Abstract
Recent advances in the ability to detect people at the early stages of HIV infection now permit the initiation of antiretroviral treatment before the full complement of antiviral immune responses has evolved. However, the influence of early treatment interventions on the developing anti-HIV immune response is unknown. This study investigates the impact of standard highly active antiretroviral therapy (HAART) during the primary stages of HIV infection on the plasma HIV-1 RNA level, CD4+ and CD8+ lymphocyte counts, and the CD8+ cell anti-HIV response. Individuals treated with HAART within 6 months of infection showed dramatic and rapid reductions in HIV-1 RNA levels along with modest increases in CD4+ cell number and decreases in CD8+ cell numbers. A significant reduction in the level of CD8+ cell noncytotoxic suppression of HIV replication was observed over time in most participants receiving HAART. Importantly, those individuals choosing not to receive therapy maintained low but detectable HIV-1 RNA levels and showed no reduction in their CD8+ cell antiviral response. These results suggest that either continued antigenic challenge is required to sustain CD8+ cell-mediated anti-HIV activity, or that HAART has some inhibitory effect on this important immunologic function during the early stages of infection.
The recent introduction of improved antiviral therapies, especially triple-drug combinations, has improved substantially the prognosis of many individuals chronically infected with HIV (1–5). These highly active antiretroviral therapies (HAARTs) take advantage of multiple drug-class combinations by using two different inhibitors of reverse transcriptase (RT) with a protease inhibitor. With successful administration and adherence to the regimen, this combination can result in dramatic reductions in plasma HIV-1 RNA levels (6–9). Although the impact of this therapeutic regimen on plasma viremia is well documented, much less is known about the effect of this treatment on the developing immune response, especially as it relates to anti-HIV activity.
The primary or acute stage of HIV infection encompasses the first weeks to months after transmission, at which time viral burdens are expanding exponentially, and antiviral immune defenses are still developing. Once the HIV-specific immune response has been established, viral loads usually decrease until a relative homeostasis is reached, marking the end of the acute phase of infection (10). The natural equilibrium of virus burden (or viral set point) reached at the conclusion of the acute phase can be indicative of the ultimate clinical course of disease (11–13) and most likely reflects both host- and pathogen-specific factors.
This study investigates the impact of HAART during very early stages of HIV infection on viral loads, CD4+ and CD8+ cell numbers, and the developing CD8+ cell noncytotoxic antiviral response. Individuals beginning this antiviral therapy regimen within 6 months of infection showed significant loss of CD8+ cell noncytotoxic activity over time concomitant with decreases in HIV-1 RNA levels. In contrast, those participants electing not to receive therapy during primary infection did not demonstrate a reduction in this cellular immune response over the same 6-month study period. These untreated individuals also showed modest decreases in viral burdens with no major change in CD4+ or CD8+ cell numbers. These data indicate that treatment of acute HIV infection with HAART can lead to a reduced CD8+ cell immune response against HIV.
Materials and Methods
Study Subjects.
Subjects undergoing the primary stages of infection with HIV were recruited through the Options Project at San Francisco General Hospital into the Primary Infection Project. All participants entered the study either before seroconversion or during the subsequent 6-month period, as determined by one or more of the following criteria: (i) plasma HIV RNA without HIV-specific antibodies; (ii) documentation of seroconversion within the past 6 months; or (iii) inclusion based on nonreactivity in a detuned (less sensitive) serum-HIV antibody ELISA (14). Whole-blood samples were collected at regular intervals during the course of the study (weeks 0, 4, 12, and 24). The purified peripheral blood mononuclear cells (PBMCs) evaluated from subjects in this study were cryopreserved and stored at the University of California, San Francisco (UCSF) AIDS Specimen Bank before use. Individuals were studied between June 1997 and June 1999, and those having blood samples available for all time periods were included. Whole blood from unexposed uninfected donors came from the Blood Centers of the Pacific. This study received approval from the Committee for Human Research, UCSF.
Plasma Antibody Levels.
Initial plasma samples collected from all subjects (“screening” time point) were diluted 1:200 as per the usual protocol and tested for anti-HIV antibodies by using a standard sensitive HIV ELISA (3A11, Abbott). Then, plasma from individuals deemed infected was analyzed by using a less-sensitive (LS) version of the ELISA at a plasma dilution of 1:20,000. This latter dilution specifically measures HIV-specific antibodies of high titer and avidity and permits the identification of those individuals who have been infected for long periods (LS-enzyme immunoassay reactive) versus individuals infected within the last 3–5 months (LS-enzyme immunoassay nonreactive; ref. 14).
Plasma Viral Load Determinations.
The plasma viral loads of all study participants were measured by using the branched DNA assay. The 2.0 version of the assay was used until January 1999, after which the 3.0 version was used (15). Individuals were informed of viral load and cell-surface marker data before the initiation of any therapy and throughout the duration of the study.
Treatment Schedule.
HAART, in most cases, consisted of zidovudine (300 mg) plus lamivudine (150 mg), combined in a single pill (combivir) administered twice daily and indinavir (800 mg) every 8 h or nelfinavir (750 mg) three times daily. Some participants also received hydroxyurea, and some used other anti-RT therapies (e.g., stavudine or didanosine; see Table 1). Week 0 was established as the date on which each individual either began HAART (“treated”) or opted not to initiate treatment (“untreated”). Untreated participants received the same evaluation and monitoring throughout the 24 weeks of the study and reported no use of antiviral agents.
