Abstract
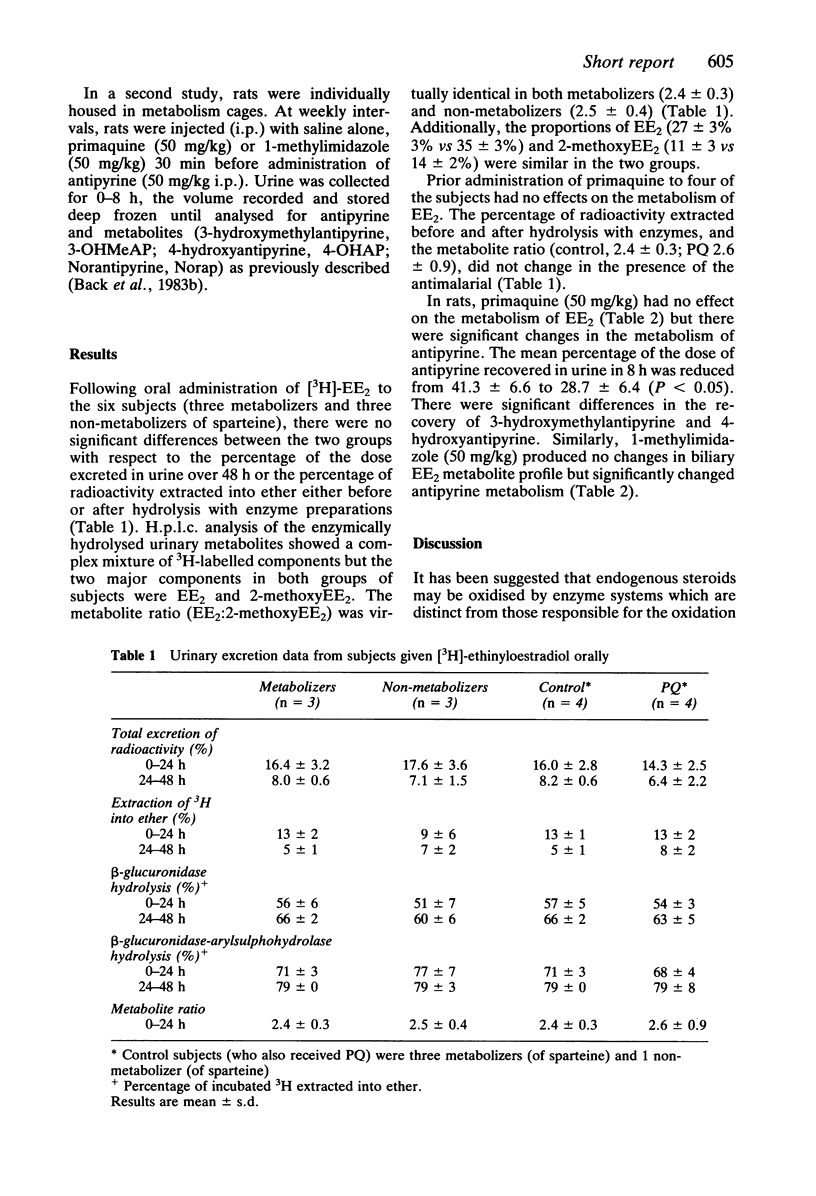
The metabolism of [3H]ethinyloestradiol (EE2) was investigated in six male subjects who had been phenotyped with respect to sparteine metabolism (three metabolizers and three non-metabolizers). Urinary metabolite profiles of EE2 were virtually identical. Following enzyme hydrolysis of sulphate and glucuronide conjugates the major urinary metabolite was 2-methoxyEE2. The ratio EE2:2-methoxyEE2 was taken as a measure of EE2 2-hydroxylation (metabolizers, 2.4 +/- 0.3; non-metabolizers, 2.5 +/- 0.4). Primaquine (45 mg), previously shown to inhibit antipyrine metabolism, had no effect on EE2 2-hydroxylation. Supporting studies in rats showed that acute administration of primaquine (50 mg/kg) and 1-methylimidazole (50 mg/kg) inhibited antipyrine but not EE2 metabolism. It is concluded that the cytochrome P-450 enzyme responsible for 2-hydroxylation of EE2 is distinct from the enzymes involved in the oxidation of sparteine and antipyrine.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Back D. B., Park B. K., Tjia J. F., Newby S. N. Sulphaphenazole and drug oxidation in man. Br J Clin Pharmacol. 1983 Oct;16(4):460–461. doi: 10.1111/j.1365-2125.1983.tb02199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back D. J., Purba H. S., Park B. K., Ward S. A., Orme M. L. Effect of chloroquine and primaquine on antipyrine metabolism. Br J Clin Pharmacol. 1983 Nov;16(5):497–502. doi: 10.1111/j.1365-2125.1983.tb02206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont V., Lemort N., Beaumont J. L. Oral contraception, circulating immune complexes, antiethinylestradiol antibodies, and thrombosis. Am J Reprod Immunol. 1982 Feb;2(1):8–12. doi: 10.1111/j.1600-0897.1982.tb00077.x. [DOI] [PubMed] [Google Scholar]
- Bolt H. M., Kappus H., Käsbohrer R. Metabolism of 17 alpha-ethinylestradiol by human liver microsomes in vitro: aromatic hydroxylation and irreversible protein binding of metabolites. J Clin Endocrinol Metab. 1974 Dec;39(6):1072–1080. doi: 10.1210/jcem-39-6-1072. [DOI] [PubMed] [Google Scholar]
