Abstract
Background
There are no systematic reviews of corticosteroids for shoulder pain that calculate the numbers needed to treat.
Aim
We wished to determine the effectiveness in terms of improvement of symptoms of intra-articular and subacromial injections of corticosteroid for rotator cuff tendonitis and frozen shoulder.
Design of study
Systematic review and meta-analysis of randomised controlled trials.
Method
Data sources included the Cochrane Controlled Trials Register, Medline, EMBASE, hand searches and author contacts. The review methods required any randomised controlled trial in which the effectiveness of subacromial or intra-articular steroid injections versus placebo and versus non-steroidal anti-inflammatory medication, could be ascertained. The outcome was improvement of symptoms. The data abstraction was done independently, as was the validity assessment. The data was pooled using Review Manager 4.1.
Results
Seven studies were reviewed for corticosteroids versus placebo and three for corticosteroids versus non-steroidal anti-inflammatory drugs (NSAIDs). The relative risk for improvement for subacromial corticosteroid injection for rotator cuff tendonitis was 3.08 (95% confidence interval [CI] = 1.94 to 4.87). The number needed to treat based on the pooled relative risk was 3.3 (95% CI = 1.8 to 7.7) patients to obtain one improvement. The relative risk for high dose (50 mg of prednisone or more) was 5.9 (95% CI = 2.8 to 12.6). The relative risk for improvement with steroids compared with NSAIDs was 1.43 (95% CI = 0.95 to 2.16). The number needed to treat for corticosteroids versus NSAIDs was 2.5 (95% CI = 1 to 9) for one significant study. The relative risks for intra-articular steroid injection for rotator cuff tendonitis were not statistically significant.
Conclusion
Subacromial injections of corticosteroids are effective for improvement for rotator cuff tendonitis up to a 9-month period. They are also probably more effective than NSAID medication. Higher doses may be better than lower doses for subacromial corticosteroid injection for rotator cuff tendonitis.
Keywords: adrenal cortex hormones; injections; meta-analysis; relative risk; review, systematic; shoulder impingement syndrome; shoulder pain
INTRODUCTION
Shoulder pain is a common source of distress. In two cross-sectional surveys based on patients registered with general practices a prevalence of 11.7% and 15%, respectively, was found.1,2
Six previous reviews of the use of corticosteroid injections in shoulders have found conflicting results.3-8 There are no systematic reviews of corticosteroids for shoulder pain that calculate the numbers needed to treat. A Cochrane review found that subacromial steroid injection was effective in improving range of abduction.3 A Health Technology Assessment published in 1997 concluded that the evidence was less than compelling and reported a number needed to treat of 33, with a confidence interval (CI) including ‘no benefit’.4 A third review reported that the evidence was scarce and of poor quality. It did consider ‘improvement’ but did not pool the results.5 The fourth review reported that local corticosteroid injections were effective in rotator cuff tendonitis although it was critical of the quality of many of the studies.6 There was no pooling of results. The fifth review was conducted by the same authors as the Cochrane review.7 They reported that subacromial steroids were better than placebo in improving the range of abduction.
We consider improvement/remission a more important patient outcome than increases in range of motion or improvements on pain scales, as it enables a number needed to treat to be calculated. This view has some support in the literature.9 The most recent review was a Cochrane review by some of the same authors from the previous Cochrane review.3 They concluded that the effect of subacromial steroids had a small benefit, but again only considered continuous outcomes, not complete remission. They also concluded that subacromial steroids were no better than non-steroidal anti-inflammatory drugs (NSAIDs).
Our objective was to systematically review the literature and statistically pool the results of improvement outcomes. The clinical question was whether or not intra-articular and subacromial injections of corticosteroid are effective compared with placebo and NSAIDs in terms of improvement of symptoms of rotator cuff tendonitis and frozen shoulder. We also wished to calculate a number needed to treat, as this has not been done before.