Table 1.
Subject characteristics by treatment status
| Characteristic | HAART, n = 21 | No HAART, n = 5 | P value |
|---|---|---|---|
| Male, n (%) | 15 (94) | 5 (100) | 0.8 |
| Female, n (%) | 1 (6) | 0 (0) | |
| Age years (mean) | 34 | 29 | 0.2 |
| Range | (25 to 53) | (23 to 35) | |
| Ethnicity | |||
| Asian | 2 (6%) | 0 (0%) | 0.1 |
| Caucasian | 14 (67%) | 4 (80%) | |
| Hispanic | 5 (24%) | 0 (0%) | |
| Pacific Islander | 0 (0%) | 1 (20%) | |
| HIV risk factor | |||
| Men sex with men | 19 (94%) | 5 (100%) | 0.6 |
| Heterosexual sex | 2 (6%) | 0 (0%) | |
| Stage of HIV at diagnosis | |||
| Preseroconversion | 4 (19%) | 0 (0%) | 0.3 |
| ≤30 days after seroconversion | 3 (14%) | 0 (0%) | |
| <6 months after seroconversion | 14 (67%) | 5 (100%) | |
| Initial treatment regimen | |||
| ZDV + 3TC + nelfinavir | 13 (62%)* | — | |
| ddI + d4T + nelfinavir + hydroxyurea | 4 (19%) | — | |
| ZDV + 3TC + indinavir | 1 (5%) | — | |
| 3TC + d4T + nevirapine | 1 (5%) | — | |
| d4T + 3TC + nelfinavir + hydroxyurea | 1 (5%) | — | |
| ZDV + 3TC + efavirenz | 1 (5%) | — |
Age (years), sex, race, HIV risk factors, stage of infection at diagnosis, and treatment, if any, are shown for HAART-treated and untreated individuals experiencing primary HIV infection. No statistically significant difference was observed between the treated and untreated groups. P values are for comparisons between the treated and untreated groups using Fisher's exact test for dichotomous categorical variables, chi square for categorical variables with more than two values, and Student's t test for continuous variables. n = number of individuals. ZDV, zidovudine; 3TC, lamivudine; ddI, didanosine; d4T, stavudine.
One of these subjects switched from ZDV to d4T after week 8.
PBMC Isolation and Subset Purification.
Cryopreserved samples of PBMCs prepared from whole blood of infected individuals by Ficoll/Hypaque (Sigma) gradient separation (16) were used. The CD4+ and CD8+ cellular fractions were purified by using anti-CD4 or anti-CD8 antibody-coated (anti-CD4/anti-CD8) immunomagnetic beads (Dynal, Great Neck, NY) as described (17). All cell preparations were cultured in RPMI medium 1640 containing 10% (vol/vol) heat-inactivated (56°C, 30 min) FCS, 100 units/ml recombinant IL-2 (generously provided by Glaxo Wellcome), 1% antibiotics (100 units/ml penicillin and 100 μg/ml streptomycin), and 2 mM glutamine, unless otherwise noted.
In Vitro CD4+ Cell Infection.
CD4+ cells from the PBMCs of unexposed uninfected donors were isolated by using anti-CD4 immunomagnetic beads and infected in vitro as described (17). Briefly, the cells were pretreated with phytohemagglutinin (Sigma) for 3 days (3 μg/ml), washed, and treated with polybrene (2 μg/ml) for 30 min. Then, 3 million cells were resuspended in 10,000 tissue culture 50% infective dose per ml of the β-chemokine-resistant SF33 strain of HIV-1 (18). This strain of HIV has been maintained in primary PBMCs since its isolation (19–21). After 1 h, these in vitro infected CD4+ cells were washed and resuspended at a concentration of 2 × 106 cells per ml in the RPMI medium 1640.
Acute Infection Assay for CD8+ Cell Noncytotoxic Anti-HIV Activity.
Acute virus suppression assays were conducted as described (17). Briefly, the CD4+ cells from uninfected control subjects were infected acutely with HIV-1SF33 and cultured in growth medium containing 100 units/ml recombinant IL-2, both alone and in the presence of CD8+ cells at CD8+:CD4+ cell ratios of 0.25:1, 0.5:1, and (when cell numbers allowed) 1:1. These cell mixtures were placed in 96-well plates in a final volume of 200 μl per well and incubated for 7–10 days at 37°C. Fluids in the cell cultures were replenished every 3–4 days by collecting half of the supernatant and replacing this fluid with fresh medium. Collected supernatants were monitored for RT activity as described (22). The percentage of suppression was calculated by comparing the average value of RT activity in culture fluids from six control wells containing replicates of infected CD4+ cells grown alone, with the average RT activity in fluids from duplicate wells containing cocultured CD8+:CD4+ cells. Levels of suppression amounting to >50% reduction in RT activity of cocultures compared with infected CD4+ cells grown alone (normally 1 × 106 to 2 × 106 cpm/ml) were considered positive. Suppression was considered high when >90% reduction of supernatant RT activity was observed.
Statistical Analysis.
Paired and unpaired Student's t tests were performed within groups to evaluate changes over time or to analyze differences between groups at individual time points, respectively, by using SAS 7 software (Cary, NC). Proportions of treated and untreated subjects achieving specified levels of CD8+ cell suppression were compared by using Fisher's exact test. Paired comparisons of proportions achieving specified levels of CD8+ cell suppression at different time points within treatment groups were made by using McNemar's test (23).
