Abstract
This study examined the relation of infant emotional responses of anger and sadness to cortisol response in 2 goal blockage situations. One goal blockage with 4-month-old infants (N = 56) involved a contingency learning procedure where infants’ learned response was no longer effective in reinstating an event. The other goal blockage with 6-month-old infants (N = 84) involved the still face procedure where infants’ reactions to their mothers’ lack of responsivity were not effective in reestablishing interaction. For both blockages, sadness was related to cortisol response, though anger was not—the greater the sadness, the higher the cortisol response. This differential relation is consistent with other evidence indicating the more positive role of anger as opposed to sadness in overcoming an obstacle.
Darwin (1872/1965) considered anger to be an adaption that evolved as part of the action patterns designed to overcome a blockage of a desired goal. Consistent with Darwin’s view, early work with children found facial expressions of anger to be associated with or accompanied by increased instrumental responding to goal blockage (Amsel, 1962; Amsel & Roussel, 1952). More recent work reviewed by Lemerise and Dodge (2000) also demonstrated that from early infancy, anger in response to goal blockage is associated with attempts to overcome the obstacle. In contrast, sadness often occurs in the absence of any attempt to overcome a blocked goal (Lewis & Goldberg, 1969; Watson, 1972; White, 1959). Sadness in children can be accompanied by a belief in the inefficacy or inadequacy of one’s efforts or ability to deal with the obstacle (Dweck, 1991, 1998; Seligman, 1975). Work by Dweck and colleagues (e.g., Burhans & Dweck, 1995; Smiley & Dweck, 1994) suggests that sadness following failure is associated with less optimal patterns of achievement motivation, perceived competence, and self-worth in children. Work by other investigators on learned helplessness and mastery motivation in children supports Dweck’s view that sadness in response to challenging situations reflects task withdrawal that is related to less optimal outcome (Kelley & Jennings, 2003; Kistner, Ziegert, Castro, & Robertson, 2001; MacTurk & Morgan, 1995; Messer, 1993). The association of anger to instrumental activity has been seen as reflecting the presence of perceived control to overcome the obstacle, whereas the association of sadness to inactivity has been seen as reflecting the absence of control to overcome the obstacle (e.g., Campos, Barrett, Lamb, Goldsmith, & Stenberg, 1983, Table 4; Saarni, Mumme, & Campos, 1997, Table 5.1). That is, anger and instrumental activity reflect the presence of control, whereas sadness and inactivity reflect the absence of control. Anger rather than sadness should be the predominant emotional response to goal blockage given that anger reflects a more positive response to overcoming the obstacle (cf. Bennett, Bendersky, & Lewis, 2002; Izard, 1977; Izard & Malatesta, 1987). Anger can be distinguished from rage in that rage is more intense, involves behaviors not directed toward a goal, and reflects the absence of control (Lewis, 1993; Retzinger, 1987; Scheff, 1987).
Besides emotional response differences to a blocked goal, there are data on differences in stress, in particular, adrenocortical response to stress, related to goal blockage. Theory and research suggest that adrenocortical activation to goal blockage is most likely to occur when perceived control to overcome the obstacle is absent (Henry, 1992; Levine, Coe, & Wiener, 1989; Levine & Wiener, 1989). For example, work reviewed by Levine et al. (1989) found that animals that do not have the control to terminate aversive stimulation show considerably enhanced adrenocortical responses relative to yoked controls who do have the control yet receive the same aversive stimulation. On the basis of this and other evidence, Henry (1992) proposed that instrumental activity in response to a blocked goal would be associated with various physiological changes including sympathetic activation (i.e., arousal) but that it would not be associated with adrenocortical activation unless or until it was apparent that the effort was not proving effective in overcoming the obstacle. Thus, if anger in response to a blocked goal reflects the perceived control to overcome the obstacle, anger would not be expected to be associated with increases in cortisol response.
If sadness to a blocked goal reflects the absence of control over the obstacle, sadness would be expected to be associated with increases in cortisol response. Consistent with Dweck’s (e.g., Burhans & Dweck, 1995; Smiley & Dweck, 1994) work, shame as well as sadness can occur in reaction to task failure (Alessandri & Lewis, 1996; Belsky, Domitrovich, & Crnic, 1997; Lewis, Alessandri, & Sullivan, 1992). Both emotional responses have been associated with problems in coping, including engaging in instrumental activity; thus, sadness and shame reflect the lack of control over the situation (Lewis, 1992; Mills, in press). Lewis and Ramsay (2002) examined the relation of shame to cortisol response in reaction to task failure. They found that greater expression of shame is related to higher cortisol response. Thus, in the present work, greater expression of sadness was expected to be related to a higher cortisol response in reaction to goal blockage. Sadness to goal blockage reflects the lack of control over the obstacle, which leads to increases in cortisol response.
Using two situations involving goal blockage, one social and the other nonsocial, the present study looked at the individual emotional expressions that infants show and examined whether there is a differential relation of particular emotional responses to cortisol response in reaction to each blockage. Two blockages were used because, if Darwin (1872/1965) is correct, the differential role of anger and sadness and their differential relation to cortisol response should exist for all events that involve the blockage of a desired goal regardless of the social or cognitive nature of the obstacle. That is, each blockage should elicit individual differences in anger and sadness as well as in other emotional expressions, and for each blockage, increases in sadness, but not anger, should be related to increases in cortisol response. This differential relation of sadness and anger to cortisol response may hold only for goal blockage situations. There could be another class or family of events where sadness is a more positive or optimal emotional response than anger. For this class of events, we would predict a relation between greater anger, but not sadness, and higher cortisol responses. Moreover, we would expect sadness as opposed to anger to be the predominant emotional response to such events. Thus, we take a contextual approach to differences in infant emotional expression (cf. Bennett et al., 2002). With respect to emotion contextual differences, goal blockage situations, involving a disruption in infant control, may prove especially sensitive in eliciting infant differences in anger and sadness as they relate to cortisol response. Research with adults suggests the importance of contextual differences, including differences in the presence or absence of control, in eliciting increases in cortisol response (Dickerson & Kemeny, 2004).
The nonsocial blockage was a frustrated contingency learning response used by Lewis and colleagues (Alessandri, Sullivan, & Lewis, 1990; Lewis, Alessandri, & Sullivan, 1990; Lewis, Sullivan, Ramsay, & Alessandri, 1992; Sullivan & Lewis, 2003). In this situation, infants learn that arm-pulling leads to an interesting event. After learning the contingency, a blockage occurs where their arm-pulling is no longer effective in producing the event. In response to the blockage, infants show increased anger and sadness as well as increased arm-pulling. Only increased anger, not sadness, is related to increased arm-pulling, suggesting that increased anger and arm-pulling reflect infants’ attempt to reinstate the outcome (Lewis et al., 1990). Moreover, increased anger and sadness to the blockage are each related to different emotional expressions once the blockage is lifted. When arm-pulling once again leads to the event, infants who showed anger to the blockage demonstrate more interest, whereas infants who showed sadness to the blockage demonstrate less joy (Lewis, Sullivan, et al., 1992). When the blockage involves the occurrence of the event noncontingent on arm-pulling, infants show increased anger but not increased arm-pulling, suggesting that the anger reflects the disruption in infants’ expected control over the contingency (Sullivan & Lewis, 2003). These results support the view that anger represents a positive response to goal blockage associated with both perceived control and instrumental activity to overcome the obstacle.
