Abstract
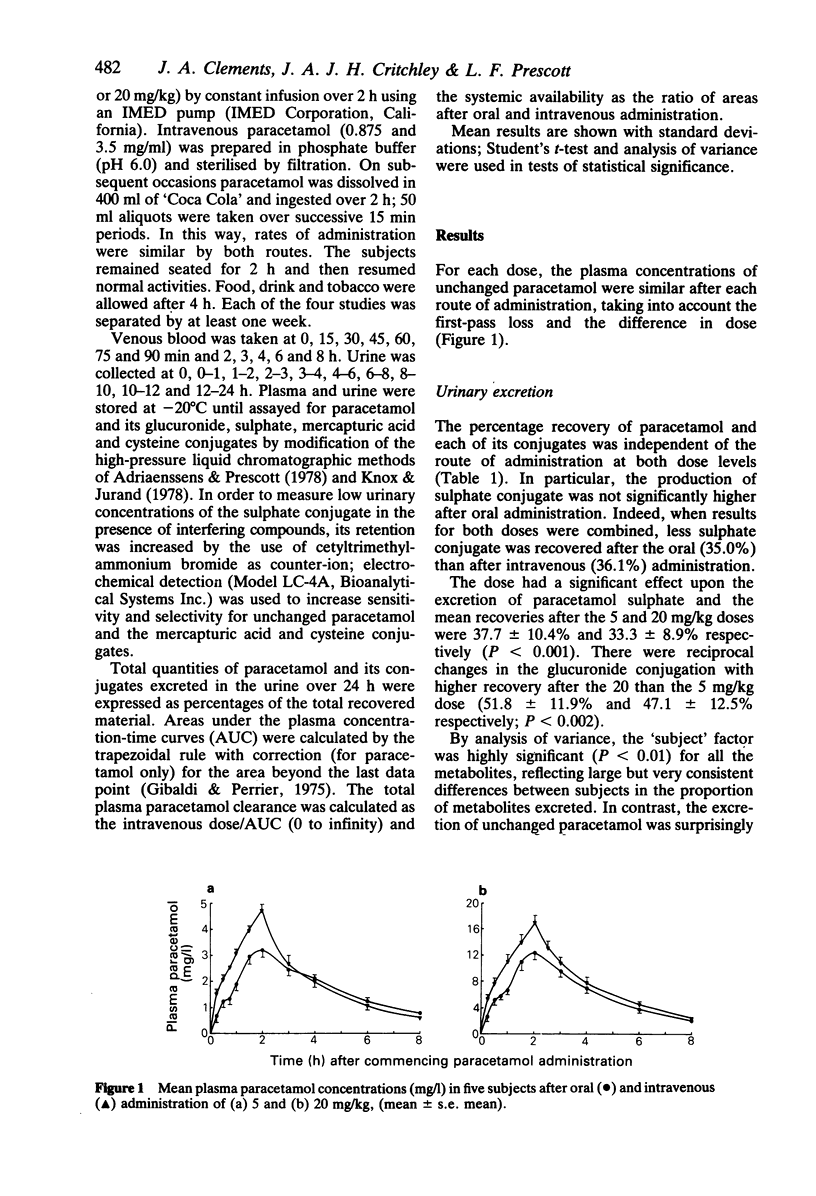
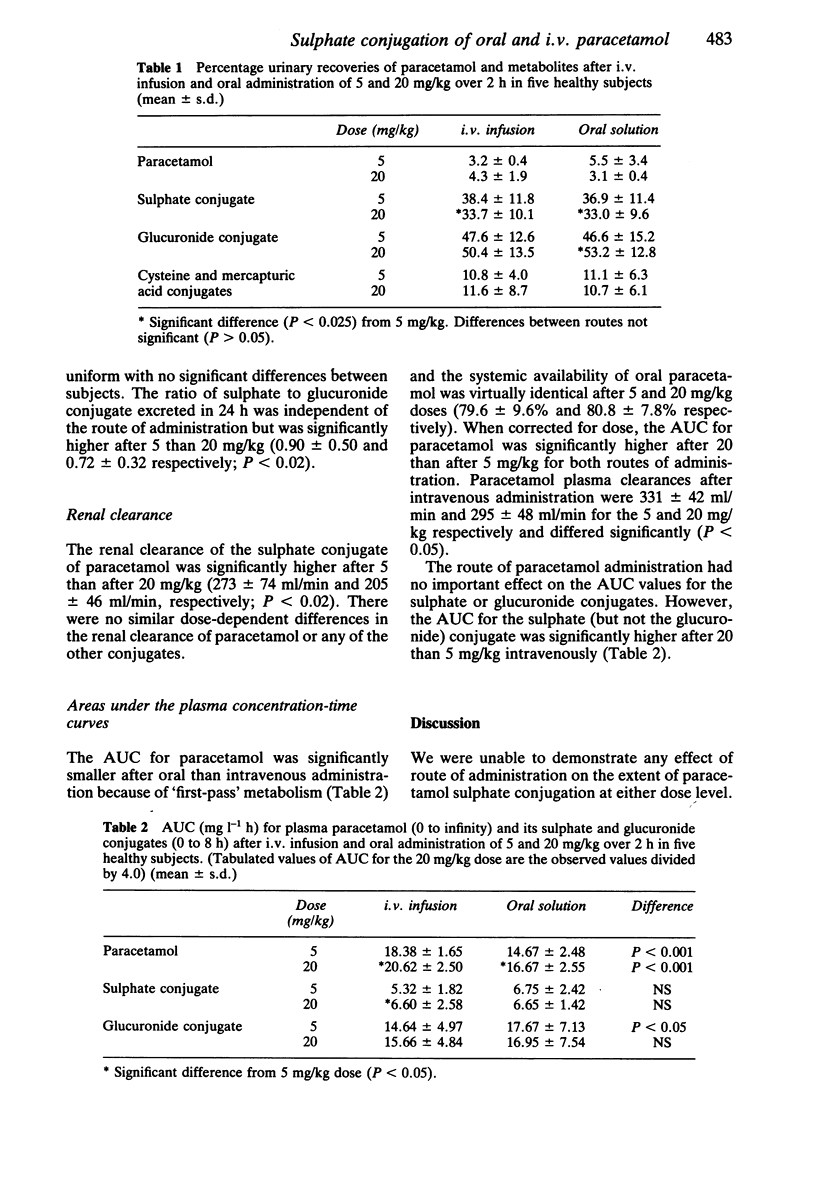
The effects of paracetamol dose (5 and 20 mg/kg) and route of administration (intravenous and oral) on the urinary excretion of paracetamol and its glucuronide, sulphate, cysteine and mercapturic acid conjugates were studied in five healthy subjects. The fractional urinary excretion of unchanged paracetamol and its conjugates was independent of the route of administration at both dose levels, suggesting that the gastrointestinal tract is not an important site for paracetamol metabolism. The percentage of the dose excreted as the sulphate conjugate was significantly higher after 5 than after 20 mg/kg (37.7% and 33.3% respectively) and this is consistent with saturation of sulphate conjugation. No significant effect of paracetamol dose upon the area under the plasma concentration-time curve (AUC), corrected for dose, was found for the sulphate or glucuronide conjugates. The total plasma clearance of paracetamol and the renal clearance of the sulphate conjugate were significantly higher after the 5 than the 20 mg/kg dose (331 +/- 42 ml/min and 295 +/- 48 ml/min; 273 +/- 74 ml/min and 205 +/- 46 ml/min respectively). The oral systemic availability of paracetamol was 80% and independent of dose.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adriaenssens P. I., Prescott L. F. High performance liquid chromatographic estimation of paracetamol metabolites in plasma. Br J Clin Pharmacol. 1978 Jul;6(1):87–88. doi: 10.1111/j.1365-2125.1978.tb01687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conolly M. E., Davies D. S., Dollery C. T., Morgan C. D., Paterson J. W., Sandler M. Metabolism of isoprenaline in dog and man. Br J Pharmacol. 1972 Nov;46(3):458–472. doi: 10.1111/j.1476-5381.1972.tb08143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George C. F., Blackwell E. W., Davies D. S. Metabolism of isoprenaline in the intestine. J Pharm Pharmacol. 1974 Apr;26(4):265–267. doi: 10.1111/j.2042-7158.1974.tb09268.x. [DOI] [PubMed] [Google Scholar]