METHOD
The Cochrane Controlled Trials Register, Medline 1966–2004, and EMBASE 1980–2004 databases were searched using the MeSH terms ‘adrenal cortex hormone’, ‘randomised controlled trial’, ‘shoulder pain’ and ‘shoulder impingement syndrome’, and the non-MeSH terms ‘rotator cuff tendonitis’, ‘frozen shoulder’ and ‘adhesive capsulitis’. Authors of studies retrieved and included were contacted regarding any known unpublished work.
The reference lists of retrieved papers were also searched for relevant papers. The selection criteria required that the studies be randomised controlled trials in which the effectiveness of corticosteroids could be assessed. This included studies of corticosteroids versus placebo or NSAIDs, and studies of local anaesthetic and corticosteroids versus local anaesthetic. The participants needed to have a diagnosis of frozen shoulder or rotator cuff tendonitis of any duration. The outcome needed to include improvement, as this was considered the most significant patient-oriented outcome. Independent assessment of included papers was undertaken and any disagreements resolved by consensus.
A validity assessment was conducted using the items from the Pedro scoring system (Table 1).10,11 Scoring of studies was undertaken using the Jadad system, which is a validated scoring system.12 Each of the included studies was assessed independently for quality by the two authors and disagreement resolved through discussion. Data extraction was also done independently and disagreement resolved through discussion. Data were analysed using Review Manager 4.1 (Update Software, Oxford). For improvement we calculated the relative risk and the number needed to treat. A fixed effects model was used throughout, as there was no significant heterogeneity.13 The a priori sensitivity analysis included an analysis by high quality studies versus low quality studies, high and low dose, and an analysis of different medical providers giving the injections. A high quality study was one with a Jadad score of three or more. The conduct of this review was undertaken according to the Quorom statement.14 CIs for the number needed to treat were calculated using the evidence-based medicine calculator on the University of Toronto website.15
Table 1.
Quality scores of shoulder studies.a
| Reference | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Jadad score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 16 | + | + | ? | + | + | + | + | + | + | + | - | 5 |
| 17 | + | + | ? | + | + | + | + | + | + | + | - | 5 |
| 19 | + | + | + | + | + | + | + | + | + | + | - | 5 |
| 20 | - | + | - | + | ? | ? | + | - | - | + | - | 0 |
| 21 | + | + | - | + | + | ? | + | + | - | + | - | 2 |
| 23 | + | + | + | - | + | + | - | - | - | + | - | 1 |
| 22 | + | + | - | + | - | - | + | + | + | + | - | 2 |
| 18 | + | + | + | + | + | + | - | + | - | + | - | 3 |
Column numbers correspond to the following: 1 = study described as double blinded; 2 = subjects were randomly allocated to groups; 3 = allocation was concealed; 4 = groups were similar at baseline; 5 = subjects were blinded; 6 = practitioners who administered the intervention were blinded; 7 = assessors were blinded; 8 = measurements of key outcomes were obtained from >85% of subjects; 9 = data were analysed by intention to treat; 10 = statistical comparisons between groups were conducted; 11 = point measures and measures of variability were provided. + Indicates the criterion was clearly satisfied; − indicates that it was not; ? indicates that it is unclear whether criterion was satisfied. Jadad score: 1. Was the study described as randomised? 2. Was the study described as double blind? 3. Was there a description of withdrawals and dropouts? Give a score of 1 point for each ‘yes’ or 0 points for each ‘no’. Give 1 extra point if randomisation or blinding appropriate. Deduct 1 point if randomisation or blinding inappropriate. Score quality 0–5. Poor quality <3.
How this fits in
There are no systematic reviews of corticosteroids for shoulder pain that calculate the numbers needed to treat. We wished to determine the effectiveness in terms of improvement of symptoms of intra-articular and subacromial injections of corticosteroids for rotator cuff tendonitis and frozen shoulder. A systematic review and meta-analysis of randomised controlled trials indicates that subacromial injections of corticosteroids are effective for improvement of rotator cuff tendonitis up to a 9-month period. They are also probably more effective than non-steroidal anti-inflammatory drugs. Higher doses may be better than lower doses for subacromial corticosteroid injection for rotator cuff tendonitis.