The social blockage was the still face manipulation developed by Tronick and colleagues (Tronick, 2003; Tronick, Als, Adamson, Wise, & Brazelton, 1978). The still face situation has been extensively used to study differences in the patterns of mother –infant interaction (e.g., Adamson & Frick, 2003; Cohn, 2003; Field, 1994; Gianino & Tronick, 1988; Muir & Lee, 2003; Tronick, 1989). The standard still face procedure involves three successive phases: engagement, still face, and re-engagement. The engagement and re-engagement phases involve infants and mothers in an ongoing face-to-face interaction consisting of behavioral exchanges between the two participants. The still face phase involves infants’ attempts to deal with their mothers’ sudden lack of responsivity. In a recent review, Adamson and Frick (2003) argued that infants’ reaction to the still face blockage is to attempt to reestablish the social interaction. Similarly, infants’ reaction to the contingency learning blockage is to attempt to reinstate the interesting outcome. There are large individual differences in infants’ emotional responses, including anger and sadness to the still face blockage, as well as in the extent of recovery or lack thereof of emotional response during the re-engagement phase (e.g., Bazhenova, Plonskaia, & Porges, 2001; Bendersky & Lewis, 1998; Haley & Stansbury, 2003; Segal et al., 1995; Weinberg & Tronick, 1996). For a sample of 6-month-old infants, Weinberg and Tronick (1996) found that facial expressions of anger and sadness were unlikely to occur during the engagement phase, increased in frequency during the still face phase, and continued to occur at approximately the same frequency during the re-engagement phase.
In the present study, we used the contingency learning and still face situations to examine our hypothesis that there would be a sadness – cortisol relation, but not an anger – cortisol relation, for each blockage. Although it would have been advantageous to use both blockages with the same infants to provide additional support for Darwin’s (1872/1965) view (e.g., that there should be cross-situation consistency in patterns of emotional and cortisol responses), we used each blockage with a different sample of infants. Infants in the two samples came from different ethnic and socioeconomic family backgrounds. Because, following Darwin, we view the differential role of anger as opposed to sadness in response to goal blockage as universal, we expected different goal blockages to elicit comparable differences in individual emotional expressions and a comparable relation of individual emotional responses to cortisol response in infants regardless of any demographic or ethnic differences.
This view is supported by research using the contingency learning and the still face situations with both White middle-class and African American lower-class infants. Using the contingency learning situation, Alessandri, Sullivan, Imaizumi, and Lewis (1993) looked at a sample of poor African American infants, and Lewis et al. (1990) looked at a sample of middle-class White infants. Findings suggest no demographic or ethnic differences in amount of emotional expressions during this situation. Similarly, using the still face situation, Bendersky and Lewis (1998) and Segal et al. (1995) each looked at a sample of poor African American infants, and Weinberg and Tronick (1996) looked at a sample of middle-class White infants. Findings also suggest no demographic or ethnic differences in amount of emotional expressions during this situation. Thus, there is no reason to expect infant demographic or ethnic differences in the relation of individual emotional responses to cortisol response in reaction to goal blockage.
In the present study, we used the contingency learning situation with 4-month-old infants and the still face situation with 6-month-old infants. There is disagreement in the literature over whether the separate emotions of anger and sadness as opposed to a more generalized negative emotional state (distress, upset) are present in early infancy (Lemerise & Dodge, 2000; Lewis, 2000). Evidence for the presence of a more generalized negative emotional state as opposed to discrete emotions includes findings in young infants that negative emotional expressions such as anger and sadness tend to co-occur, that blended expressions are common, and that they are not specific to particular contexts (Camras, Malatesta, & Izard, 1991; Camras, Sullivan, & Michel, 1993). On the other hand, our past contingency learning findings reviewed earlier suggest that anger and sadness are already distinct emotions as early as 2 months of age. For example, anger is related to increased arm-pulling, whereas sadness is not related to arm-pulling, for infants from 2 to 8 months of age (Lewis et al., 1990). In addition to these findings, our past contingency learning work includes evidence of short-term cross-age stability of individual differences in infant anger and sadness to the blockage at successive 2-month intervals between 2 and 8 months (Sullivan, Lewis, & Alessandri, 1992). There is no argument that anger and sadness are distinct emotions by 8 months. Thus, their cross-age stability down to 2 months indicates that these emotions already are distinct even at the youngest age. Consistent with our findings, other work has found that anger is present at 4 and 7 months but not at 1 month (Stenberg & Campos, 1990, Stenberg, Campos, & Emde, 1983). In this work at the older ages, facial components of anger occurred more frequently than facial components of other emotions in response to infants’ mother or a stranger removing a biscuit from infants’ mouths. Moreover, there is evidence for stability of individual differences in anger and sadness to inoculation from 2 to 6 to 19 months (Izard, Hembree, & Huebner, 1987). Finally, a recent study found evidence that sadness was distinct from other negative emotion at 6 months (Buss et al., 2003). This study found relations involving sadness, high cortisol, and relatively greater right than left frontal EEG activation in support of the view that sadness is a withdrawal as opposed to an approach emotion (Davidson, 1995). Thus, there is evidence that anger and sadness are already distinct emotions by 4 to 6 months. As the finding that other constructs (e.g., arm-pulling) are differentially related to the expression of anger and sadness indicates that they are distinct emotions, the finding for a differential relation of cortisol response to anger and sadness would provide additional evidence that they already are distinct emotions by this point in development.
Study 1
Method
Participants
Participants consisted of 56 four-month-old infants (M age = 4.0 months, SD =.3; 29 females, 27 males). The racial ethnicity of the sample was 91% White, 2% Hispanic, and 7% Asian. All mothers had received at least a high school degree, with most (68%) having received a college degree. Thirteen additional infants were excluded because of equipment problems (n = 4), fussy behavior (n = 3), and failure to complete the procedure (n = 6). For this and the following study, mothers were recruited for participation during their hospital stay following the birth of their infant. At the time, all infants were being cared for in the hospital well-baby nursery. Infants did not have any known developmental problem at the time of the assessment. Informed consent was obtained from mothers before beginning the assessment.
Procedure
Contingency learning
The contingency learning situation involved pulling on a string attached to infants’ wrist where each arm-pull could activate a 3-s audiovisual display that consisted of a color slide of an infant’s smiling face and a recording of children’s voices singing (Alessandri et al., 1990; Lewis, Alessandri, et al., 1990, 1992; Sullivan & Lewis, 2003). The situation had three successive phases: baseline, learning, and extinction. The baseline phase was a 2-min period of nonreinforcement during which arm-pulling did not activate the display. The learning phase was a 6-min period of contingent stimulation during which arm-pulling did activate the display. The extinction phase was a 2-min period of nonreinforcement (i.e., procedurally identical to the baseline phase). During the procedure, infants were seated in a reclining infant seat in a three-sided booth facing a rear-projection screen mounted in the rear wall of the booth with an audiocassette player positioned behind the screen. Infants’ faces were video-recorded from a camera behind the rear wall through an opening below the screen. A parent sat behind the infants out of the infants’ view.
Infant emotional expressions were coded from the videotapes using AFFEX (Izard, Dougherty, & Hembree, 1983), the whole face version of the Maximally Discriminative Facial Movement Coding System (MAX; Izard, 1995). Past work using MAX has provided evidence of the reliability of the facial coding system in the contingency learning situation (e.g., Lewis, Alessandri, et al., 1990, 1992; Sullivan & Lewis, 2003) as well as the still face situation (e.g., Bendersky & Lewis, 1998; Weinberg & Tronick, 1996). Past work also has provided evidence for the reliability of MAX and AFFEX in other situations (e.g., Izard et al., 1995; Izard et al., 1987). Given that we had used MAX in our previous contingency learning work, we were confident that using the less time-consuming AFFEX would not sacrifice reliability or accuracy.