Results
Study Population.
The 26 subjects in the primary stages of HIV infection were followed for a 24-week period. Of these individuals, 21 chose to receive HAART, and 5 elected no therapy. Table 1 presents demographic and specific treatment data on the entire study population as well as the individual treatment groups. Included in the study group are 2 females and 24 males with an overall mean age of 33 years. Of the participants, 31% (8 of 36) reported belonging to non-white ethnic groups. No statistically significant difference was observed between the two populations for any of these parameters at the start of the study.
Viral Loads.
Plasma viral load was evaluated in each individual starting just before the initiation of therapy or the decision not to begin treatment (week 0) and at regular intervals through week 24. Table 2 presents the means and ranges of HIV-1 RNA levels (copies per ml) as well as the percentage of individuals demonstrating undetectable (<500 copies per ml) viral loads for each of the two subject groups: HAART-treated or untreated participants. Subjects receiving antiretroviral therapy demonstrated substantial reductions in HIV-1 RNA levels. Over half of the participants (12 of 21) reached undetectable levels (<500 copies per ml) by the 4th week of the study. Pretherapy levels of viral RNA averaged ≈137,513 copies per ml in the HAART-treated population. Overall, HIV-1 RNA levels were <500 copies per ml in all but one treated individual by week 12 (Table 2). During the period under study, subjects receiving therapy reported an average of >90% adherence to the treatment regimen (data not shown).
Table 2.
Viral load measurements in HAART-treated and untreated subjects
| Category | Week 0 | Week 4 | Week 12 | Week 24 |
|---|---|---|---|---|
| HAART-treated (n = 21) | ||||
| Mean HIV-1 RNA | 137,513 | 1,193 | 500 | 568 |
| HIV-1 RNA range | <500–>1,600,000 | <500–5,136 | <500–513 | <500–1,947 |
| Subjects with HIV-1 RNA <500 | 14% (3 of 21) | 57% (12 of 21) | 95% (20 of 21) | 95% (20 of 21) |
| Untreated (n = 5) | ||||
| Mean HIV-1 RNA | 65,795 | 23,888 | 24,630 | 14,031 |
| HIV-1 RNA range | <500–296,549 | <500–73,740 | <500–71,150 | <500–27,222 |
| Subjects with HIV-1 RNA <500 | 20% (1 of 5) | 20% (1 of 5) | 20% (1 of 5) | 20% (1 of 5) |
| P value comparing mean HIV-1 RNA | 0.34 | 0.005 | <0.001 | <0.001 |
The mean and viral-load ranges (RNA copies per ml) are shown for both HAART-treated and untreated populations over time, as well as the fraction of individuals with detectable levels of plasma virus. Week 0 time point is before the initiation of therapy or when no therapeutic intervention was chosen for the treated and untreated groups, respectively. P values were calculated by comparing HIV-1 RNA levels for the treated and untreated groups at each time point by using the Student's t test. n = number of individuals. Subjects with an HIV-1 RNA <500 copies per ml were counted as having an HIV-1 RNA level of 500 copies per ml for calculations of the mean HIV-1 RNA level.
Initial plasma HIV-1 RNA levels were somewhat lower for individuals opting not to receive treatment (mean of ≈66,000 copies per ml) (Table 2), but this amount did not differ significantly from that of the treated population (P = 0.34). HIV-1 RNA levels in one of the five individuals opting not to receive HAART remained undetectable for the duration of the study (Table 2). The level of plasma HIV-1 RNA decreased in the untreated group to a mean of ≈14,000 copies per ml (0.4-log reduction) by week 24 of the study. This decrease over time was not statistically significant. In comparing the treated and untreated groups, a statistically significant difference in plasma HIV RNA levels was reached by week 4 of the study (P = 0.005).
CD4+ and CD8+ Cell Numbers.
PBMCs collected from both study populations were monitored for the number of cells expressing CD4 and CD8 surface markers over time. Table 3 shows the mean number and ranges of CD4- and CD8-expressing cells found in treated and untreated populations at weeks 0, 4, 12, and 24. Initial CD8+ cell numbers were modestly higher in the treated population, whereas CD4+ cell numbers showed no substantial difference between the two groups. Treated and untreated populations showed similar increases in CD4+ cell numbers over the course of the study (15 and 11%, respectively). However, these groups differed with respect to changes in CD8+ cell numbers. Although the HAART-treated individuals showed a 16% decrease in the mean number of CD8+ cells over the 24 weeks, untreated patients demonstrated a 17% increase in this same cell subset (Table 3). Overall, the numbers of both CD4+ and CD8+ cells were higher at week 24 in the untreated population. None of these changes in cellular subsets reached statistical significance either within or between groups.
Table 3.