- Bolt H. M., Kassel H. Effects of insecticide synergists on microsomal oxidation of estradiol and ethynylestradiol and on microsomal drug metabolism. Xenobiotica. 1976 Jan;6(1):33–38. doi: 10.3109/00498257609151609. [DOI] [PubMed] [Google Scholar]
- Bolt H. M. Metabolism of estrogens--natural and synthetic. Pharmacol Ther. 1979;4(1):155–181. doi: 10.1016/0163-7258(79)90018-4. [DOI] [PubMed] [Google Scholar]
- Breimer D. D. Interindividual variations in drug disposition. Clinical implications and methods of investigation. Clin Pharmacokinet. 1983 Sep-Oct;8(5):371–377. doi: 10.2165/00003088-198308050-00001. [DOI] [PubMed] [Google Scholar]
- Eichelbaum M., Bertilsson L., Säwe J., Zekorn C. Polymorphic oxidation of sparteine and debrisoquine: related pharmacogenetic entities. Clin Pharmacol Ther. 1982 Feb;31(2):184–186. doi: 10.1038/clpt.1982.29. [DOI] [PubMed] [Google Scholar]
- Gelbke H. P., Ball P., Knuppen R. 2-Hydroxyoestrogens. Chemistry, biogenesis, metabolism and physiological significance. Adv Steroid Biochem Pharmacol. 1977;6:81–154. [PubMed] [Google Scholar]
- Liehr J. G. 2-Fluoroestradiol. Separation of estrogenicity from carcinogenicity. Mol Pharmacol. 1983 Mar;23(2):278–281. [PubMed] [Google Scholar]
- Maggs J. L., Grabowski P. S., Park B. K. Protection of catechol oestrogen from oxidation during enzymic hydrolysis of biliary conjugates. J Steroid Biochem. 1983 Aug;19(2):1235–1237. doi: 10.1016/0022-4731(83)90422-3. [DOI] [PubMed] [Google Scholar]
- Maggs J. L., Grabowski P. S., Rose M. E., Park B. K. The biotransformation of 17 alpha-ethynyl[3H]estradiol in the rat: irreversible binding and biliary metabolites. Xenobiotica. 1982 Oct;12(10):657–668. doi: 10.3109/00498258209042044. [DOI] [PubMed] [Google Scholar]
- Maggs J. L., Grimmer S. F., Orme M. L., