Abstract
Background
Non-adherence with medication prescribed for chronic disease is ubiquitous and undermines the benefits of effective therapy.
Aim
To evaluate the influence of an educational booklet on thyroxine adherence and health in patients with primary hypothyroidism.
Design of study
Unblinded randomised clinical trial of individual patients (by stratified permutated blocks) to receive an ‘educational booklet’ or ‘usual care’.
Setting
Three general practices in the north-west of England serving 497 adults with primary hypothyroidism (prevalence 1.5%).
Method
A total of 332 adults who had been prescribed thyroxine for hypothyroidism were allocated to either a group that was posted a hypothyroid booklet addressing lay health beliefs or to a group that received usual care. Outcomes were mean within-subject change over 3 months in thyroid stimulating hormone (TSH), the SF-36 domains of vitality and general health, and a hypothyroid symptoms index. All results were concealed until the end of the trial.
Results
A total of 332 randomised patients were analysed by ‘intention to treat’ (TSH available for 330 patients). Groups were comparable at baseline, although ‘undetectable TSH’ was higher in the intervention than the control group (20% versus 13%). Mean change in TSH was −0.11mIU/L (intervention) and −0.12mIU/L (control). An absolute difference of 0.01mIU/L (95% confidence interval [CI] −0.93 to 0.94 mIU/L). Analysis adjusted (ANCOVA) for baseline TSH produced a difference of −0.12mIU/L (95% CI = −1.97 to 1.95). Changes in SF-36 and hypothyroid index were minimal. Trial participants were younger than non-participants and more likely to have a previous TSH in the normal range.
Conclusion
Brief intervention with an educational booklet has no influence on thyroxine adherence or health in patients with primary hypothyroidism. These findings do not support the routine distribution of health educational materials to improve medication adherence.
Keywords: Health education, hypothyroidism, patient compliance, randomised controlled trial, thyroxine
INTRODUCTION
Low adherence with self-administered medication is widespread.1,2 It affects all chronic conditions, with adherence rates averaging 50%.1-4 Non-adherence with medication undermines the benefits of medical therapy depending on both the efficacy of treatment and the actual level of non-adherence.2 Intervention to improve adherence could potentially produce large benefits for both individual patients and society.1
Evidence that medication adherence can be improved in routine clinical practice is lacking.1 Despite its importance, relatively few rigorous intervention trials to promote adherence have been undertaken.1 Rigorous randomised controlled trials of adequate size are required to detect clinically important benefits from interventions applicable to clinical practice, particularly in primary care where the majority of prescribing for chronic conditions occurs. Previous randomised controlled trials have usually been small and evaluated complex interventions that are not easily transferable into routine clinical practice.1
The greatest potential for improving medication adherence may lie in the widespread implementation of brief interventions that motivate large numbers of people to increase their medication adherence by small amounts,5,6 rather than by intensive intervention focused on a small number of extremely non-adherent individuals.5,6 The widespread use of clinical computer systems in primary care means that it is already feasible to target health education materials at patients with specific conditions.5 Such educational interventions, however, need to take account of the lay health beliefs that individuals with chronic conditions hold about their medicines.7
How this fits in
The practice of not adhering to self-administered medication for chronic conditions is extremely common. Depending on the efficacy of treatment and the level of non-adherence, it undermines the benefits of medical therapy. Several small clinical trials have suggested that printed materials can influence adherence. Provision of an educational booklet to patients with primary hypothyroidism had no influence on their thyroxine adherence or health. The evidence that medication adherence can be improved within routine clinical practice remains limited.
Our objective was to determine if patients prescribed thyroxine for primary hypothyroidism benefit from receiving an educational booklet addressing lay health beliefs about medicine taking in comparison with usual care.
METHOD
Study population and setting
Prescribing data from three computerised suburban group practices in north-west England were searched to identify adults (aged 18 years or over) who were prescribed thyroxine for primary hypothyroidism (due to thyroidectomy, radio-iodine treatment, or autoimmune thyroiditis). The identification and recruitment of patients is shown in Figure 1. All patients provided informed written consent to participate in the study.