AFFEX coding was done from the videotapes in slow motion with volume off second-by-second for the duration of the procedure. Following the coding system, individual emotional expressions were then identified on the basis of the AFFEX formulas. The individual emotional expressions were joy, surprise, anger, and sadness. In addition to the individual expressions of anger and sadness, anger-sadness blends were coded. We were interested in examining the different types of emotional expressions that infants were likely to show across the phases of the contingency learning situation as well as assessing the relation of a given individual emotional response to cortisol response while controlling for the other emotional responses to the blockage. For each emotional expression, the number of expressions per minute was determined for each phase of the situation. For each expression, a summary measure of emotional response to the blockage was indexed by the change in the frequency of the expression from the learning to the extinction phase (extinction minus learning).
The coding was done by three experienced coders who were unaware of the cortisol data and of the predicted relation between emotional and cortisol responses. Reliability was assessed by computing ê statistics on a random sample of 9 infants scored by two coders. The average ê coefficient for the ratings of the facial expressions combined over the total session was .86 (joy, κ =.92; surprise, κ =.69; anger, κ =.87; sadness, κ =.93; anger-sadness blend, κ =.91). All kappas indicated above-chance agreement for all expressions scored.
Cortisol
Two salivary cortisol samples were obtained from the infants: the first shortly after arrival at the laboratory before the start of the contingency learning procedure, and the second 20 min after the end of the procedure. This 20-min interval was chosen to index infants’ peak response to the stress of the extinction phase of the procedure (cf. Gunnar, Broderson, Krueger, & Rigatuso, 1996; Lewis & Ramsay, 1995, 1999). To collect each sample, an absorbent dental cotton roll was applied to the tongue, cheeks, and gums of the infants. A syringe was then used to express the saliva into labeled test tubes. Usually the application of only one cotton roll was necessary for collection of a sufficient quantity of saliva for analysis. Each sample collection took no longer than 1 min. Oral stimulants to increase saliva flow were not used so as to avoid any problem with the cortisol assay associated with their use (Schwartz, Granger, Susman, Gunnar, & Laird, 1998). No infant was fed in the minutes before collecting the samples so that milk contamination of the assay was not an issue (Magnano, Diamond, & Gardner, 1989). Samples were immediately refrigerated and then frozen for storage until they were shipped in dry ice to our laboratory (Covance, Chantilly, VA) for the cortisol assay. The assay used the Salivary Cortisol Mnemonic 6410 protocol. The assay was run in duplicate for which a minimum sample volume of 100 ul was needed. The criterion for reanalysis was a coefficient of variation greater than 20%. The lower limit of quantitation of the assay is .007 μg/dl. The intra- and inter-assay coefficients of variation are both less than 10%.
The cortisol data were screened for outlying values defined as any value greater than 3 SDs above the mean for a given time point (Gunnar, Mangelsdorf, Larson, & Hertsgaard, 1989; Magano, Gardner, & Karmel, 1992; Lewis & Ramsay, 1995). To maintain the sample size, each outlying value (a total of five values) was replaced by a value that was proportional to the one nonoutlying value relative to the mean values for the infants with both samples. This was done by solving for a missing individual pre- or post-stressor value using the following formula: individual prestressor value/individual poststressor value = mean prestressor value/mean poststressor value. There was no difference in the results regardless of whether the data for the outliers were included in the analyses.
Mean pre- and post-stressor cortisol levels were .70 (SD =.35) and .71 (SD =.31) μg/dl, respectively. There was not a reliable pre- to post-stressor increase in cortisol level for the group as a whole (by one-way analysis of variance [ANOVA]). The absence of a group cortisol response has typically been found for the stressors that have been used in studies with infants and children (e.g., Davis, Bruce, & Gunnar, 2002; Gunnar et al., 1989; Lewis & Ramsay, 2002; Schmidt et al., 1999; Spangler & Grossmann, 1993; Stansbury & Harris, 2000). Nonetheless, these studies have all successfully related differences in cortisol response to other constructs. In the present work, we were interested in the relation between individual differences in infant cortisol response and emotional responses to goal blockage, regardless of the absence of significant mean change in cortisol level over time.
Cortisol response was indicated by the difference (post- minus pre-stressor) between the two cortisol samples. Cortisol and other physiological responses are subject to the law of initial value (LIV; Lacey, 1956; Lewis & Ramsay, 1995; Lewis & Thomas, 1990; Wilder, 1956). The LIV involves a negative relation between pre-stressor cortisol level and cortisol response. For the present sample, there was a significant LIV effect (r = −.72, df = 54, p<.001). To remove this effect, as in past work (cf. Lacey, 1956; Lewis & Thomas, 1990), regression analysis was used to obtain a residualized cortisol response measure that was independent of pre-stressor cortisol level such that the correlation between the residualized measure and prestressor level was zero. This residualized response measure (M = 0, SD = 1) was used in the data analysis. This residualized measure is numerically the same as the residualized measure that results from regressing poststressor level on pre-stressor level. Skewness in the two cortisol levels was not a factor in the results. A residualized response score obtained from log10 transformations of the two cortisol values yielded the same results as those described here.
Time of day
To accommodate the schedules of mothers and infants, it was necessary for the testing to be done throughout the day (8:45 a.m. to 6:30 p.m.). Arrival time at the laboratory was noted to examine whether there were differences in cortisol response or emotional responses as a function of time of day. There was no relation of arrival time to the cortisol response measure (r = −.03) or to any emotional response measure (rs =.19, −.12, .05, −.04, and .19 for joy, surprise, anger, sadness, and anger-sadness blends, respectively). Data analyses including arrival time (partial correlation, predictor in regression, covariate in analysis of covariance [ANCOVA]) yielded the same results as those described here. Therefore, time of day was not considered further.
Results and Discussion
The following results are described: (a) change in the individual emotional expressions by phase of the contingency learning procedure, (b) relation of emotional and cortisol responses to the blockage of the extinction phase of the procedure, and (c) cortisol response as a function of increased sadness as opposed to anger to the blockage.
Change in Emotional Expressions to the Blockage
Figure 1 shows the frequency of occurrence of each emotional expression for the baseline, learning, and extinction phases of the contingency learning procedure. There was reliable change in the frequency of each emotional expression across the three phases (by repeated measures multivariance analysis of variance [MANOVA]): joy, Wilks’s F(2, 54) = 4.18, p<.03; surprise, p<.001; anger, p<.001; sadness, p<.003; and anger-sadness blends, p<.002. The interest here was in emotional expression changes from learning to extinction that reflect emotional response to the blockage. Both joy and surprise decreased from learning to extinction (ps<.006 and .001 by one-way ANOVA, respectively). Anger, sadness, and anger-sadness blends increased from learning to extinction (p<.008 or better).
Figure 1.
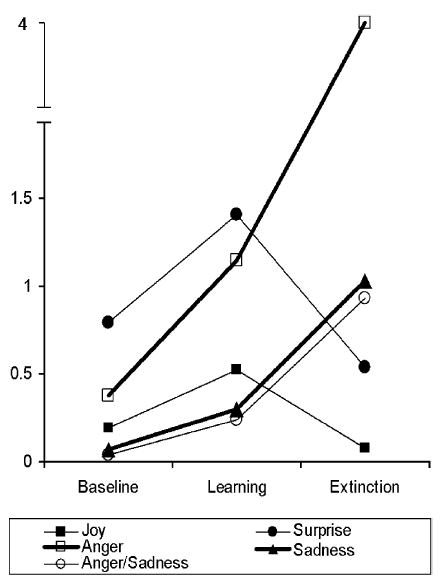
Frequency of occurrence of each emotional expression by phase of the contingency learning procedure.