RESULTS
The search for trials and reasons for exclusion are presented in Figure 1, with the seven trials found to meet the ‘versus placebo or NSAID’ inclusion criteria.16-22 An additional paper was found which met the inclusion criteria for corticosteroids versus NSAIDs.23
Figure 1.
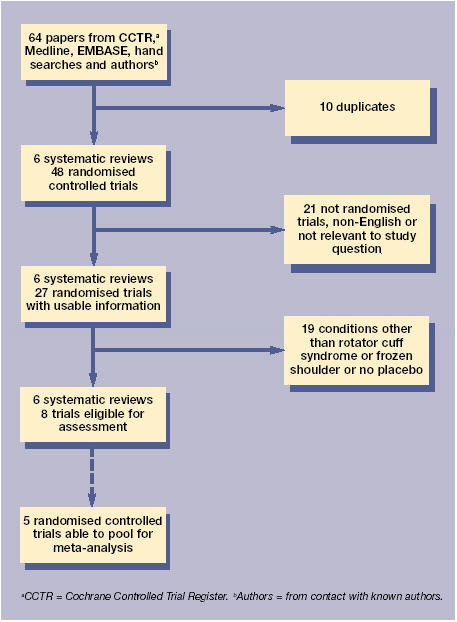
Process of inclusion of studies and usable information.
There were five studies that had data on improvement for subacromial injections versus placebo for rotator cuff tendonitis (Table 2). The terms that we interpreted as improvement (Supplementary Table 1) were ‘responder’,16 ‘decreased pain’,17 ‘remission’,19 ‘excellent result’20 and ‘complete remission’.21 There were two interventions in the Plafki et al study20 and hence in Table 2 the placebo group is halved to avoid double counting of that group. There was a significant improvement, with a relative risk of 3.08 (95% CI = 1.94 to 4.87). The numbers needed to treat for the statistically significant studies were between 1.4 (95% CI = 1 to 2) and 2.2 (95% CI = 1 to 5). The numbers needed to treat obtained from the pooled relative risk using a control event rate of 14.3%24 were 3.3 (95% CI = 1.8 to 7.7). There were no important harms other than transient redness and discomfort. None of the studies reported tendon rupture. Pooling our three high scoring (high quality) studies resulted in a pooled relative risk = 5.9 (95% CI = 2.8 to 12.6).16,17,19 Examination of study doses showed that the study with the highest dose (100 mg of prednisone equivalent) had the highest relative risk16 and the study with the lowest dose20 (26.66 mg of prednisone equivalent) had a non-significant result. Pooling of the three high dose (also the high quality studies) had a relative risk of 5.9 (95% CI = 2.8 to 12.6). A sensitivity analysis was conducted with the study by Blair et al17 removed as the outcomes for the corticosteroid group were assessed on average at 33 weeks while the placebo group was assessed at 28 weeks. The pooled relative risk was similar to that with Blair et al included.
Table 2.
Improvement for subacromial steroid for rotator cuff tendonitis.
| Reference | Control n/total | Treatment n/total | Weight % | Relative risk (fixed) (95% CI) |
| 16 | 14/20 | 0/20 | 2.67 | 29.00 (1.85 to 455.25) |
| 17 | 16/19 | 8/21 | 40.63 | 2.21 (1.24 to 3.94) |
| 19 | 7/25 | 2/25 | 10.69 | 3.50 (0.80 to 15.23) |
| 20a | 11/16 | 0/5 | 3.95 | 8.12 (0.56 to 117.70) |
| 20b | 8/16 | 0/5 | 3.95 | 6.00 (0.40 to 88.93) |
| 21 | 9/28 | 7/27 | 38.10 | 1.24 (0.54 to 2.86) |
| Total | 65/124 | 17/103 | 100.00 | 3.08 (1.94 to 4.87) |
Test for hetrogeneity χ2 = 9.14, degrees of freedom = 5 (P = 0.10). Test for overall effect Z = 4.79 (P<0.00001). aArm with dexamethasone. bArm with triamcinolone.