Mean CD4+ and CD8+ cell numbers in HAART-treated and untreated subjects
| Category | Week 0 | Week 4 | Week 12 | Week 24 | Change, % |
|---|---|---|---|---|---|
| HAART-treated (n = 21) | |||||
| CD4+ cells (range) | 581 (288–1,000) | 675 (432–1,275) | 658 (342–1,170) | 667 (300–1,015) | +15 |
| CD8+ cells (range) | 867 (232–2,280) | 773 (378–3,009) | 725 (288–1,152) | 725 (286–1,610) | −16 |
| Untreated (n = 5) | |||||
| CD4+ cells (range) | 636 (300–1,452) | 654 (357–1,300) | 679 (357–1,118) | 707 (420–1,288) | +11 |
| CD8+ cells (range) | 697 (378–1,155) | 651 (374–858) | 797 (425–1,000) | 815 (500–1,150) | +17 |
Mean and ranges of CD4+ and CD8+ cell counts (per μl) are shown for both HAART-treated and untreated groups over time. The week 0 time point is before the initiation of therapy or when no therapeutic intervention was chosen for the treated and untreated groups, respectively. The final column shows percentage of increase or decrease for each group in mean number of CD4+ or CD8+ cells, comparing week 0 with week 24. No statistically significant difference was observed either within or between treatment groups at any time point. n = number of individuals.
CD8+ Cell Noncytotoxic Responses in HAART-Treated Subjects.
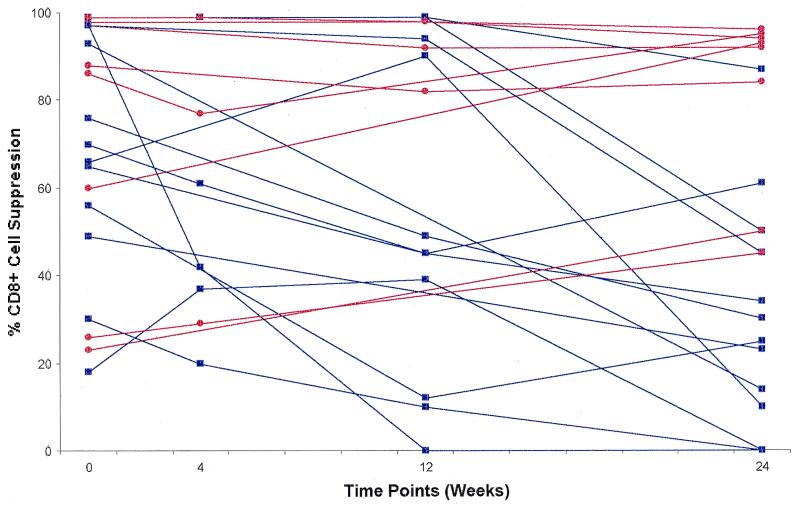
CD8+ cells from the peripheral blood of 21 individuals receiving HAART during the primary stages of HIV infection were analyzed for their ability to suppress HIV replication in a noncytotoxic assay (see Materials and Methods). Fig. 1 shows CD8+ cell-suppression results at a CD8+:CD4+ cell ratio of 0.5:1 for the 21 treated individuals beginning at week 0 (just before the start of therapy) and at 4, 12, and 24 weeks of HAART. The 0.5:1 ratio was chosen for presentation of these data, because it represented a ratio consistently evaluated in all individuals at each time point. Similar patterns of anti-HIV activity were observed at all ratios tested (0.25:1 to 1:1, data not shown), although low recovery of CD8+ cells in some samples precluded evaluation at a ratio of 1:1.
Figure 1.
Effect of HAART treatment on CD8+ cell noncytotoxic anti-HIV activity in primary infection. The percentage of suppression of HIV-1SF33 replication at a CD8+:CD4+ cell ratio of 0.5:1 is presented for 21 individuals experiencing the primary stages of HIV infection and receiving highly active antiretroviral therapy for a period of 24 weeks. Week 0 represents the level of CD8+ cell suppression before the initiation of HAART, compared with weeks 4, 12, or 24 of treatment. Statistical significance was determined by comparing the actual suppression values of all participants at week 0 with these same measurements at week 24 (P = 0.01). Red line, maintained or enhanced antiviral response; blue line, decreased antiviral response.
Most individuals receiving HAART (16 of 21, 76%) exhibited >50% suppression by CD8+ cells at week 0, the time closest to initial infection (Table 4). Moreover, the majority of the treated subjects (15 of 21, 71%) exhibited their peak levels of CD8+ cell suppression at this time point (week 0; see Fig. 1). Of these subjects, only four exhibited either relative maintenance or elevation of these levels of suppression at the end of the study (week 24). In fact, only 8 of the 21 treated individuals (38%) demonstrated any significant CD8+ cell suppression (>50%) at week 24, compared with 76% of these same individuals at the start of the study (Table 4). A statistically significant loss in the level of CD8+ cell suppression from week 0 to 24 was found in this population (P = 0.01).
Table 4.
CD8+ cell noncytotoxic anti-HIV suppression in HAART-treated and untreated subjects
| Extent of CD8+ cell suppression, % | Subject category | Week 0 | Week 4 | Week 12 | Week 24 |
|---|---|---|---|---|---|
| >50 | Treated | 16 of 21 (76%) | 4 of 9 (44%) | 8 of 15 (53%) | 8 of 21 (38%)* |
| Untreated | 3 of 5 (60%) | 4 of 4 (100%) | 5 of 5 (100%) | 5 of 5 (100%)** | |
| ≥90 | Treated | 8 of 21 (38%) | 2 of 9 (22%) | 7 of 15 (47%) | 5 of 21 (24%) |
| Untreated | 1 of 5 (20%) | 2 of 4 (50%) | 3 of 5 (60%) | 3 of 5 (60%) |
Values shown are the fraction and percentage of individuals whose CD8+ cells demonstrate >50% or ≥90% inhibition of HIV-1SF33 (CXCR4-tropic, β chemokine-resistant virus) replication in an acute infection assay. Inhibition of virus replication was assessed by evaluating RT levels in culture supernatants (see Materials and Methods) on days 3, 7, and 10 after infection. The final level of suppression was determined on the day of peak reverse transcriptase activity in wells containing only control infected CD4+ cells (typically 1 × 106 to 2 × 106 cpm/ml).