Relation Between Emotional and Cortisol Responses
Table 1 gives the relation of the emotional responses (i.e., extinction minus learning) to cortisol response. Correlations (Pearson r) between emotional responses and cortisol response yielded a significant relation for sadness (r =.37, df = 54, p<.006) but not for anger or for the other emotional expressions. Greater increases in sadness were associated with higher cortisol responses to the blockage. Partial correlations were performed between each emotional response and cortisol response, controlling for the other emotional responses. These correlations also yielded a significant relation for sadness (partial r =.43, df = 50, p<.002) but not for anger or for the other emotional expressions. To follow up these results, a regression analysis was performed with emotional responses for the individual emotional expressions as the predictors and cortisol response as the dependent variable. This regression yielded a significant R2 of .20, F(5, 50) = 2.46, p<.05. Emotional response only for sadness was related to cortisol response (β=.53, p<.002).
Table 1.
Relation Between Emotional Responses and Cortisol Response to the Contingency Learning Blockage
| Emotion | Pearson r | Partial r | Regression b |
|---|---|---|---|
| Joy | −.09 | −.13 | −.13 |
| Surprise | −.04 | −.07 | −.07 |
| Anger | .05 | −.17 | −.20 |
| Sadness | .37** | .43** | .53** |
| Anger/Sadness | .02 | −.14 | −.15 |
p<.01.
Study 2
Method
Participants
Participants consisted of 84 six-month-old infants (M age = 5.9 months, SD =.4; 35 females, 49 males). Six months is the most frequent age at which the still face procedure has been used (Adamson & Frick, 2003). The racial ethnicity of the sample was 9.5% White, 78.6% African American, 8.3% Hispanic, and 3.6% Other. Approximately half the mothers (51.2%) had not received a high school degree, with the remainder (48.8%) having received at least a high school degree. Two additional infants were excluded because of failure to provide the two cortisol saliva samples.
Procedure
Still face
The still face situation consisted of three successive phases: engagement, still face, and re-engagement (Tronick, 2003; Tronick et al., 1978). The engagement phase was a 2-min period of face-to-face mother – infant interaction. The still face phase was a 2-min period during which the mother remained quiet and still while maintaining a head-down posture with a neutral facial expression. The re-engagement phase involved a 90-s period of renewed mother – infant interaction. During the procedure, infants were seated in a reclining infant seat secured to a table. Mothers were seated in a chair to the front of the table at eye level with their infants. The chair was at a slight angle to the table so that a clear view of the infants’ faces could be obtained for videotaping by a camera positioned behind a one-way mirror directly in front of the infants.
Infant emotional expressions were coded from the videotapes using MAX (Izard, 1995). Coding of the brows, eyes, and mouth was done from the videotapes in slow motion with volume off second-by-second for the last 15 s of the engagement phase and for the entire duration of the still face and re-engagement phases. Only the last 15 s of the engagement phase was coded because it was considered representative of infants’ undisturbed emotional behavior. Following the coding system, facial expressions that were likely to occur with some frequency in the situation were then identified on the basis of the MAX formulas. The emotional expressions were joy, interest, anger, and sadness. Because of the slight time variation of the phases, the proportion of time each emotional expression occurred was determined for each phase of the procedure. For each emotional expression, a summary measure of emotional response to the blockage (comparable to that used for the contingency learning blockage) was indexed by the change in the frequency of the expression from the engagement to the still face phase (still face minus engagement).
The coding was done by two experienced coders who were unaware of the cortisol data and of the predicted relation between emotional and cortisol responses. The coders had achieved high interrater reliability (κs>.80) with each other and with other coders in the scoring of data from previous studies.
Cortisol
Two salivary cortisol samples were obtained from the infants, the first shortly after arrival at the laboratory before the start of the still face procedure, and the second 20 min after the end of the still face phase of the procedure. Samples were collected, stored, and assayed as described earlier. The cortisol data were screened for outlying values, and outlying values (a total of three) were replaced as described earlier. There was no difference in the results regardless of whether the data for the outliers were included in the analyses. Mean pre- and post-stressor cortisol levels were .56 (SD =.35) and .60 (SD =.41) μg/dl, respectively. There was not a reliable pre- to post-stressor increase in cortisol level for the group as a whole (by one-way ANOVA). Cortisol response was indicated by the difference (post- minus pre-stressor) between the two cortisol samples. Because there was a significant LIV effect (r = −.38, df = 82, p<.001), regression analysis was used to obtain a residualized cortisol response measure that was independent of prestressor cortisol level. This residualized response measure was used in the data analysis. Skewness in the two cortisol levels was not a factor in the results. A residualized response score obtained from log10 transformations of the two cortisol values yielded the same results as those described here.
Time of day
Testing was conducted throughout the day. There was no relation of arrival time at the laboratory (8:30 a.m. to 4:45 p.m.) to the cortisol response measure (r = −.05) or to any emotional response measure (rs = −.01, −.07, .11, and −.02 for joy, interest, anger, and sadness, respectively). Data analyses including arrival time yielded the same results as those described here.
Results and Discussion
The following results are described: (a) change in the individual emotional expressions by phase of the still face procedure, (b) relation of emotional and cortisol responses to the blockage of the still face phase of the procedure, (c) cortisol response as a function of increased sadness as opposed to anger to the blockage, and (d) relation of both emotional response to the blockage and recovery of emotional response after the blockage to cortisol response.
Change in Emotional Expressions to the Blockage
Figure 2 shows the proportion of time each emotional expression was shown for the engagement, still face, and re-engagement phases of the still face procedure. There was reliable change in the proportion of time the emotional expressions of joy and anger were shown across the three phases, Wilks’s Fs(2, 82) = 40.98 and 7.07, ps<.001 and .002, respectively, by repeated measures MANOVA. Joy decreased from engagement to still face and increased from still face to re-engagement (p<.001 in each case by one-way ANOVA). Anger increased from engagement to still face (p<.002) but did not change reliably from still face to re-engagement. Interest and sadness did not show reliable change across the three phases. With respect to sadness, there was a trend (p<.09 by one-way ANOVA) for sadness to increase from engagement to still face.
Figure 2.
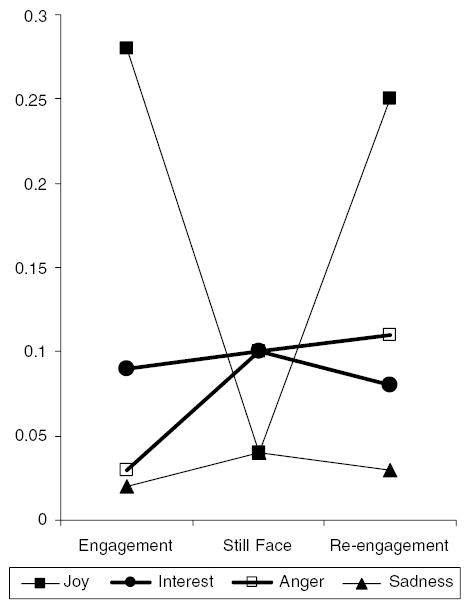
Proportion of time each emotional expression was shown by phase of the still face procedure.
