Abstract
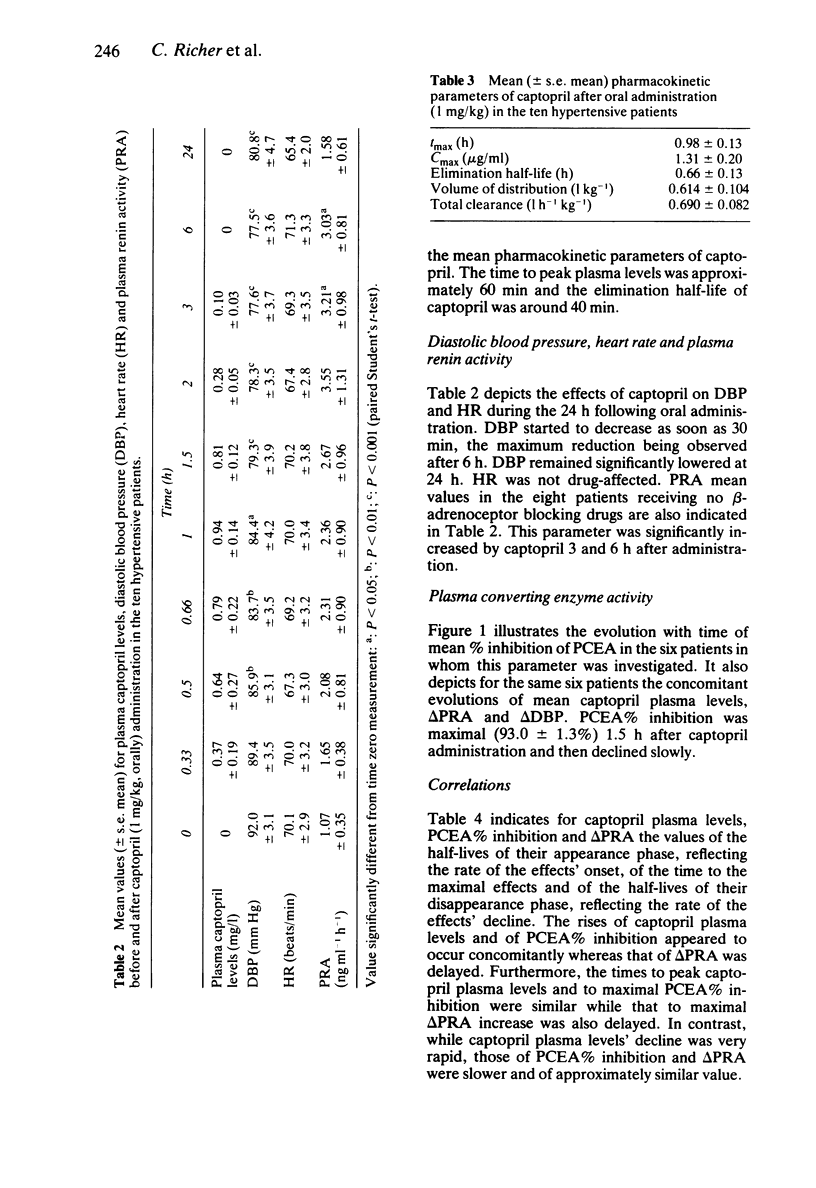
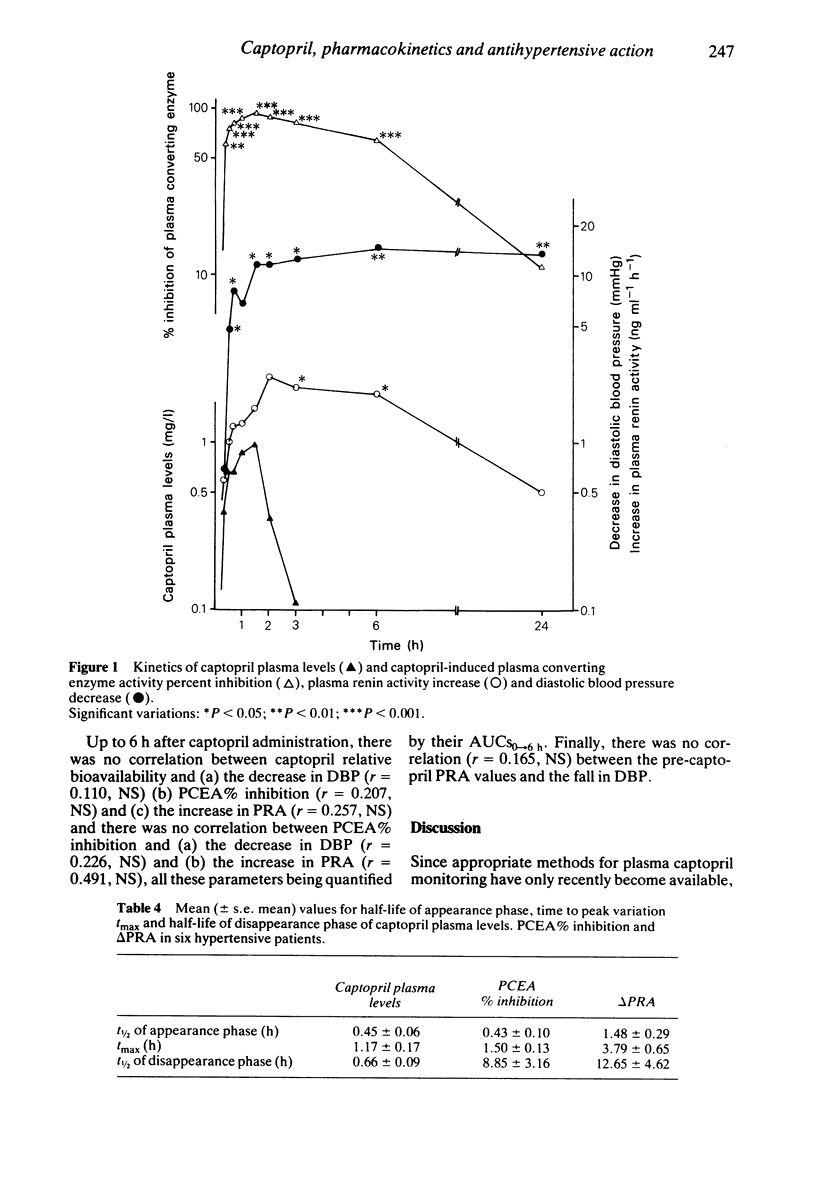
The kinetics of captopril plasma levels and of the drug-induced plasma converting enzyme activity (PCEA), plasma renin activity (PRA) and diastolic blood pressure (DBP) modifications were studied over 24 h after oral administration of captopril, 1 mg/kg, to ten hypertensive patients. Free unchanged captopril pharmacokinetic parameters were: t1/2, alpha: 0.45 +/- 0.06 h; tmax: 0.98 +/- 0.13 h; Cmax: 1.31 +/- 0.20 mg l-1; t1/2,z: 0.66 +/- 0.13 h; V: 0.614 +/- 0.104 1 kg-1 and CLtot: 0.690 +/- 0.082 l h-1 kg-1. At 6 h captopril was no longer detectable in plasma. The onset of PCEA inhibition and of DBP decrease closely followed the rise of captopril's plasma levels, while that of PRA increase was delayed. In contrast, while captopril rapidly disappeared from plasma, its biological and antihypertensive effects were long-lasting. The lack of correlation between the relative bioavailability of captopril and the induced reduction in DBP (evaluated by the corresponding AUCs) suggests that free unchanged captopril plasma monitoring is not an adequate indicator of hypertensive patients' potential responsiveness to captopril's blood pressure lowering effects.