, P = 0.01 in comparing CD8+ cell anti-HIV response between week 0 and week 24 (McNemar's test);
, P = 0.04 in comparing treated and untreated subjects at week 24 (Fisher's exact test).
CD8+ Cell Noncytotoxic Responses in Untreated Subjects.
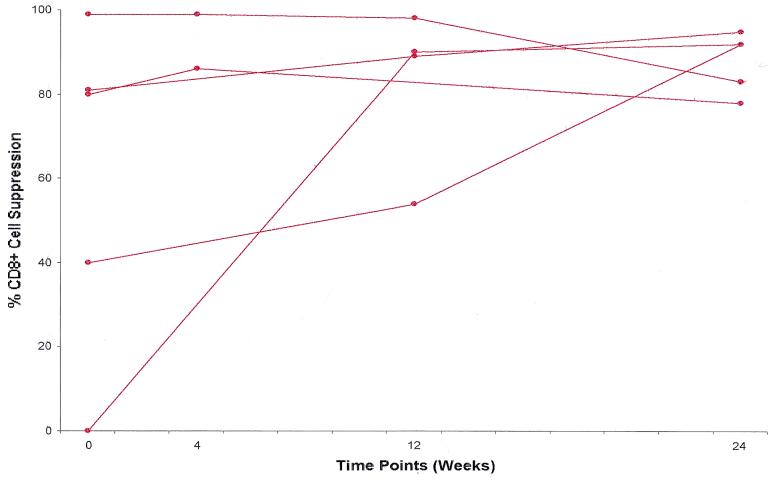
Five individuals diagnosed during the primary stages of HIV infection elected not to receive therapy. Identical studies to those performed in the treated group were conducted with peripheral blood samples collected from the untreated participants. Noncytotoxic anti-HIV suppression levels were obtained from these subjects from the time at which they elected not to receive therapy (designated week 0) through several follow-up time points, as was used with treated subjects. Fig. 2 shows the percentage of CD8+ cell noncytotoxic suppression of virus replication over time at the CD8+:CD4+ cell ratio of 0.5:1.
Figure 2.
CD8+ cell noncytotoxic anti-HIV activity in untreated primary infection subjects. The percentage of suppression of HIV-1SF33 replication at a CD8+:CD4+ cell ratio of 0.5:1 is presented for five individuals experiencing the primary stages of HIV infection and receiving no therapeutic intervention. Week 0 represents the level of CD8+ cell suppression at the time each patient elected not to receive treatment, compared with weeks 4, 12, and 24 of follow-up. No statistical significance was observed when comparing the actual suppression values of all participants at week 0 with these same measurements at week 24 (P = 0.1).
As with the treated individuals, most untreated participants (3 of 5) demonstrated significant levels of CD8+ cell-mediated suppression (>50% reduction in virus replication) at the start of the study. Additionally, all four of the tested subjects had CD8+ cell-suppression levels ≥50% by week 4 of the study (see Fig. 2 and Table 4). However, unlike the HAART-treated group, the entire untreated population either sustained this relative level or showed an increase in the magnitude of CD8+ cell antiviral activity over the course of 24 weeks. No statistically significant difference was found between the starting (week 0) and ending (week 24) levels of CD8+ cell suppression in these individuals (P = 0.11). All untreated individuals demonstrated >50% suppression of virus replication at week 24; three of these five maintained high levels of antiviral activity in excess of 90%. At week 24, CD8+ cell suppression at >50% was significantly more frequent in the untreated compared with the treated population (P = 0.04). No significant difference was observed in initial (week 0) levels of CD8+ cell suppression between the two populations (P = 0.1).
Overall Observations on CD8+ Cell Anti-HIV Responses in Primary Infection Subjects.
In general, two patterns of CD8+ cell suppression can be discerned in participants experiencing the primary stages of HIV infection: maintenance or enhancement of the CD8+ cell suppression vs. reduction in this antiviral response. In the HAART-treated group, the majority of subjects (13 of 21, 62%) had an overall decrease in the level of their CD8+ cell suppression over time, whereas only 4 of 21 (19%) demonstrated any substantial enhancement of this immune response (see Fig. 1). Conversely, the untreated clinical group demonstrated enhanced noncytotoxic suppression activity in three of five cases, with the remainder of the group exhibiting maintenance of this response over time (Fig. 2).
Discussion
HAART plays a well documented role in significantly reducing plasma HIV-1 RNA levels in HIV-infected individuals (6–9). Moreover, treatment of acute infection can reduce clinical symptoms and declines in CD4+ cell numbers (24–26). However, little is known about its potential effect on either the state of the immune system or, more specifically, the natural anti-HIV response. Anti-HIV drugs have been shown to reduce PBMC proliferative responses to mitogen (27). In fact, most of the salient HIV-specific immune responses likely take place very early in infection, during the acute or primary stages shortly after virus transmission. This point is illustrated in the observation that the viral set point within an infected individual, reflecting the contribution of both host and viral factors, is established normally within the first months after transmission (10). Likewise, this balance point can be prognostic for the eventual clinical outcome (12, 13). The major immunologic components involved in limiting HIV infection seem to be HIV-specific CD8+ T cells, potentially assisted by CD4+ helper cells (28–31). Importantly, the two populations that might be expected to illustrate productive anti-HIV responses, clinically healthy HIV-infected long term survivors and highly exposed but uninfected individuals, both demonstrate strong natural CD8+ cell responses (both cytotoxic and noncytotoxic) against the virus (17, 32–36). In this study, we have investigated the status of the salient CD8+ cell anti-HIV noncytotoxic response and compared the relative levels of this virus-inhibiting activity in HAART-treated vs. untreated individuals during the earliest stages of infection.