Relation Between Emotional and Cortisol Responses
Table 2 gives the relation of the emotional responses (i.e., still face minus engagement) to cortisol response. Correlations (Pearson r) between emotional responses and cortisol response yielded a significant relation for sadness (r =.37, df = 82, p<.001) but not for anger or for the other emotional expressions. Greater increases in sadness were associated with higher cortisol responses to the blockage. Partial correlations were performed between each emotional response and cortisol response, controlling for the other emotional responses. These correlations also yielded a significant relation for sadness (partial r =.44, df = 79, p<.001) but not for anger. To follow up these results, a regression analysis was performed with emotional responses for the individual emotional expressions as the predictors and cortisol response as the dependent variable. This regression yielded a significant R2 of .21, F(4, 79) = 5.26, p<.002. Emotional response for sadness was related to cortisol response (β=.49, p<.001). The partial correlation and regression analyses (but not the correlation analyses) also indicated that increases in joy were related to higher cortisol responses to the blockage.
Table 2.
Relation Between Emotional Responses and Cortisol Response to the Still Face Blockage
| Emotion | Pearson r | Partial r | Regression b |
|---|---|---|---|
| Joy | .14 | .23* | .21* |
| Interest | −.02 | .19 | .19 |
| Anger | −.02 | −.01 | −.01 |
| Sadness | .37*** | .44*** | .49*** |
p<.05.
p<.001.
Relation of Emotional Response or Emotional Recovery to Cortisol Response
The relation between emotional responses and cortisol response could reflect emotional response to the blockage or recovery of emotional response after the blockage. It was of interest to determine the relation of emotional response and recovery of response for each emotional expression to cortisol response. To address this question, a regression analysis was performed with separate emotional response and recovery scores for each emotional expression as the predictors and cortisol response as the dependent variable. The measure of emotional response was the difference in frequency of emotion between the engagement and still face phases (still face minus engagement, as analyzed earlier). The measure of emotional recovery was the difference in frequency of emotion between the still face and re-engagement phases (re-engagement minus still face). These scores were obtained for each emotional expression. This regression yielded a significant R2 of .25, F(8, 75) = 3.18, p<.005. Emotional response of sadness was related to cortisol response (β=.53, p<.001), whereas emotional recovery of sadness was not (β=.05). Emotional response and emotional recovery of anger, interest, and joy were not related to cortisol response (βs = −.19 to .13).
Emotional Expressions During the Still Face and Contingency Learning Blockages
In Darwin’s (1872/1965) view, anger should be the predominant emotion expressed in response to goal blockage. This was examined for the three emotional expressions—anger, sadness, and joy—that were coded for both the still face and the contingency learning blockage. For each blockage, the frequency of each emotional expression relative to the frequency of the other emotional expressions was compared. For anger, sadness, and joy, respectively, the mean ratios of the amount of the emotion to the average amount of the other emotions were, respectively, 4.88, 1.44, and 1.82 for the still face blockage, and 4.31, .33, and .06 for the contingency learning blockage. A two-way (blockage, emotion) MANOVA performed on these data yielded a significant main effect only for blockage, Wilks’s F(2, 137) = 4.37, p<.02. This effect reflected the fact that anger was the predominant emotion expressed to each blockage. Despite the absence of a significant Blockage × Emotion interaction, the data suggested that whereas anger was expressed comparably across the two blockages, sadness and joy were expressed more to the still face than to the contingency learning blockage (p<.02 and p =.053, respectively, by one-way ANOVA).
General Discussion
The results indicate a relatively specific relation of the expression of greater sadness, but not anger and other emotional expressions, to higher cortisol responses in reaction to the blockage of a desired goal in young infants. The specificity of the relation of sadness to cortisol response was found for two goal blockages: one in a contingency learning situation and the other in the still face situation. Results were comparable for the two blockages in that both obstacles elicited increases in anger and sadness, with increases in sadness, but not anger, related to increases in cortisol response. That greater anger was not associated with higher cortisol responses is consistent with Darwin’s view (1872/1965) that anger is a more positive response to goal blockage. Past work has found that anger to a blocked goal is associated with perceived control and instrumental activity to overcome the obstacle as well as with more positive emotion after the obstacle has been removed (Lewis, Alessandri, et al., 1990, 1992; Sullivan & Lewis, 2003). The relative absence of stress might be expected with this pattern of responding. On the other hand, that greater sadness was associated with higher cortisol responses is consistent with Henry’s (1992) view that cortisol activation to goal blockage occurs when efforts to overcome the obstacle are ineffective. Sadness to goal blockage reflects the lack of control over the obstacle, which leads to increases in cortisol response.
Following Darwin (1872/1965), we believe that the differential role of anger and sadness in reaction to goal blockage will prove to be universal. If so, the present results would have to be obtained for infants regardless of differences in their ethnic and socioeconomic family backgrounds. Using the contingency learning situation, Alessandri et al. (1993) looked at a sample of poor African American infants, and Lewis et al. (1990) looked at a sample of middle-class White infants. Similarly, using the still face situation, Bendersky and Lewis (1998), Segal et al. (1995), and the present work all looked at samples of poor African American infants, and Weinberg and Tronick (1996) looked at a sample of middle-class White infants. Findings suggest no demographic or ethnic differences in amount of emotional expressions during both situations. Thus, there is no reason to expect infant demographic or ethnic differences in the relation of individual emotional responses to cortisol response in reaction to goal blockage. In fact, in the present work, the differential relation of anger versus sadness to cortisol response in reaction to goal blockage was comparable across two studies that used infants from different ethnic and demographic backgrounds. This is not to say that ethnic or demographic differences might not be important in other aspects of infant emotional expression (Camras, Oster, Campos, Miyake, & Bradshaw, 1992; Lewis, Ramsay, & Kawakami, 1993).
If the differential role of anger and sadness in reaction to goal blockage is universal, the present findings would have to be obtained with other goal blockages regardless of the nature of the obstacle. That is, regardless of the blockage, there should be a sadness – cortisol relation but not an anger – cortisol relation. Various situations, including those that are frustrating, could involve goal blockage. For us, the major defining feature of a blockage situation is that the situation involves a disruption in infant expectations of control. The relation between emotional and cortisol responses should be examined in a variety of blockage situations as the basis for confirming the universality of the differential anger-sadness to cortisol relation proposed here. As noted earlier, that the expression of anger is not related to higher cortisol responses may hold only for the anger that is expressed in reaction to goal blockage. There could be another class of events where anger is not, but sadness is, a more positive or optimal emotional response such that greater anger, but not sadness, is related to higher cortisol responses. For example, imagine your emotional response to someone declining to do you a favor that you had asked. Here, anger could well be a less optimal emotional response than sadness, with anger as opposed to sadness being associated with higher cortisol responses. In infants, physical pain such as to inoculation (Lewis & Ramsay, 1995, 1999) might prove to be another such class of events.