There was only one study for intra-articular corticosteroid injection and the effect was not significant.22 Pooling of the three trials of corticosteroids versus NSAIDs (Table 3) found a pooled relative risk for improvement of 1.43 (95% CI = 0.95 to 2.16).16,19,23 The numbers needed to treat to obtain one remission by giving a corticosteroid injection compared with an NSAID was 2.5 for the one significant study. Pooling of the two high quality studies of corticosteroids versus NSAID had a relative risk = 1.9 (95% CI = 1.06 to 3.4).16,19 As no non-English language studies were included it was decided to do a sensitivity analysis with the one study from a non-English speaking country (a German study published in an English language journal) being left out of the analysis.20 This did not make a substantial difference, with the relative risk = 3.3 (95% CI 1.2 = 9.2). A funnel plot revealed a possible absence of small trials with small effects. All of the clinicians giving the injections were rheumatologists, orthopaedic surgeons, internal medicine specialists or rehabilitation specialists. There was no difference between these groups.
Table 3.
Improvement for subacromial steroid injection versus NSAIDfor rotator cuff tendonitis.
| Reference | Control n/total | Treatment n/total | Weight % | Relative risk (fixed) (95% CI) |
| 16 | 14/20 | 6/20 | 28.60 | 2.33 (1.13 to 4.83) |
| 19 | 7/25 | 5/25 | 23.80 | 1.40 (0.51 to 3.82) |
| 23 | 9/15 | 10/15 | 47.60 | 0.90 (0.52 to 1.55) |
| Total | 30/60 | 21/60 | 100.00 | 1.43 (0.95 to 2.16) |
Test for hetrogeneity c2 = 4.49, degrees of freedom = 2 (P = 0.11). Test for overall effect Z = 1.70 (P = 0.09).
DISCUSSION
Summary of main findings
Our results show a significant benefit for subacromial corticosteroid injections versus placebo for painful shoulder. This is the first review to show a benefit for steroid injections in terms of the dichotomous variable improvement. The numbers needed to treat range between 1.4 and 2.2 patients, and, hence are clinically very significant. The numbers needed to treat in similar ranges were obtained by applying the pooled relative risk to the control event rates. It is also the first review to suggest that higher doses of corticosteroids may give greater improvement. The interpretation of the subgroups of higher doses requires caution as there was a range of doses and episode duration in the three studies. It is probably not possible in a series of clinical trials to identify safety issues such as tendon rupture. One reviewer claims that corticosteroid injections into the rotator cuff have not been shown to be deleterious but that it is logical to limit the number of local corticosteroid injections.6 Our findings also suggest that, rather than having no benefit when compared with NSAIDs, there may in fact be a large benefit, with a number needed to treat of 2.5. However, this interpretation is based on two high quality studies, although only one was statistically significant. We could find no steroids versus placebo studies for adhesive capsulitis.
Strengths and limitations of this study
A limitation of this review is possible publication bias, in that by missing unpublished or negative trials we may have overestimated the beneficial effect of subacromial corticosteroid injections. An analysis leaving out the one study from a non-English speaking country did not alter the findings. However, we are confident that most research in this field was identified by our comprehensive, systematic search strategy including hand searching and author contacts. All of the studies were conducted in outpatient settings and hence our findings are generalisable to those settings.
Comparison with existing literature
Our findings differ from the other reviews in that we report improvement. We feel that this is a more important patient-oriented outcome than increases in range of movement and/or pain reduction as it allows a number needed to treat to be calculated.25 Only the two reviews by Green et al3,7 and the one by Buchbinder et al8 attempted to pool the results of the papers. They did not pool the papers by Blair et al,17 Plafki et al20 and Vecchio et al21 in part because they did not have sufficient data to analyse for continuous data. We feel their omission of these papers is not warranted as they contain discrete data that are relevant to effectiveness and possibly more pertinent as they enable a number needed to treat to be calculated.