Most notably, primary-infection subjects receiving HAART showed an overall reduction in the levels of CD8+ cell antiviral activity after 24 weeks of therapy. Although this finding was not present in some individual cases, CD8+ cells from 13 of 21 (62%) of those subjects receiving HAART showed a reduction in anti-HIV inhibition potential over the course of this 6-month study; a statistically significant decrease was observed in the group overall (P = 0.01). This observation can be compared with the untreated population in which only one of the five patients demonstrated a small decline in CD8+ cell suppression, from 99 to 83% by week 24 (Fig. 2). An overall trend toward elevated levels of CD8+ cell suppression was observed in this latter untreated group. Although we had only a small number of subjects who chose not to receive therapy, previous studies in our laboratory with large numbers of HIV-infected individuals who have remained healthy for long periods of time indicate the stability of this CD8+ cell noncytotoxic anti-HIV response over time (17).
Several recent reports on the use of long-term HAART during either primary or chronic HIV infection also found reductions in B cell and T cell antiviral responses (26, 37–40). In one study of chronic HIV infection, the noncytotoxic CD8+ cell immune response was noted to decrease with time after treatment (39). Likewise, cytotoxic T lymphocyte responses have been found to diminish during long-term HAART exposure, both in primary and chronic infection stages (26, 38, 39). Conversely, Rosenberg et al. (31) reported that some HIV-specific CD4+ cell helper responses are enhanced with HAART treatment during acute infection. This study differs from ours in two major ways: (i) no untreated participants in the acute stages of infection were available for comparison, and (ii) the three individuals studied (31) were all treated before seroconversion. This latter point raises the possibility that therapeutic intervention before the appearance of specific anti-HIV antibodies may affect the immune response differently than when used after the antiviral immunity has developed.
One confounding feature of the present study was that those participants opting for no therapy generally had lower initial viral loads than those choosing therapy, although at least one untreated individual exhibited a viral burden near 300,000 copies per ml at week 0 (Table 2). Nevertheless, these starting differences in plasma HIV-1 RNA levels between the treated and untreated groups were not statistically significant. However, it remains possible that those individuals who received treatment could still be biologically different from the subjects who chose no therapy. The observed variability is most likely due to the fact that individuals were informed of their clinical data before making therapy choices and were therefore more likely to elect no treatment if their viral burdens were small. However, if the level of initial HIV-1 RNA was solely indicative of CD8+ cell-suppression responses, we would expect to see strong antiviral activity in all individuals with initially low HIV-1 RNA values. This expectation was not necessarily the case. Several treated subjects began the study with low-level plasma HIV-1 RNA and modest CD8+ cell anti-HIV responses. They then experienced a reduction in their HIV-specific immune response over time, suggesting a link between treatment and inhibition of CD8+ cell suppression. Of particular interest, one individual who received HAART and showed a precipitous drop in CD8+ cell responses recently elected to stop therapy after 48 weeks of undetectable HIV-1 RNA. This individual experienced a return of strong CD8+ cell antiviral responses with, as yet, no rebound in virus (unpublished observations).
Importantly, untreated subjects under study, all of whom demonstrated strong CD8+ cell noncytotoxic suppression, maintained persistent but low-level viremia. It seems reasonable that a persistent low-level viremia may have contributed to maintenance of the CD8+ cell antiviral response in these untreated individuals. Thus, some virus replication could help maintain this natural antiviral immune response. In this regard, the four individuals on HAART who maintained CD8+ cell antiviral activity are noteworthy. Conceivably, they were not as adherent to therapy, or they had brief bursts of viral replication that were not noted at the time points tested for HIV-1 RNA levels.
The overall combination of CD8+ cell cytotoxic (T lymphocyte) and noncytotoxic activity and appropriate CD4+ cell help likely contribute to the control of HIV infection in vivo (10). These immunologic markers are potentially important considerations, along with viral burdens and CD4+ cell numbers, for determining clinical prognosis, because they specifically measure the robustness of the anti-HIV-specific cellular response. Based on the relative ease of cell-to-cell transfer versus-free virus-mediated spread (41), more than one immune mechanism most likely is needed to reduce the number of infected cells in the host. The effects of limiting the noncytotoxic CD8+ cell responses are unknown. HAART has proven to be highly effective for decreasing HIV-1 RNA levels and increasing CD4+ cells, both commonly viewed as surrogate markers of disease progression. However, the influence of antiviral therapy on immunologic control of the virus must be considered. Therefore, the strategic timing of HAART initiation such that key immune responses are not blunted remains a salient clinical-research issue. Moreover, approaches to boost anti-HIV immune responses for individuals on HAART merit further attention.