For the differential role of anger and sadness in reaction to goal blockage to be universal, it would have to be characteristic of individual infants. Evidence for this would include the presence of cross-situation and cross-age consistency of individual differences in patterns of emotional and cortisol responses to goal blockage (cf. Sullivan et al., 1992). If these patterns are characteristic of individual infants, they should prove to be differentially related to various outcomes at older ages (cf. Hill & Braungart-Rieker, 2002; Moore, Cohn, & Campbell, 2001). If so, we would propose that early differences in the presence or absence of perceived control reflect differences in volition or will underlying goal-directed activity. As such, we would expect these early differences in reaction to goal blockage to be related subsequently to other manifestations of will, for example, persistence in mastery situations (MacTurk & Morgan, 1995; Messer, 1993; Yarrow, Klein, Lomonaco, & Morgan, 1975). That is, early anger and low cortisol should be related subsequently to task persistence, whereas early sadness and high cortisol should be related subsequently to task withdrawal. It is of interest to examine differences not just in emotional response to goal blockage but also in emotional recovery of response once the blockage has been removed. The present still face study found differences in the extent to which there was recovery of the individual emotional expressions, including the rebound in joy and the continued occurrence of anger and sadness, that are consistent with past work (Bendersky & Lewis, 1998; Weinberg & Tronick, 1996). It was not possible to examine emotional recovery in the present contingency learning study because the contingency learning procedure did not involve a postextinction phase where arm-pulling was once again effective in producing the interesting event. Past work that has used such a postextinction phase found patterns of emotional recovery, including increases in joy and decreases in anger and sadness, comparable to those seen in the re-engagement phase of the still face procedure (Lewis, Alessandri, et al., 1990, 1992). These recovery results indicate that emotional responses to the blockage likely reflect the disruption in infant expectations of control over the contingency rather than simply increased boredom or upset over time. That emotional responses to the blockage reflect disruption in infant expectations of control also is indicated by other results that indicate emotional responses to the blockage occur only for experimental infants receiving contingent reinforcement during learning as opposed to time-yoked control infants receiving non-contingent reinforcement during learning (Lewis et al., 1990).
In the present still face study, it was possible to examine the relation of emotional response and recovery to cortisol response for the individual emotional expressions. Having found a relation of sadness to cortisol response, we were able to determine whether this relation was specific to emotional response versus emotional recovery of sadness. The cortisol increase was related to the elicitation of sadness, not its recovery. This specificity of the relation suggests that it reflects the disruption in infant expectations of control over the interaction with their mothers rather than increased boredom or upset over time.
Although relations of positive emotional responses such as joy to cortisol response were not expected, for the present still face situation there was some evidence of increased joy associated with higher cortisol responses in reaction to the blockage. It is possible that increased joy to the blockage reflects some infants’ attempts to reinstate interaction with their mothers, which led to increased stress because the attempt was not successful. The expression of joy was more frequent to the still face than to the contingency learning blockage. This difference could reflect the social nature of the still face situation, where joy during the blockage serves the goal for some infants to reinstate interaction with their mothers. If this interpretation is correct, it would provide additional evidence for Henry’s (1992) view that a cortisol response to goal blockage does not occur unless or until efforts to overcome the obstacle are not effective.
Whereas past work has related infant negative emotionality or temperament to cortisol response (e.g., Gunnar, Porter, Wolf, Rigatuso, & Larson, 1995; Keenan, Grace, & Gunthorpe, 2003; Ramsay & Lewis, 2001), with the one exception noted earlier (Buss et al., 2003), past infant cortisol work has not related infant emotional response for individual emotions to cortisol response. Haley and Stansbury (2003) assessed cortisol response to the still face procedure in a sample of 5- to 6-month-old infants. A relatively global measure of infant negative emotionality that did not distinguish anger and sadness was obtained. It is not surprising, in view of the present results and those by Buss et al. (2003), that these investigators did not report any relations of negative emotionality to cortisol response. They did find relations of negative emotionality to heart rate. In this regard, as noted earlier, Henry’s (1992) view suggests that increased anger, but not increased sadness, to goal blockage would be expected to be associated with sympathetic activation reflecting the arousal involved in attempting to overcome the obstacle. That anger and sadness expressions are differentially related to sympathetic and adrenocortical responses would provide evidence that they are distinct emotions reflecting different emotional states in addition to previous findings for a differential relation of anger as opposed to sadness to instrumental behavior in reaction to goal blockage.
References
- Adamson LB, Frick JE. The still face: A history of a shared experimental paradigm. Infancy. 2003;4:451 – 473. [Google Scholar]
- Alessandri SM, Lewis M. Differences in pride and shame in maltreated and nonmaltreated pre-schoolers. Child Development. 1996;67:1857 – 1869. [PubMed] [Google Scholar]
- Alessandri SM, Sullivan MW, Imaizumi S, Lewis M. Learning and emotional responsivity in cocaine exposed infants. Developmental Psychology. 1993;29:989 – 997. [Google Scholar]
- Alessandri SM, Sullivan MW, Lewis M. Violation of expectancy and frustration in early infancy. Developmental Psychology. 1990;26:738 – 744. [Google Scholar]
- Amsel A. Frustrative nonreward in partial reinforcement and discrimination learning. Psychological Review. 1962;69:306 – 328. doi: 10.1037/h0046200. [DOI] [PubMed] [Google Scholar]
- Amsel A, Roussel J. Motivational properties of frustration. Journal of Experimental Psychology. 1952;43:363 – 368. doi: 10.1037/h0059393. [DOI] [PubMed] [Google Scholar]
- Bazhenova OV, Plonskaia O, Porges SW. Vagal reactivity and affective adjustment in infants during interaction challenges. Child Development. 2001;72:1314 – 1326. doi: 10.1111/1467-8624.00350. [DOI] [PubMed] [Google Scholar]
- Belsky J, Domitrovich C, Crnic K. Temperament and parenting antecedents of individual differences in three-year-old boys’ pride and shame reactions. Child Development. 1997;68:456 – 466. [PubMed] [Google Scholar]
- Bendersky M, Lewis M. Arousal modulation in cocaine-exposed infants. Developmental Psychology. 1998;34:555 – 564. [PMC free article] [PubMed] [Google Scholar]
- Bennett DS, Bendersky M, Lewis M. Facial expressivity at 4 months: A context by expression analysis. Infancy. 2002;3:97 – 113. doi: 10.1207/S15327078IN0301_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burhans KK, Dweck CS. Helplessness in early childhood: The role of contingent worth. Child Development. 1995;66:1719 – 1738. [PubMed] [Google Scholar]
- Buss KA, Schumacher JRM, Dolski I, Kalin NH, Goldsmith HH, Davidson RJ. Right frontal brain activity, cortisol, and withdrawal behavior in 6-month-old infants. Behavioral Neuroscience. 2003;117:11 – 20. doi: 10.1037//0735-7044.117.1.11. [DOI] [PubMed] [Google Scholar]
- Campos, J. J., Barrett, K. C., Lamb, M. E., Goldsmith, H. H., & Stenberg, C. (1983). Socioemotional development. In P. H. Mussen (Series Ed.) & M. M. Haith & J. J. Campos (Vol. Eds.) Handbook of child psychology: Vol. 2. Infancy and developmental psychobiology (4th ed., pp. 783 – 915). New York: Wiley.
- Camras, L. A., Malatesta, C. Z., & Izard, C. E. (1991). The development of facial expressions in infancy. In R. S. Feldman & B. Rimé (Eds.), Fundamentals of nonverbal behavior (pp. 73 – 105). New York: Cambridge University Press.
- Camras LA, Oster H, Campos JJ, Miyake K, Bradshaw D. Japanese and American infants’ responses to arm restraint. Developmental Psychology. 1992;28:578 – 583. [Google Scholar]
- Camras LA, Sullivan J, Michel G. Do infants express discrete emotions? Adult judgments of facial, vocal, and body actions. Journal of Nonverbal Behavior. 1993;17:171 – 186. [Google Scholar]
- Cohn JF. Additional components of the still-face effect: Commentary on Adamson and Frick. Infancy. 2003;4:493 – 497. [Google Scholar]
- Darwin, C. R. (1965). The expression of the emotions in man and animals Chicago: University of Chicago Press. (Original work published 1872)
- Davidson, R. J. (1995). Cerebral asymmetry, emotion and affective style. In R. J. Davidson & K. Hugdahl (Eds.), Brain asymmetry (pp. 361 – 387). Cambridge, MA: MIT Press.
