisory role and Professor Murray Tilyard for help with patient participation. We would also like to express our appreciation to the GPs involved and the patients who generously gave of their time.
Supplementary Information
Additional information accompanies this article at http://www.rcgp.org.uk/journal/index.asp
Ethics committee and reference number
The study was approved by the Southern Regional Health Authority Ethics Committee (95/03/29)
Competing interests
None
REFERENCES
- 1.Stewart RB, Moore MT, Marks RG, Hale WE. Nocturnal leg cramps in an ambulatory elderly population: An evaluation of risk factors. Journal of Geriatric Drug Therapy. 1993;7:23–46. [Google Scholar]
- 2.Maule B. Nocturnal cramp: quinine versus folklore. Practitioner. 1990;234:420–421. [PubMed] [Google Scholar]
- 3.Daniel H. Simple cure for nocturnal leg cramps. N Engl J Med. 1979;301:216. [PubMed] [Google Scholar]
- 4.McGee SR. Muscle cramps. Arch Intern Med. 1990;150:511–518. [PubMed] [Google Scholar]
- 5.Mandal AK, Abernathy T, Nelluri SN, Stitzel V. Is quinine effective and safe in leg cramps? J Clin Pharmacol. 1995;35:588–593. doi: 10.1002/j.1552-4604.1995.tb05015.x. [DOI] [PubMed] [Google Scholar]
- 6.Man-Son-Hing M, Wells G. Meta-analysis of efficacy of quinine for treatment of nocturnal leg cramps in elderly people. BMJ. 1995;310:13–17. doi: 10.1136/bmj.310.6971.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Man-Son-Hing M, Wells G, Lau A. Quinine for nocturnal leg cramps: a meta-analysis including unpublished data. J Gen Intern Med. 1998;13:600–606. doi: 10.1046/j.1525-1497.1998.00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdulla A, Jones P, Pearce V. Leg cramps in the elderly: prevalence, drug and disease associations. Int J Clin Pract. 1999;53:494–496. [PubMed] [Google Scholar]
- 9.MedlinePlus Drug Information. Quinine. http://www.nlm.nih.gov/medlineplus/druginfo/medmaster/a682322.html (accessed 11 February 2005)
- 10.Townsend B, Sturm J, Whyte S. Quinine associated blindness. Aust Fam Physician. 2004;33:627–628. [PubMed] [Google Scholar]
- 11.Fung M-C, Holbrook JH. Placebo-controlled trial of quinine therapy for nocturnal leg cramps. West J Med. 1989;151:42–44. [PMC free article] [PubMed] [Google Scholar]
- 12.Jones K, Castleden C. A double blind comparison of quinine sulphate and placebo in muscle cramps. Age Ageing. 1983;12:155–158. doi: 10.1093/ageing/12.2.155. [DOI] [PubMed] [Google Scholar]
- 13.Larson EB. N-of-1 Clinical trials. A technique for improving medical therapeutics. West J Med. 1990;152:52–56. [PMC free article] [PubMed] [Google Scholar]
- 14.Guyatt G, Sackett D, Adachi J, et al. A clinician's guide for conducting randomized trials in individual patients. Can Med Assoc J. 1988;139:497–503. [PMC free article] [PubMed] [Google Scholar]
- 15.Jaeschke R, Cook D, Sackett DL. The potential role of single-patient randomized controlled trials (n-of-1 RCTs) in clinical practice. J Am Board Fam Pract. 1992;5:227–229. [PubMed] [Google Scholar]
- 16.Dovey SM, Tilyard MW. The computer research network of the Royal New Zealand College of General Practitioners: an approach to general practice research in New Zealand. Br J Gen Pract. 1996;46:749–752. [PMC free article] [PubMed] [Google Scholar]
- 17.Hall AP, Czerwinski AW, Madonia EC, Evensen KL. Human plasma and urine quinine levels following tablets, capsules, and intravenous infusion. Clin Pharmacol Ther. 1973;14:580–585. doi: 10.1002/cpt1973144part1580. [DOI] [PubMed] [Google Scholar]
- 18.Dunn NR. Effectiveness of quinine for night cramps. Br J Gen Pract. 1993;43:127–128. [PMC free article] [PubMed] [Google Scholar]
- 19.Johannessen T. Controlled trials in single subjects. 1. Value in clinical medicine. BMJ. 1991;303:173–174. doi: 10.1136/bmj.303.6795.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahon J, Laupacis A, Donner A, Wood T. Randomised study of n of 1 trials versus standard practice. BMJ. 1996;312:1069–1074. doi: 10.1136/bmj.312.7038.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.March L, Irwig L, Schwarz J, et al. N of 1 trials comparing a non-steroidal anti-inflammatory drug with paracetamol in osteoarthritis. BMJ. 1996;309:1041–1046. doi: 10.1136/bmj.309.6961.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