The present study suggests that special consideration should be given in decisions on when and whether to use HAART. Although reduction in free virus is important for the management of transmission and control of virus spread, this favorable result should not overshadow the valuable contribution of an effective natural host-immune response to the pathogen. In the untreated subjects included in this study, low-level HIV-1 RNA was observed concomitant with an increase in CD8+ cell number (Table 3) and a strong CD8+ cell antiviral response (Table 4). Initiating HAART during the primary stages of infection may blunt the still-developing anti-HIV immune activity. Recently, HAART has been reported to show an inhibitory effect on immune activity (42). This decrease in the anti-HIV noncytotoxic CD8+ cell response, caused by either limited antigenic challenge or some other effect of therapy on CD8+ cell responses, could be detrimental to the overall course of the infection.
Acknowledgments
We thank Marcy Webb for help with the statistical analyses and Ann Murai and Kaylynn Peter for secretarial assistance. These studies were supported by grants from the National Institutes of Health (A141531-03), California State University-wide AIDS Research Program (PH97-SF-203), and the University of California, San Francisco, Gladstone Immunology and Virology Institute's Center for AIDS Research (P30 MH59037), and Glaxo Wellcome, Agouron Pharmaceuticals (La Jolla, CA), Merck, and Bristol-Myers Squibb provided the medications for participants.
Abbreviations
- HAART
highly active antiretroviral therapy
- PBMC
peripheral blood mononuclear cell
- RT
reverse transcriptase
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.021550598.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.021550598
References
- 1.Hammer S M, Katzenstein D A, Hughes M D, Gundacker H, Schooley R T, Haubrich R H, Henry W K, Lederman M M, Phair J P, Niu M, et al. N Engl J Med. 1996;335:1081–1090. doi: 10.1056/NEJM199610103351501. [DOI] [PubMed] [Google Scholar]
- 2.Hogg R S, O'Shaughnessy M V, Gataric N, Yip B, Craib K, Schechter M T, Montaner J S G. Lancet. 1997;349:1294. doi: 10.1016/S0140-6736(05)62505-6. [DOI] [PubMed] [Google Scholar]
- 3.Detels R, Munoz A, McFarlane G, Kingsley L A, Margolick J B, Giorgi J, Schrager L K, Phair J P. J Am Med Assoc. 1998;280:1497–1503. doi: 10.1001/jama.280.17.1497. [DOI] [PubMed] [Google Scholar]
- 4.Palella F J, Jr, Delaney K M, Moorman A C, Loveless M O, Fuhrer J, Satten G A, Aschman D J, Holmberg S D. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 5.Schwarcz S K, Hsu L C, Vittinghoff E, Katz M H. Am J Epidemiol. 2000;152:178–185. doi: 10.1093/aje/152.2.178. [DOI] [PubMed] [Google Scholar]
- 6.Collier A C, Coombs R W, Schoenfeld D A, Bassett R L, Timpone J, Baruch A, Jones M, Facey K, Whitacre C, McAuliffe V J, et al. N Engl J Med. 1996;334:1011–1018. doi: 10.1056/NEJM199604183341602. [DOI] [PubMed] [Google Scholar]
- 7.Deeks S G, Smith M, Holodniy M, Kahn J O. J Am Med Assoc. 1997;277:145–153. [PubMed] [Google Scholar]
- 8.Gulick R M, Mellors J W, Havlir D, Eron J J, Gonzalez C, McMahon D, Richman D D, Valentine F T, Jonas L, Meibohm A, et al. N Engl J Med. 1997;337:734–739. doi: 10.1056/NEJM199709113371102. [DOI] [PubMed] [Google Scholar]
- 9.Gulick R M, Mellors J W, Havlir D, Eron J J, Meibohm A, Condra J H, Valentine F T, McMahon D, Gonzalez C, Jonas L, et al. Ann Intern Med. 2000;133:35–39. doi: 10.7326/0003-4819-133-1-200007040-00007. [DOI] [PubMed] [Google Scholar]
- 10.Kahn J O, Walker B D. N Engl J Med. 1998;339:33–39. doi: 10.1056/NEJM199807023390107. [DOI] [PubMed] [Google Scholar]
- 11.Lee T H, Sheppard H W, Reis M, Dondero D, Osmond D, Busch M P. J Acquired Immune Defic Syndr. 1994;7:381–388. [PubMed] [Google Scholar]
- 12.Mellors J W, Rinaldo C R, Jr, Gupta P, White R M, Todd J A, Kingsley L A. Science. 1996;272:1167–1170. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- 13.Mellors J W, Munoz A, Girogi J V, Margolick J B, Tassoni C J, Gupta P, Kingsley L A, Todd J A, Saah A J, Detels R, et al. Ann Intern Med. 1997;126:946–954. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 14.Janssen R S, Satten G A, Stramer S L, Rawal B D, O'Brien T R, Weiblen B J, Hecht F M, Jack N, Cleghorn F R, Kahn J O, et al. J Am Med Assoc. 1998;280:42–48. doi: 10.1001/jama.280.1.42. [DOI] [PubMed] [Google Scholar]