- Davis EP, Bruce J, Gunnar MR. The anterior attention network: Associations with temperament and neuroendocrine activity in 6-year-old children. Developmental Psychobiology. 2002;40:43 – 56. doi: 10.1002/dev.10012. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355 – 391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dweck, C. S. (1991). Self-theories and goals: Their role in motivation, personality, and development. In R. A. Dienstbier (Ed.), Nebraska Symposium on Motivation (pp. 199 – 235). Lincoln: University of Nebraska Press. [PubMed]
- Dweck, C. S. (1998). The development of early self-conceptions: Their relevance for motivational processes. In J. Heckhausen & C. S. Dweck (Eds.), Motivation and self-regulation across the life span (pp. 257 – 280). New York: Cambridge University Press.
- Field T. The effects of mother’s physical and emotional unavailability on emotion regulation. In N. A. Fox (Ed.), The development of emotion regulation: Biological and behavioral considerations. Monographs of the Society for Research in Child Development. 1994;59(Serial No 240):208 –227. [PubMed] [Google Scholar]
- Gianino, A., & Tronick, E. Z. (1988). The mutual regulation model: The infant’s self and interactive regulation and coping and defensive capacities. In T. M. Field, P. M. McCabe, & N. Schneiderman (Eds.), Stress and coping across development (pp. 47 – 68). Hillsdale, NJ: Erlbaum.
- Gunnar MR, Broderson L, Krueger K, Rigatuso J. Dampening of adrenocortical responses during infancy: Normative changes and individual differences. Child Development. 1996;67:877 – 889. [PubMed] [Google Scholar]
- Gunnar MR, Mangelsdorf S, Larson M, Hertsgaard L. Attachment, temperament, and adrenocortical activity in infancy: A study of psychoendocrine regulation. Developmental Psychology. 1989;25:355 – 363. [Google Scholar]
- Gunnar MR, Porter FL, Wolf CM, Rigatuso J, Larson MC. Neonatal stress reactivity: Predictions to later emotional temperament. Child Development. 1995;66:1 – 13. doi: 10.1111/j.1467-8624.1995.tb00851.x. [DOI] [PubMed] [Google Scholar]
- Haley DW, Stansbury K. Infant stress and parent responsiveness: Regulation of physiology and behavior during still-face and reunion. Child Development. 2003;74:1534 – 1546. doi: 10.1111/1467-8624.00621. [DOI] [PubMed] [Google Scholar]
- Henry JP. Biological basis of the stress response. Integrative Physiological and Behavioral Science. 1992;27:66 – 83. doi: 10.1007/BF02691093. [DOI] [PubMed] [Google Scholar]
- Hill AL, Braungart-Rieker JM. Four-month attentional regulation and its prediction of three-year compliance. Infancy. 2002;3:261 – 273. doi: 10.1207/S15327078IN0302_9. [DOI] [PubMed] [Google Scholar]
- Izard, C. E. (1977). Human emotions New York: Plenum.
- Izard, C. E. (1995). The Maximally Discriminative Facial Movement Coding System (MAX) (Rev. ed.). Newark: University of Delaware, Instructional Resources Center.
- Izard, C. E., Dougherty, L. M., & Hembree, E. A. (1983). A system for identifying affect expressions by holistic judgments (Affex) Unpublished manuscript, University of Delaware.
- Izard CE, Fantauzzo CA, Castle JM, Haynes OM, Rayias MF, Putnam PH. The ontogeny and significance of infants’ facial expressions in the first 9 months of life. Developmental Psychology. 1995;31:997 – 1013. [Google Scholar]
- Izard CE, Hembree EA, Huebner RR. Infants’ emotional expressions to acute pain: Developmental change and stability of individual differences. Developmental Psychology. 1987;23:105 – 113. [Google Scholar]
- Izard, C. E., & Malatesta, C. Z. (1987). Perspectives on emotional development I: Differential emotions theory of early emotional development. In J. D. Osofsky (Ed.), Handbook of infant development (2nd ed., pp. 494 – 554). New York: Wiley.
- Keenan K, Grace D, Gunthorpe D. Examining stress reactivity in neonates: Relations between cortisol and behavior. Child Development. 2003;74:1930 – 1942. doi: 10.1046/j.1467-8624.2003.00647.x. [DOI] [PubMed] [Google Scholar]
- Kelley SA, Jennings KD. Putting the pieces together: Maternal depression, maternal behavior, and toddler helplessness. Infant Mental Health Journal. 2003;24:74 – 90. [Google Scholar]
- Kistner JA, Ziegert DI, Castro R, Robertson B. Helplessness in early childhood: Prediction of symptoms associated with depression and negative self-worth. Merrill-Palmer Quarterly. 2001;47:336 – 354. [Google Scholar]
- Lacey JI. The evaluation of autonomic responses: Toward a general solution. Annals of the New York Academy of Science. 1956;67:125 – 163. doi: 10.1111/j.1749-6632.1956.tb46040.x. [DOI] [PubMed] [Google Scholar]
- Lemerise, E. A., & Dodge, K. A. (2000). The development of anger and hostile interactions. In M. Lewis & J. M. Haviland-Jones (Eds.), Handbook of emotions (2nd ed., pp. 594 – 606). New York: Guilford.
- Levine, S., Coe, C., & Wiener, S. G. (1989). Psycho-neuroendocrinology of stress: A psychobiological perspective. In F. R. Bush & S. Levine (Eds.), Psycho-endocrinology (pp. 341 – 377). New York: Academic Press.
- Levine, S., & Wiener, S. G. (1989). Coping with uncertainty: A paradox. In D. S. Palermo (Ed.), Coping with uncertainty: Behavioral and developmental perspectives (pp. 1 – 16). Hillsdale, NJ: Erlbaum.
- Lewis, M. (1992). Shame. The exposed self New York: Free Press.
- Lewis, M. (1993). The development of anger and rage. In R. A. Glick & S. P. Roose (Eds.), Rage, power, and aggression: The role of affect in motivation, development, and adaptation (pp. 148 – 168). New Haven, CT: Yale University Press.
- Lewis, M. (2000). The emergence of human emotions. In M. Lewis & J. M. Haviland-Jones (Eds.), Handbook of emotions (2nd ed., pp. 265 – 280). New York: Guilford.