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brunner D. B., Desponds G., Biollaz J., Keller I., Ferber F., Gavras H., Brunner H. R., Schelling J. L. Effect of a new angiotensin converting enzyme inhibitor MK 421 and its lysine analogue on the components of the renin system in healthy subjects. Br J Clin Pharmacol. 1981 May;11(5):461–467. doi: 10.1111/j.1365-2125.1981.tb01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell B. C., Shepherd A. N., Reid J. L. Effects of the angiotensin converting enzyme inhibitor, captopril, in essential hypertension. Br J Clin Pharmacol. 1982 Feb;13(2):213–217. doi: 10.1111/j.1365-2125.1982.tb01359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
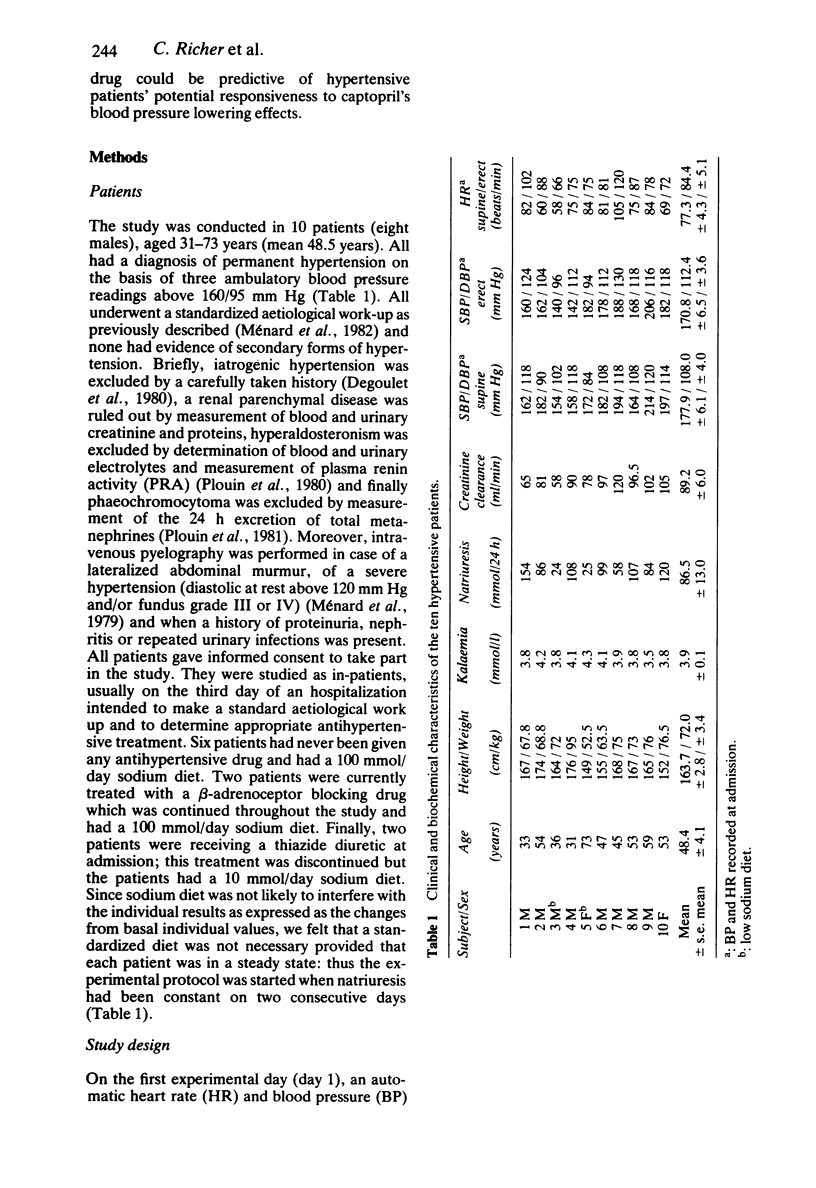
- Degoulet P., Menard J., Berger C., Plouin P. F., Devries C., Hirel J. C. Hypertension management: the computer as a participant. Am J Med. 1980 Apr;68(4):559–567. doi: 10.1016/0002-9343(80)90306-x. [DOI] [PubMed] [Google Scholar]
- Duchin K. L., Singhvi S. M., Willard D. A., Migdalof B. H., McKinstry D. N. Captopril kinetics. Clin Pharmacol Ther. 1982 Apr;31(4):452–458. doi: 10.1038/clpt.1982.59. [DOI] [PubMed] [Google Scholar]
- Gavras H., Biollaz J., Waeber B., Brunner H. R., Gavras I., Davies R. O. Antihypertensive effect of the new oral angiotensin converting enzyme inhibitor "MK-421". Lancet. 1981 Sep 12;2(8246):543–547. doi: 10.1016/s0140-6736(81)90937-5. [DOI] [PubMed] [Google Scholar]