The reviews by McQuay et al,4 Goupille and Sibilia6 and van der Heijden et al5 commented on the poor quality of the literature and did not attempt to pool their findings.4-6
From our data the duration of benefit of subacromial corticosteroid injections appears to be from 3 to 38 weeks. The longer term benefit may not be so enduring, since a 2-year follow-up study of an effective (in the short-term) corticosteroid injection found no long-term difference between manipulation and physiotherapy, and that up to half of the patients experienced recurrent complaints.26 High doses of corticosteroids (50 mg equivalent of prednisone or greater) may be more effective than lower doses.
Implications for future research and clinical practice
In summary, our findings suggest that subacromial injections of corticosteroids are probably effective in rotator cuff tendonitis. They are probably more effective than NSAID medication. There is insufficient evidence to determine the effectiveness of intra-articular injections for rotator cuff tendonitis or for frozen shoulder. Our finding that using improvement as an outcome rather than pain or range of motion was significant suggests that authors of other musculoskeletal reviews may wish to consider a broader range of outcome measures. Further research is needed to examine different doses and repeated injections. Outcomes need to include dichotomous results so that numbers needed to treat can be calculated. The small numbers needed to treat may make GPs more likely to use subacromial steroids for rotator cuff syndrome as it is a relatively easy procedure to perform.
Supplementary Material
Supplementary information
Additional information accompanies this article at http://www.rcgp.org.uk/journal/index.asp
Funding body
This work was funded by the Accident Rehabilitation and Compensation Insurance Corporation
Competing interests
None
REFERENCES
- 1.Badcock LJ, Lewis M, Hay EM, et al. Chronic shoulder pain in the community: a syndrome of disability or distress? Ann Rheum Dis. 2002;61:128–131. doi: 10.1136/ard.61.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palmer KT, Cooper C, Walker-Bone K, et al. Use of keyboards and symptoms in the neck and arm: evidence from a national survey. Occup Med (Lond) 2001;5:392–395. doi: 10.1093/occmed/51.6.392. [DOI] [PubMed] [Google Scholar]
- 3.Green S, Buchbinder R, Glazier R, Forbes A. Interventions for shoulder pain: systematic review. Cochrane Musculoskeletal Group Cochrane Database of Systematic Reviews. 2002:3. doi: 10.1002/14651858.CD001156. [DOI] [PubMed] [Google Scholar]
- 4.McQuay HJ, Moore RA, Eccleston C, et al. Systematic review of outpatient services for chronic pain control. Health Technol Assess. 1997;1:i–iv. 1–135. [PubMed] [Google Scholar]
- 5.Van der Heijden GJ, van der Windt DA, Kleijnen J, et al. Steroid injections for shoulder disorders: a systematic review of randomised clinical trials. Br J Gen Pract. 1996;46:309, 316. [PMC free article] [PubMed] [Google Scholar]
- 6.Goupille P, Sibilia J. Local corticosteroid injections in the treatment of rotator cuff tendinitis (except for frozen shoulder and calcific tendinitis). Groupe Rhumatologique Francais de l'Epaule (G.R.E.P.) Clin Exp Rheumatol. 1996;14:561–566. [PubMed] [Google Scholar]
- 7.Green S, Buchbinder R, Glazier R, Forbes A. Systematic review of randomised controlled trials of interventions for painful shoulder: selection criteria, outcome assessment, and efficacy. BMJ. 1998;316:354–360. doi: 10.1136/bmj.316.7128.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchbinder R, Green S, Youd JM Cochrane Collaboration. The Cochrane Library. Issue 1. Oxford: Update Software; 2003. Corticosteroid injections for shoulder pain. (Cochrane Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guyatt G, Juniper EF, Walter SD, et al. Interpreting treatment effects in randomised trials. BMJ. 1998;316:690–693. doi: 10.1136/bmj.316.7132.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herbert RD, Gabriel M. Effects of stretching before and after exercising on muscle soreness and risk of injury: systematic review. BMJ. 2002;325:468. doi: 10.1136/bmj.325.7362.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verhagen AP, de Vet HC, de Bie RA, et al. The Delphi list: a criteria list for quality assessment of randomised clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol. 1998;51:1235–1241. doi: 10.1016/s0895-4356(98)00131-0. [DOI] [PubMed] [Google Scholar]