- Lewis M, Alessandri SM, Sullivan MW. Violation of expectancy, loss of control, and anger in young infants. Developmental Psychology. 1990;26:745 – 751. [Google Scholar]
- Lewis M, Alessandri SM, Sullivan MW. Differences in shame and pride as a function of children’s gender and task difficulty. Child Development. 1992;63:630 – 638. [PubMed] [Google Scholar]
- Lewis M, Goldberg S. Perceptual-cognitive development in infancy: A generalized expectancy model as a function of mother-infant interaction. Merrill-Palmer Quarterly. 1969;15:81 – 100. [Google Scholar]
- Lewis M, Ramsay DS. Developmental change in infants’ responses to stress. Child Development. 1995;66:657 – 670. doi: 10.1111/j.1467-8624.1995.tb00896.x. [DOI] [PubMed] [Google Scholar]
- Lewis M, Ramsay DS. Effect of maternal soothing on infant stress response. Child Development. 1999;70:11 – 20. doi: 10.1111/1467-8624.00002. [DOI] [PubMed] [Google Scholar]
- Lewis M, Ramsay D. Cortisol response to embarrassment and shame. Child Development. 2002;73:1034 – 1045. doi: 10.1111/1467-8624.00455. [DOI] [PubMed] [Google Scholar]
- Lewis M, Ramsay DS, Kawakami K. Differences between Japanese infants and Caucasian American infants in behavioral and cortisol response to inoculation. Child Development. 1993;64:1722 – 1731. [PubMed] [Google Scholar]
- Lewis M, Sullivan MW, Ramsay DS, Alessandri SM. Individual differences in anger and sad expressions during extinction: Antecedents and consequences. Infant Behavior and Development. 1992;15:443 – 452. [Google Scholar]
- Lewis M, Thomas D. Cortisol release in infants in response to inoculation. Child Development. 1990;61:50 – 59. [PubMed] [Google Scholar]
- MacTurk, R. B., & Morgan, G. A. (Eds.). (1995). Mastery motivation: Origins, conceptualizations, and applications Norwood, NJ: Ablex.
- Magnano CL, Diamond EJ, Gardner JM. Use of salivary cortisol measurements in young infants: A note of caution. Child Development. 1989;60:1099 – 1101. [PubMed] [Google Scholar]
- Magnano CL, Gardner JM, Karmel BZ. Differences in salivary cortisol levels in cocaine-exposed and noncocaine-exposed NICU infants. Developmental Psychobiology. 1992;25:93 – 103. doi: 10.1002/dev.420250203. [DOI] [PubMed] [Google Scholar]
- Messer, D. (Ed.). (1993). Mastery motivation in early childhood: Development, measurement and social processes London: Routledge.
- Mills, R. S. L. (in press). Taking stock of the developmental literature on shame. Developmental Review
- Moore GA, Cohn JF, Campbell SB. Infant affective responses to mother’s still face at 6 months differentially predict externalizing and internalizing behaviors at 18 months. Developmental Psychology. 2001;37:706 – 714. [PubMed] [Google Scholar]
- Muir D, Lee K. The still-face effect: Methodological issues and new applications. Infancy. 2003;4:483 – 491. [Google Scholar]
- Ramsay, D., & Lewis, M. (2001). Temperament, stress, and soothing. In T. D. Wachs & D. Kohnstamm (Eds.), Temperament in context (pp. 23 – 41). Mahwah, NJ: Erlbaum.
- Retzinger, S. R. (1987). Resentment of laughter: Video studies of the shame-rage spiral. In H. B. Lewis (Ed.), The role of shame in symptom formation (pp. 151 – 181). Hillsdale, NJ: Erlbaum.
- Saarni, C., Mumme, D. L., & Campos, J. J. (1997). Emotional development: Action, communication, and understanding. In W. Damon (Series Ed.) & N. Eisenberg (Vol. Ed.), Handbook of child psychology: Vol. 3. Social, emotional, and personality development (5th ed., pp. 237 – 309). New York: Wiley.
- Schmidt LA, Fox NA, Sternberg EM, Gold PW, Smith CC, Schulkin J. Adrenocortical reactivity and social competence in seven year-olds. Personality and Individual Differences. 1999;26:977 – 985. [Google Scholar]
- Schwartz EB, Granger DA, Susman EJ, Gunnar MR, Laird B. Assessing salivary cortisol in studies of child development. Child Development. 1998;69:1503 – 1513. [PubMed] [Google Scholar]
- Scheff, T. J. (1987). The shame-rage spiral: A case study of an interminable quarrel. In H. B. Lewis (Ed.), The role of shame in symptom formation (pp. 109 – 150). Hillsdale, NJ: Erlbaum.
- Segal LB, Oster H, Cohen M, Caspi B, Myers M, Brown D. Smiling and fussing in seven-month-old preterm and full-term black infants in the still-face situation. Child Development. 1995;66:1829 – 1843. [PubMed] [Google Scholar]
- Seligman, M. E. P. (1975). Helplessness: On depression, development, and death San Francisco: Freeman.
- Smiley PA, Dweck CS. Individual differences in achievement goals among young children. Child Development. 1994;65:1723 – 1743. doi: 10.1111/j.1467-8624.1994.tb00845.x. [DOI] [PubMed] [Google Scholar]
- Spangler G, Grossmann KE. Behavioral organization in securely and insecurely attached infants. Child Development. 1993;64:1439 – 1450. doi: 10.1111/j.1467-8624.1993.tb02962.x. [DOI] [PubMed] [Google Scholar]
- Stansbury K, Harris ML. Individual differences in stress reactions during a peer entry episode: Effects of age, temperament, approach behavior, and self-perceived peer competence. Journal of Experimental Child Psychology. 2000;76:50 – 63. doi: 10.1006/jecp.1999.2541. [DOI] [PubMed] [Google Scholar]
- Stenberg, C. R., & Campos, J. J. (1990). The development of anger expressions in infancy. In N. L. Stein, B. Leventhal, & T. Trabasso (Eds.), Psychological and biological approaches to emotion (pp. 247 – 282). Hillsdale, NJ: Erlbaum.
- Stenberg CR, Campos JJ, Emde RN. The facial expression of anger in seven-month-old infants. Child Development. 1983;54:178 – 184. doi: 10.1111/j.1467-8624.1983.tb00347.x. [DOI] [PubMed] [Google Scholar]
- Sullivan MW, Lewis M. Contextual determinants of anger and other negative expressions in young infants. Developmental Psychology. 2003;39:693 – 705. doi: 10.1037/0012-1649.39.4.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan MW, Lewis M, Alessandri SM. Cross-age stability in emotional expressions during learning and extinction. Developmental Psychology. 1992;28:58 – 63. [Google Scholar]
- Tronick E. Emotions and emotional communication in infants. American Psychologist. 1989;44:112 – 119. doi: 10.1037//0003-066x.44.2.112. [DOI] [PubMed] [Google Scholar]
- Tronick EZ. Things still to be done on the still-face effect. Infancy. 2003;4:475 – 482. [Google Scholar]
- Tronick E, Als H, Adamson L, Wise S, Brazelton TB. The infant’s response to entrapment between contradictory messages in face-to-face interaction. Journal of the American Academy of Child Psychiatry. 1978;17:1 – 13. doi: 10.1016/s0002-7138(09)62273-1. [DOI] [PubMed] [Google Scholar]
- Watson JS. Smiling, cooing, and the “game”. Merrill-Palmer Quarterly. 1972;18:323 – 339. [Google Scholar]
- Weinberg MK, Tronick EZ. Infant affective reactions to the resumption of maternal interaction after the still-face. Child Development. 1996;67:905 – 914. [PubMed] [Google Scholar]
- White R. Motivation reconsidered: The concept of competence. Psychological Review. 1959;66:297 – 334. doi: 10.1037/h0040934. [DOI] [PubMed] [Google Scholar]
- Wilder J. The law of initial value in neurology and psychiatry. Journal of Nervous and Mental Diseases. 1956;125:73 – 86. doi: 10.1097/00005053-195701000-00009. [DOI] [PubMed] [Google Scholar]
- Yarrow, L. J., Klein, R., Lomonaco, S., & Morgan, G. (1975). Cognitive and motivational development in early childhood. In B. Z. Friedlander, G. M. Sterritt, & G. E. Kirk (Eds.), Exceptional infant: Assessment and intervention (Vol. 3) (pp. 491 – 502) New York: Bruner/Mazel.