- Imbs J. L., Bakish D., Schmidt M., Schwartz J. Low temperature sustains inhibition of angiotensin-converting-enzyme activity in serum from patients taking captopril. N Engl J Med. 1981 Jul 23;305(4):229–229. doi: 10.1056/NEJM198107233050425. [DOI] [PubMed] [Google Scholar]
- Jarrott B., Anderson A., Hooper R., Louis W. J. High-performance liquid chromatographic analysis of captopril in plasma. J Pharm Sci. 1981 Jun;70(6):665–667. doi: 10.1002/jps.2600700622. [DOI] [PubMed] [Google Scholar]
- Jarrott B., Drummer O., Hooper R., Anderson A. I., Miach P. J., Louis W. J. Pharmacokinetic properties of captopril after acute and chronic administration to hypertensive subjects. Am J Cardiol. 1982 Apr 21;49(6):1547–1549. doi: 10.1016/0002-9149(82)90385-x. [DOI] [PubMed] [Google Scholar]
- Kawahara Y., Hisaoka M., Yamazaki Y., Inage A., Morioka T. Determination of captopril in blood and urine by high-performance liquid chromatography. Chem Pharm Bull (Tokyo) 1981 Jan;29(1):150–157. doi: 10.1248/cpb.29.150. [DOI] [PubMed] [Google Scholar]
- Kripalani K. J., McKinstry D. N., Singhvi S. M., Willard D. A., Vukovich R. A., Migdalof B. H. Disposition of captopril in normal subjects. Clin Pharmacol Ther. 1980 May;27(5):636–641. doi: 10.1038/clpt.1980.90. [DOI] [PubMed] [Google Scholar]
- Millar J. A., Derkx F. H., McLean K., Reid J. L. Pharmacodynamics of converting enzyme inhibition: the cardiovascular, endocrine and autonomic effects of MK421 (enalapril) and MK521. Br J Clin Pharmacol. 