- 12.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports on randomised clinical trials. Is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 13.Mulrow CD, Oxman AD Cochrane Collaboration. The Cochrane Library. Issue 4. Oxford: Update software; 1997. Cochrane collaboration handbook. (Updated 1 March 1997) [Google Scholar]
- 14.Moher D, Cook DJ, Eastwood S, et al. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet. 1999;354:1896–900. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 15.Centre for Evidence Based Medicine. EBM calculator v1.2 http://www.cebm.utoronto.ca/palm/ebmcalc/ (accessed 24 Jan 2005)
- 16.Adebajo AO, Nash P, Hazleman BL. A prospective double blind dummy placebo controlled study comparing triamcinolone hexacetonide injection with oral diclofenac 50 mg TDS in patients with rotator cuff tendinitis. J Rheumatol. 1990;17:1207–1210. [PubMed] [Google Scholar]
- 17.Blair B, Rokito AS, Cuomo F, et al. Efficacy of injections of corticosteroids for subacromial impingement syndrome. J Bone Joint Surg. 1996;78:1685–1689. doi: 10.2106/00004623-199611000-00007. [DOI] [PubMed] [Google Scholar]
- 18.De Jong BA, Dahmen R, Hogeweg JA, Marti RK. Intra-articular triamcinolone acetonide injection in patients with capsulitis of the shoulder: a comparative study of two dose regimens. Clin Rehabil. 1998;12(3):211–215. doi: 10.1191/026921598673772420. [DOI] [PubMed] [Google Scholar]
- 19.Petri M, Dobrow R, Neiman R, et al. Randomised, double-blind, placebo-controlled study of the treatment of the painful shoulder. Arthritis Rheum. 1987;30:1040–1045. doi: 10.1002/art.1780300911. [DOI] [PubMed] [Google Scholar]
- 20.Plafki C, Steffen R, Willburger RE, Wittenberg RH. Local anaesthetic injection with and without corticosteroids for subacromial impingement syndrome. Int Orthop. 2000;24(1):40–42. doi: 10.1007/s002640050010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vecchio PC, Hazleman BL, King RH. A double-blind trial comparing subacromial methylprednisolone and lignocaine in acute rotator cuff tendinitis. Br J Rheumatol. 1993;32(8):743–745. doi: 10.1093/rheumatology/32.8.743. [DOI] [PubMed] [Google Scholar]
- 22.Berry H, Fernandes L, Bloom B, et al. Clinical study comparing acupuncture, physiotherapy, injection and oral anti-inflammatory therapy in shoulder-cuff lesions. Curr Med Res Opin. 1980;7:121–126. doi: 10.1185/03007998009112038. [DOI] [PubMed] [Google Scholar]
- 23.White RH, Paull DM, Fleming KW. Rotator cuff tendinitis: comparison of subacromial injection of a long acting corticosteroid versus oral indomethacin therapy. J Rheumatol. 1986;13:608–613. [PubMed] [Google Scholar]
- 24.Picavet HS, Schouten JS. Musculoskeletal pain in the Netherlands: prevalences, consequences and risk groups, the DMC(3)-study. Pain. 2003;102:167–178. doi: 10.1016/s0304-3959(02)00372-x. [DOI] [PubMed] [Google Scholar]
- 25.Liang MH, Lew RA, Stucki G, Fortin PR, Daltroy L. Measuring clinically important changes with patient-oriented questionnaires. Med Care. 2002;40(4 Suppl):II45–II51. doi: 10.1097/00005650-200204001-00008. [DOI] [PubMed] [Google Scholar]
- 26.Winters JC, Sobel JS, Groenier KH, et al. Comparison of physiotherapy, manipulation, and corticosteroid injection for treating shoulder complaints in general practice randomised, single blind study. BMJ. 1997;314:1320–1325. doi: 10.1136/bmj.314.7090.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