- 15.Triques K, Coste J, Perret J L, Segarra C, Mpoudi E, Reynes J, Delaporte E, Butcher A, Dreyer K, Herman S, et al. J Clin Microbiol. 1999;37:110–116. doi: 10.1128/jcm.37.1.110-116.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castro B A, Weiss C D, Wiviott L D, Levy J A. J Clin Microbiol. 1988;26:2371–2376. doi: 10.1128/jcm.26.11.2371-2376.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mackewicz C E, Ortega H W, Levy J A. J Clin Invest. 1991;87:1462–1466. doi: 10.1172/JCI115153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mackewicz C E, Barker E, Levy J A. Science. 1996;274:1393–1395. doi: 10.1126/science.274.5291.1393. [DOI] [PubMed] [Google Scholar]
- 19.Levy J A, Hoffman A D, Kramer S M, Landis J A, Shimabukuro J M, Oshiro L S. Science. 1984;225:840–842. doi: 10.1126/science.6206563. [DOI] [PubMed] [Google Scholar]
- 20.Levy J A, Shimabukuro J. J Infect Dis. 1985;152:734–738. doi: 10.1093/infdis/152.4.734. [DOI] [PubMed] [Google Scholar]
- 21.Tateno M, Levy J A. Virology. 1988;167:299–301. doi: 10.1016/0042-6822(88)90084-0. [DOI] [PubMed] [Google Scholar]
- 22.Hoffman A D, Banapour B, Levy J A. Virology. 1985;147:326–335. doi: 10.1016/0042-6822(85)90135-7. [DOI] [PubMed] [Google Scholar]
- 23.Fliess J L. Statistical Methods for Rates and Proportions. New York: Wiley; 1981. [Google Scholar]
- 24.Niu M T, Stein D S, Schnittman S M. J Infect Dis. 1993;168:1490–1501. doi: 10.1093/infdis/168.6.1490. [DOI] [PubMed] [Google Scholar]
- 25.Kinloch-De Loes S, Hirschel B J, Hoen B, Cooper D A, Tindall B, Carr A, Saurat J-H, Clumeck N, Lazzarin A, Mathiesen L, et al. N Engl J Med. 1995;333:408–413. doi: 10.1056/NEJM199508173330702. [DOI] [PubMed] [Google Scholar]
- 26.Markowitz M, Vesanen M, Tenner-Racz K, Cao Y, Binley J M, Talal A, Hurley A, Ji X, Chaudhry M R, Yaman M, et al. J Infect Dis. 1999;179:527–537. doi: 10.1086/314628. [DOI] [PubMed] [Google Scholar]
- 27.Heagy W, Crumpacker C, Lopez P A, Finberg R W. J Clin Invest. 1991;87:1916–1924. doi: 10.1172/JCI115217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borrow P, Lewicki H, Hahn B H, Shaw G M, Oldstone M B A. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koup R A, Safrit J T, Cao Y, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mackewicz C E, Yang L C, Lifson J D, Levy J A. Lancet. 1994;344:1671–1673. doi: 10.1016/s0140-6736(94)90459-6. [DOI] [PubMed] [Google Scholar]
- 31.Rosenberg E S, Billingsley J M, Caliendo A M, Boswell S L, Sax P E, Kalams S A, Walker B D. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 32.Clerici M, Giorgi J V, Chou C C, Gudeman V K, Zack J A, Gupta P, Ho H N, Nishanian P G, Berzofsky J A, Shearer G M. J Infect Dis. 1992;165:1012–1019. doi: 10.1093/infdis/165.6.1012. [DOI] [PubMed] [Google Scholar]
- 33.Clerici M, Levin J M, Kessler H A, Harris A, Berzofsky J A, Landay A L, Shearer G M. J Am Med Assoc. 1994;271:42–46. [PubMed] [Google Scholar]
- 34.Rowland-Jones S, Sutton J, Ariyoshi K, Dong T, Gotch F, McAdam S, Whitby D, Sabally S, Gallimore A, Corrah T, et al. Nat Med. 1995;1:59–64. doi: 10.1038/nm0195-59. [DOI] [PubMed] [Google Scholar]
- 35.Shearer G M, Clerici M. Immunol Today. 1996;17:21–24. doi: 10.1016/0167-5699(96)80564-0. [DOI] [PubMed] [Google Scholar]
- 36.Stranford S, Skurnick J, Louria D, Osmond D, Chang S, Sninsky J, Ferrari G, Weinhold K, Lindquist C, Levy J. Proc Natl Acad Sci USA. 1999;96:1030–1035. doi: 10.1073/pnas.96.3.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morris L, Binley J M, Clas B A, Bonhoeffer S, Astill T P, Kost R, Hurley A, Cao Y, Markowitz M, Ho D D, Moore J P. J Exp Med. 1998;188:233–245. doi: 10.1084/jem.188.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spiegel H M, DeFalcon E, Ogg G S, Larsson M, Beadle T J, Tao P, McMichael A J, Bhardwaj N, O'Callaghan C, Cox W I, et al. J Infect Dis. 1999;180:359–368. doi: 10.1086/314867. [DOI] [PubMed] [Google Scholar]
- 39.Wilkinson J, Zaunders J J, Carr A, Cooper D A. J Infect Dis. 1999;180:68–75. doi: 10.1086/314833. [DOI] [PubMed] [Google Scholar]
- 40.Pitcher C J, Quittner C, Peterson D M, Connors M, Koup R A, Maino V C, Picker L J. Nat Med. 1999;5:518–525. doi: 10.1038/8400. [DOI] [PubMed] [Google Scholar]
- 41.Sato H, Orenstein J, Dimitrov D, Martin M. Virology. 1992;186:712–724. doi: 10.1016/0042-6822(92)90038-q. [DOI] [PubMed] [Google Scholar]
- 42.Tovo R-A. AIDS. 2000;14:743–744. doi: 10.1097/00002030-200004140-00014. [DOI] [PubMed] [Google Scholar]