Figure 1.
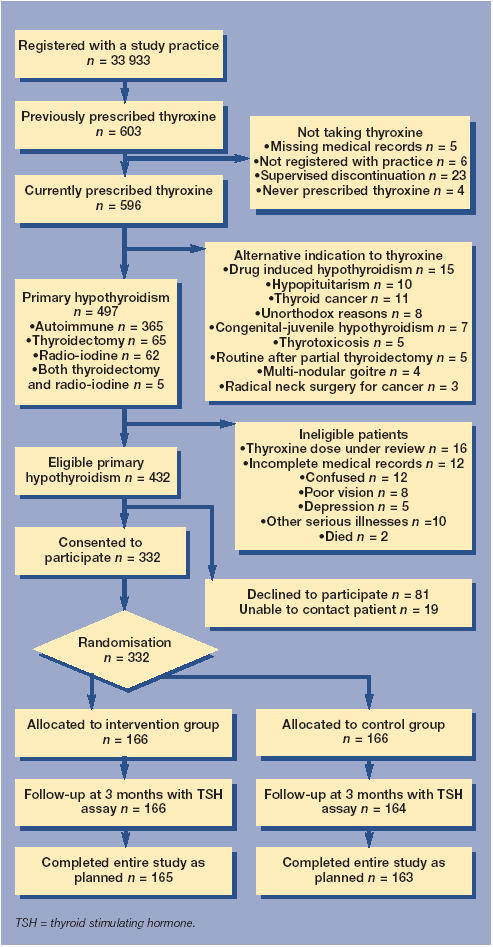
Study flow chart.
Broad inclusion criteria
Patients on a stable dose of thyroxine, whose dose had remained unchanged over the previous 8 weeks and who were not under active medical review were eligible for inclusion. Details of initial diagnosis and subsequent management were extracted from their medical records. Patients unable to provide informed written consent and those with serious illnesses were excluded. Patients were informed that the study was ‘to assess the benefits of giving additional information to people with an underactive thyroid gland’. The first patient was randomised on 12 March 1998; the final patient completed the study on 18 September 1998.
Home assessment
All participants received two home assessments 3 months apart. The sequence of events at all home visits followed a standardised approach: patients completed a questionnaire (that included the SF-36 and the hypothyroid symptom index), had their resting pulse and weight recorded, and provided a venous blood sample for a thyroid stimulating hormone (TSH) assay. All assessments were undertaken by a single investigator.
Stratified random permutated blocks
The study was a randomised controlled clinical trial in which half of the participants were allocated to receive the hypothyroid booklet by post a few days after initial assessment and the other half were to receive usual medical care (without receipt of the booklet until the end of the trial). Random allocation was by permutated blocks (stratified by GP) using schedules generated in advance from a random numbers table. The principal investigator generated and executed the schedules, which were concealed from participants and GPs.
The trial was unblinded as patients (and potentially their physicians if they directly asked their patients about receipt of the booklet) were aware of study allocation after randomisation. The laboratory staff performing the TSH assays were blind to patient allocation for the entire study. All TSH results were held and concealed on the laboratory computer until the trial ended; consequently neither the principal investigator, patients, nor GPs were aware of TSH results until completion of the whole trial.
Intervention with an educational booklet
The hypothyroid patient information booklet was developed following a review of the lay health beliefs that patients hold concerning medicine taking,7 existing patient educational materials,8,9 current consensus on the management of hypothyroidism,10,11 and the principles for producing effective printed educational materials.12 The theory underpinning the development of the educational booklet was the health action model, which conceptualises the influences of cognitive, affective, and normative factors on intended and actual health related behaviour.13,14
The seven-page booklet included a medication reminder sticker and calendar. It specifically addressed lay health beliefs about medicine taking. It had a high level of general readability with a Flesch reading ease score of 62 (equivalent to a mass circulation British tabloid newspaper).13 The booklet conformed to guidelines from the Plain English Campaign (www.plainenglish.co.uk) and the Centre for Health Information Quality (www.hfht.org). The booklet was successfully piloted in five patients with primary hypothyroidism before being used in the trial. The GPs in the study practices had no involvement in the development or distribution of the booklet.