- George C. F. Drug metabolism by the gastrointestinal mucosa. Clin Pharmacokinet. 1981 Jul-Aug;6(4):259–274. doi: 10.2165/00003088-198106040-00002. [DOI] [PubMed] [Google Scholar]
- Knox J. H., Jurand J. Determination of paracetamol and its metabolites in urine by high-performance liquid chromatography using ion-pair systems. J Chromatogr. 1978 Feb 11;149:297–312. doi: 10.1016/s0021-9673(00)80994-2. [DOI] [PubMed] [Google Scholar]
- Levy G., Yamada H. Drug biotransformation interactions in man. 3. Acetaminophen and salicylamide. J Pharm Sci. 1971 Feb;60(2):215–221. doi: 10.1002/jps.2600600212. [DOI] [PubMed] [Google Scholar]
- Perucca E., Richens A. Paracetamol disposition in normal subjects and in patients treated with antiepileptic drugs. Br J Clin Pharmacol. 1979 Feb;7(2):201–206. doi: 10.1111/j.1365-2125.1979.tb00922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott L. F. Kinetics and metabolism of paracetamol and phenacetin. Br J Clin Pharmacol. 1980 Oct;10 (Suppl 2):291S–298S. doi: 10.1111/j.1365-2125.1980.tb01812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins M. D., Henderson D. B., Hijab A. R. Pharmacokinetics of paracetamol (acetaminophen) after intravenous and oral administration. Eur J Clin Pharmacol. 1977 Apr 20;11(4):283–286. doi: 10.1007/BF00607678. [DOI] [PubMed] [Google Scholar]
- Williams F. M., Briant R. H., Dollery C. T., Davies D. S. The influence of the route of administration on urinary metabolites of isoetharine. Xenobiotica. 1974 Jun;4(6):345–353. doi: 10.3109/00498257409052110. [DOI] [PubMed] [Google Scholar]