1982 Sep;14(3):347–355. doi: 10.1111/j.1365-2125.1982.tb01990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoyama K., Hirakata H., Iseki K., Fujimi S., Omae T., Kobayashi M., Kawahara Y. Blood concentration and urinary excretion of captopril (SQ 14,225) in patients with chronic renal failure. Hypertension. 1981 Jul-Aug;3(4):456–459. doi: 10.1161/01.hyp.3.4.456. [DOI] [PubMed] [Google Scholar]
- Plouin P. F., Duclos J. M., Menard J., Comoy E., Bohuon C., Alexandre J. M. Biochemical tests for diagnosis of phaeochromocytoma: urinary versus plasma determinations. Br Med J (Clin Res Ed) 1981 Mar 14;282(6267):853–854. doi: 10.1136/bmj.282.6267.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen S., Damkjaer Nielsen M., Giese J. Captopril combined with thiazide lowers renin substrate concentration: implications for methodology in renin assays. Clin Sci (Lond) 1981 May;60(5):591–593. doi: 10.1042/cs0600591. [DOI] [PubMed] [Google Scholar]
- Roulston J. E., MacGregor G. A. The measurement of angiotensin-converting enzyme in subjects receiving captopril. N Engl J Med. 1980 Aug 14;303(7):397–397. doi: 10.1056/NEJM198008143030717. [DOI] [PubMed] [Google Scholar]
- Waeber B., Brunner H. R., Brunner D. B., Curtet A. L., Turini G. A., Gavras H. Discrepancy between antihypertensive effect and angiotensin converting enzyme inhibition by captopril. Hypertension. 1980 Mar-Apr;2(2):236–242. doi: 10.1161/01.hyp.2.2.236. [DOI] [PubMed] [Google Scholar]