TSH as the primary outcome
TSH is the best single measure of the level of thyroxine replacement in primary hypothyroidism.10,11 The delayed TSH response (of 4–6 weeks) to changes in thyroxine dose permits some of the inherent difficulties in assessing medication adherence to be circumvented — in particular, the phenomenon that patients who do not adhere to their regimen will often take their medication prior to clinical assessment (so-called ‘white coat adherence’). TSH was also selected as the primary outcome measure for other reasons:
The classical hormonal feedback mechanism means that there is a direct causal relationship between thyroxine and TSH;
Modern ultrasensitive TSH assays can detect small changes (0.1mIU/L) in TSH;
Change in TSH is a normally distributed continuous variable suitable for parametric analysis;
Changes in adherence could be detected even in patients already taking a sufficient proportion of their prescribed thyroxine to maintain their TSH within the reference range.
Quality control of TSH assays
TSH samples were analysed in a single biochemistry laboratory (Southport and Formby District General Hospital, Southport, UK) using an Abbot AxSYM System Ultrasensitive TSH assay (microparticle enzyme immunoassay, [hTSH-MEIA]). The AxSYM system was dedicated purely to hormonal assays. The AxSYM microprocessor was standardised using two master calibrators and six standard calibrators referenced against World Health Organisation samples (WHO-TSH 80/558). Three quality control calibrators (WHO-TSH 80/558) were tested daily. The laboratory holds full clinical pathology accreditation (www.cpa-uk.co.uk) and participates in the UK National External Quality Assessment Service (UK-NEQAS, www.ukneqas.org.uk) for thyroid hormones. The AxSYM System remained within the UK-NEQAS monthly monitored standards for the entire study period.
Sample size calculation
A pilot survey in a separate general practice of 66 patients with primary hypothyroidism (80% on a stable thyroxine dose) found a mean within-subject change in TSH of 0.33mIU/L (standard deviation [SD] 4.42mIU/L) ranging from −15.4 to 15.0mIU/L over 12 months. Several GPs, who were not involved in the trial, indicated that they would increase thyroxine if TSH was consistently 1.5mIU/L above the reference range. With a type I error of 5% and a type II error of 20% (power 80%), a target sample size of 274 patients was required to detect a minimum difference of 1.5mIU/L in the mean within-subject change in TSH between groups.15
Statistical analysis
No data analysis was undertaken until completion of the whole trial. Change in individual patients' TSH between baseline and follow-up (within-subject) was identified a priori as the primary outcome measure. Analysis was by the ‘intention to treat’ of individual patient data.15 Data analysis was performed using Microsoft Excel for Windows and SPSS for Windows. Within-subject change in TSH was normally distributed. Baseline TSH differences between the two groups were adjusted using ANCOVA in SPSS.16 The TSH reference range (0.5–4.7mIU/L, Abbott Laboratories, Abbott Park, Illinois, US) was used to categorise baseline TSH (as undetectable, suppressed, normal and elevated TSH) for pre-planned subgroup analysis.
The two short-form 36 (SF-36) domains of vitality and general health were selected in advance to avoid multiple comparisons. SF-36 scores were calculated by analysis of complete cases (a higher SF-36 score indicating better health on a 100-point scale).17
The hypothyroid symptom index was a modified version of the validated Billewicz index.18 It consisted of 10 hypothyroid symptoms that were scored with the same weightings as in the original index (a reduced score indicating a reduction in hypothyroid symptoms on 72-point scale).18
RESULTS
Study sample
The three general practices (comprising 18 physicians) served a combined registered population of 33 933 (with a similar age–sex structure to the general population of England). The trial flow chart shows the identification and progress of patients in the study (Figure 1). A total of 497 patients prescribed thyroxine for primary hypothyroidism (autoimmune thyroiditis, thyroidectomy, radio-iodine therapy, both thyroidectomy and radio-iodine therapy) were identified. The point prevalence for treated primary hypothyroidism was 1.5% (95% CI = 1.3 to 1.6%). A total of 332 (67%) patients participated in the trial. Participants were on average 8 years younger than non-participants, were more likely to have had their TSH checked within the previous 12 months as part of their routine medical care, and were more likely to have had a recent TSH level within the reference range.
Baseline characteristics of patients at randomisation
The two groups were comparable at baseline (Table 1). Although the prescribed dose of thyroxine was similar in both groups (1.7mcg/kg in the intervention group versus 1.6mcg/kg for controls), more patients in the intervention group had undetectable TSH at baseline compared with the control group (20% versus 13%). A total of 329 (99%) participants had biochemically confirmed hypothyroidism, the three patients lacking biochemical confirmation had all undergone total thyroidectomy in the past.
Table 1.
Baseline characteristics of patients randomised with primary hypothyroidism (n = 332).
| Intervention group (n = 166) | Control group (n = 166) | |||
|---|---|---|---|---|
| Patient characteristics | n | % | n | % |
| Mean age, years (SD) | 61.3 (13.8) | – | 62.3 (12.3) | – |
| Female | 152 | 92 | 153 | 92 |
| No formal educational qualificationsa | 43 | 26 | 50 | 30 |
| Home owner | 147 | 89 | 144 | 87 |
| Diagnosis of primary hypothyroidism | ||||
| Autoimmune thyroiditis | 123 | 74 | 113 | 68 |
| Mean age at diagnosis, years (SD) | 51.8 (14.2) | – | 52.9 (13.5) | – |
| Median duration, years (IQR) | 6 (3–13) | – | 6 (3–12) | – |
| Symptomatic at diagnosisb | 109 | 66 | 103 | 62 |
| Previous history of thyrotoxicosis | 37 | 22 | 37 | 22 |
| Provision of medical care | ||||
| GP care alone over last 12 months | 145 | 87 | 138 | 83 |
| TSH checked within last 12 months | 135 | 81 | 134 | 81 |
| Median numbers of GP visits in 12 months (IQR) | 6 (4–9) | – | 6 (3–9) | – |
| Thyroxine only prescribed medication | 36 | 22 | 42 | 25 |
| Thyroxine therapy and baseline TSH | ||||
| Mean dose thyroxine, mcg/kg (SD) | 1.7 (0.6) | – | 1.6 (0.6) | – |
| Undetectable TSH (0mIU/L) | 34 | 20 | 22 | 13 |
| Suppressed TSH (0.1 to 0.4mIU/L) | 30 | 18 | 29 | 17 |
| Normal TSH (0.5 to 4.7mIU/L) | 83 | 50 | 96 | 58 |
| Elevated TSH (4.8mIU/L or more) | 19 | 11 | 19 | 11 |
| Generic and disease-specific health | ||||
| Mean SF-36: vitality (SD) | 46.3 (21.9) | – | 49.4 (23.0) | – |
| Mean SF-36: general health (SD) | 57.0 (23.2) | – | 60.0 (22.6) | – |
| Mean Hypothyroid Index (SD)c | −8.4 (16.9) | – | −10.6 (13.1) | – |
IQR = interquartile range. SD = standard deviation. TSH = thyroid stimulating hormone.
Information missing for 8 participants.
Information missing for 27 participants.
Hypothyroid Index (10 items) is a modified version of the Billewicz Hypothyroid Index.18
Patient follow-up
The time interval between baseline and follow-up assessments was a median of 96 days for both groups. On average only 2 days elapsed between telephone contacts and home visits. Most patients remained on the same dose of thyroxine throughout the trial (89% intervention; 92% control). Only two control group patients were completely lost to follow-up (one patient died and one developed severe depression). Baseline and follow-up TSH was available for 330 patients and completed questionnaires available for 328 patients (an additional two patients failed to complete the final questionnaire due to depression and confusion).
At the second assessment 22% of patients in both groups admitted to knowing another trial participant; however, no patients reported showing or being shown the intervention booklet by another participant in the trial.
Change in TSH (n = 330)
The mean within-subject change in TSH was −0.11mIU/L in the intervention group and −0.12mIU/L in the control group with an absolute difference between groups of 0.01mIU/L (95% CI = −0.93 to 0.94mIU/L) (Table 2). At baseline more patients in the intervention than the control group had undetectable TSH levels (Table 1). Baseline TSH also demonstrated regression to the mean, being negatively correlated with change in TSH over follow-up (Pearson correlation coefficient −0.42; 95% CI = −0.34 to −0.51). Consequently analysis of covariance (ANCOVA)16 was employed to adjust for differences in TSH at baseline. The absolute difference in mean within-subject change in TSH, (adjusted for baseline TSH using ANCOVA) was −0.12mIU/L (95% CI = −1.97 to 1.95).
Table 2.
Main outcome measures of mean ‘within-subject’ change in TSH, SF-36, and Hypothyroid Index (n = 330).
| Intervention group | Control group | ||||||
|---|---|---|---|---|---|---|---|
| n | Mean change | SD | n | Mean change | SD | Difference in means (95% CI) | |
| TSH (mIU/L)a | 166 | −0.11 | 3.47 | 164 | −0.12 | 5.05 | 0.01 (−0.93 to 0.94) |
| SF-36: vitality | 164 | 2.6 | 14.6 | 163 | −0.4 | 15.9 | 2.9 (−0.4 to 6.3) |
| SF-36: general health | 163 | 1.5 | 14.6 | 163 | 0.1 | 14.2 | 1.4 (−1.7 to 4.6) |
| Hypothyroid Indexb | 166 | −2.9 | 12.1 | 163 | −0.6 | 12.1 | −2.3 (−4.9 to 0.3) |
TSH = thyroid stimulating hormone. SF-36 = Short Form 36. SD= standard deviation.
TSH is the primary outcome measure.
Hypothyroid Index (10 items) is a modified version of the Billewicz Hypothyroid Index.18
The overall results remained essentially the same with analysis restricted to patients remaining on the same thyroxine dose and also in those with detectable levels of TSH at baseline.
Symptomatic change
Patients who received the booklet showed some symptomatic improvement when compared to those receiving routine care. This improvement was seen for both vitality and general health on the SF-36 and also on the hypothyroid index. However, all these changes were small and not statistically significant (Table 2).
Subgroup analysis by baseline TSH
As the overall comparison failed to demonstrate any difference, a subgroup analysis should be interpreted cautiously. For patients with elevated TSH at baseline (TSH ≥4.8mIU/L), the mean reduction in TSH was 3.09mIU/L (95% CI = −2.57 to 8.76mIU/L) higher in the control than the intervention group (Table 3), although this is based on data from only 38 patients. Changes in TSH in the other three subgroups were much smaller (Table 3). None of the changes in TSH in subgroup analysis were statistically significant.
Table 3.
Subgroup analysis of mean ‘within-subject’ change in TSH categorised by baseline TSH (n = 330).
| Intervention group | Control group | ||||||
|---|---|---|---|---|---|---|---|
| n | Mean change | SD | n | Mean change | SD | Difference in means (95% CI) | |
| Undetectable (0.0) | 34 | 0.11 | 0.55 | 22 | 0.02 | 0.04 | 0.09 (−0.15 to 0.32) |
| Suppressed (0.1 to 0.4) | 30 | 0.10 | 0.23 | 29 | 0.33 | 1.22 | −0.23 (−0.69 to 0.22) |
| Normal (0.5 to 4.7) | 83 | 0.24 | 2.62 | 94 | 0.79 | 4.77 | −0.55 (−1.72 to 0.61) |
| Elevated (≥4.8) | 19 | −2.37 | 8.49 | 19 | −5.46 | 8.73 | 3.09 (−2.57 to 8.76) |
TSH = thyroid stimulating hormone (mIU/L). SD = standard deviation.
Hypothyroid information booklet
At follow-up, 153 (92%) patients in the intervention group recalled receiving the booklet and 148 (89%) recalled reading it; 70% read it more than once. In response to a satisfaction questionnaire administered at the end of the trial, almost all the patients who recalled reading the booklet had found it easy to read (99%) and understand (99%). Most found the booklet personally relevant (82%) and many had kept it for future reference (88%).
DISCUSSION
Summary of main findings
Intervention in primary care with a carefully designed educational booklet (intended to increase thyroxine adherence in primary hypothyroidism) had no clinically or statistically important influence on either adherence (as measured by TSH) or health related quality of life and symptoms of hypothyroidism. Changes in the primary outcome measure of TSH were minimal and unadjusted analysis excluded any clinically important change in TSH (of 1.0mIU/L or more in either direction). This lack of clinical impact occurred despite the intervention booklet being very highly regarded by patients. Further subgroup analysis failed to demonstrate any benefit from the booklet in patients with either detectable or elevated TSH levels at baseline assessment.
Strengths and the limitations of the study
The intervention booklet had considerable merit. It was produced after a careful review of the literature on hypothyroidism and lay health beliefs about medicine taking. The booklet met explicit criteria for the creation of effective educational materials and had a high level of readability. It was highly rated by those patients who received it. Although our results relate specifically to this booklet we would be surprised if a modified booklet could have a substantially greater impact.
Randomisation produced two comparable groups, both of whom received the same level of clinical care other than the planned intervention. Although the trial was unblinded, there was no evidence of any unintended influences on physician management (such as changes to the thyroxine dose prescribed), or ‘contamination’ of the control group by exposure to the intervention booklet. The primary outcome measure (TSH) was an objective assessment that was not subject to influence by knowledge of patient allocation — all TSH samples were assayed in an identical manner, laboratory staff were unaware of patient allocation, and TSH results were concealed until the completion of the trial.
Participants in the trial appeared to be somewhat more compliant than non-participants: trial participants were more likely to have had their TSH checked within the previous 12 months and for their most recent TSH to be within the reference range. Our findings that the intervention did not positively alter adherence behaviour could be anticipated if trial participants were already highly adherent with their thyroxine. However, in response to a previously validated questionnaire19 concerning the difficulties that people often have in taking thyroxine tablets (administered at the end of the trial), 22% (72/327) of participants admitted to thyroxine non-adherence — a level of self-reported non-adherence that is similar to other chronic conditions where non-adherence is recognised as a major challenge.19,20
As almost all of our patients had previous biochemical confirmation of their hypothyroidism, ‘diagnostic misclassification’ is also unlikely to account for our study findings.
The level of TSH suppression in the study practices was unanticipated. In the pilot study, only 3% of patients had undetectable TSH levels on ultrasensitive assay (compared with 17% at baseline in the trial). This baseline ‘TSH flooring effect’ limited the responsiveness of TSH to changes in thyroxine adherence due to the inability to detect subsequent reductions in TSH. However, the overall results remained the same, with the analysis restricted only to patients with detectable baseline TSH. Adjustment for baseline differences in TSH between the intervention and control groups did not alter the overall results of the trial.
The careful design and conduct of this clinical trial is reflected in the high participation rate with minimal losses to follow-up. We would anticipate that use of the same intervention elsewhere would produce broadly similar results. Patients with biochemically confirmed hypothyroidism who took part in our study are likely to be similar to those treated elsewhere. Our high prevalence (higher than in previous studies)21,22 suggests that we successfully identified the majority of patients with treated hypothyroidism. Our broad inclusion criteria permitted the recruitment of a wide spectrum of patients with primary hypothyroidism, a large proportion of whom (67%) participated. Consequently, the trial was adequately powered to produce a precise and generalisable estimate of the effect of intervention.
Comparison with the existing literature
Although no previous clinical trials have attempted to improve thyroxine adherence in primary hypothyroidism, evidence from other randomised controlled trials suggests that intervention with printed materials can be effective. Five randomised controlled trials (one of factorial design, hence six comparisons) involving chronic conditions23-27 have attempted to improve medication adherence with printed health educational materials with an objective measure of adherence. All the trials are relatively small (range 87 to 210 patients), but four reported statistically significant changes in medication adherence in patients with hypertension,26 those discharged from hospital,23 and long-term users of benzodiazepines.24,25 The factorial study found no benefit from an asthma booklet.27
Implications for future research and clinical practice
This trial suggests that the provision of additional printed educational materials to patients with established primary hypothyroidism has no influence on their adherence to thyroxine replacement therapy (or any influence on their health). Although physicians have a duty to ensure that patients receive adequate information about their health, the trial does not support the routine provision of additional health educational materials to patients with primary hypothyroidism in order to improve their adherence or well-being. The evidence that medication adherence can be influenced within routine clinical practice remains limited.
Acknowledgments
We are grateful to all the patients and practice staff for participating in this trial.
Additional information
Further information about the trial is available elsewhere.13 The original trial documentation is archived at Aberdeen University under the institutions research governance framework. This documentation would permit the reconstruction and reanalysis of the trial from the original data. The trial adheres to the revised CONSORT (2001) reporting requirements (www.consort-statement.org).
Funding body
The study was funded with a grant from the Clare Wand Fund of the British Medical Association.
Ethics committee
Written ethical approval for the trial was granted by North Sefton, Research Protocol 233 (16 December 1997) and South Sefton, EC.184.97 (24 February 1998) Local Research Ethics Committees.
Competing interests
None
REFERENCES
- 1.Haynes R, McDonald H, Garg A, Montague P. The Cochrane Library. Issue 3. Chichester: John Wiley & Sons; 2004. Interventions for helping patients to adhere to prescriptions for medications. Cochrane Review. [Google Scholar]
- 2.Haynes RB, Taylor DW, Sackett DL, editors. Compliance in health care. Baltimore: The Johns Hopkins University Press; 1979. [Google Scholar]
- 3.Sackett DL. The hypertensive patient: 5. Compliance with therapy. Can Med Assoc J. 1979;121(3):259–261. [PMC free article] [PubMed] [Google Scholar]
- 4.Inui TS, Carter WB, Pecoraro RE, et al. Variations in patient compliance with common long-term drugs. Med Care. 1980;18(10):986–993. doi: 10.1097/00005650-198010000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Lennox AS, Osman LM, Reiter E, et al. Cost effectiveness of computer tailored and non-tailored smoking cessation letters in general practice: randomised controlled trial. BMJ. 2001;322:1396. doi: 10.1136/bmj.322.7299.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rose G. The strategy of preventive medicine. Oxford: Oxford University Press; 1992. [Google Scholar]
- 7.Blaxter M, Britten N. Lay beliefs about drugs and medicines and the implications for community pharmacy: A review paper. Report Number 5. Manchester: Pharmacy Practice Research Resource Centre University of Manchester; 1996. [Google Scholar]
- 8.Toft A. Understanding thyroid disorders. Guildford: Family Doctor Publications; 1996. [Google Scholar]
- 9.Bayliss RIS. Thyroid disease: the facts. Oxford: Oxford University Press; 1982. [Google Scholar]
- 10.Vanderpump MP, Ahlquist JA, Franklyn JA, Clayton RN. Consensus statement for good practice and audit measures in the management of hypothyroidism and hyperthyroidism. The Research Unit of the Royal College of Physicians of London, the Endocrinology and Diabetes Committee of the Royal College of Physicians of London, and the Society for Endocrinology. BMJ. 1996;313:539–544. doi: 10.1136/bmj.313.7056.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singer PA, Cooper DS, Levy EG, et al. Treatment guidelines for patients with hyperthyroidism and hypothyroidism. Standards of Care Committee, American Thyroid Association. JAMA. 1995;273(10):808–812. [PubMed] [Google Scholar]
- 12.Ley P, Llewelyn S. Improving patients' understanding recall satisfaction and compliance. In: Broome A, Llewelyn S, editors. Health psychology. 2nd edn. London: Chapman & Hall; 1995. pp. 75–98. [Google Scholar]
- 13.Crilly M. Thyroxine adherence study: a randomised controlled clinical trial in primary care. Manchester: University of Manchester; 2003. (MD thesis) [Google Scholar]
- 14.Tones K, Tilford S. Effectiveness, efficiency and equity. 2nd edn. London: Chapman and Hall; 1994. Health education. [Google Scholar]
- 15.Pocock SJ. Clinical trials. A practical approach. Chichester: John Wiley & Sons; 1983. [Google Scholar]
- 16.Vickers A, Altman D. Statistics notes: Analysing controlled trials with baseline and follow up measurements. BMJ. 2001;323:1123–1124. doi: 10.1136/bmj.323.7321.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ware J, Snow KK, Kosinski M, Gandek B. SF-36 Health survey manual and interpretation guide. Boston: The Health Institute, New England Medical Center; 1993. [Google Scholar]
- 18.Billewicz WZ, Chapman RS, Crooks J, et al. Statistical methods applied to the diagnosis of hypothyroidism. Q J Med. 1969;38(150):255–266. [PubMed] [Google Scholar]
- 19.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Haynes RB, Taylor DW, Sackett DL, et al. Can simple clinical measurements detect patient noncompliance? Hypertension. 1980;2(6):757–764. doi: 10.1161/01.hyp.2.6.757. [DOI] [PubMed] [Google Scholar]
- 21.Parle JV, Franklyn JA, Cross KW, et al. Thyroxine prescription in the community: serum thyroid stimulating hormone level assays as an indicator of undertreatment or overtreatment. Br J Gen Pract. 1993;43:107–109. [PMC free article] [PubMed] [Google Scholar]
- 22.Tunbridge WM, Evered DC, Hall R, et al. The spectrum of thyroid disease in a community: the Whickham survey. Clin Endocrinol (Oxf) 1977;7(6):481–493. doi: 10.1111/j.1365-2265.1977.tb01340.x. [DOI] [PubMed] [Google Scholar]
- 23.Raynor DK, Booth TG, Blenkinsopp A. Effects of computer generated reminder charts on patients' compliance with drug regimens. BMJ. 1993;306:1158–1161. doi: 10.1136/bmj.306.6886.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cormack MA, Sweeney KG, Hughes-Jones H, Foot GA. Evaluation of an easy, cost-effective strategy for cutting benzodiazepine use in general practice. Br J Gen Pract. 1994;44:5–8. [PMC free article] [PubMed] [Google Scholar]
- 25.Bashir K, King M, Ashworth M. Controlled evaluation of brief intervention by general practitioners to reduce chronic use of benzodiazepines. Br J Gen Pract. 1994;44:408–412. [PMC free article] [PubMed] [Google Scholar]
- 26.Gabriel M, Gagnon JP, Bryan CK. Improved patients' compliance through use of a daily drug reminder chart. Am J Public Health. 1977;67(10):968–969. doi: 10.2105/ajph.67.10.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jenkinson D, Davison J, Jones S, Hawtin P. Comparison of effects of a self management booklet and audiocassette for patients with asthma. BMJ. 1988;297:267–270. doi: 10.1136/bmj.297.6643.267. [DOI] [PMC free article] [PubMed] [Google Scholar]


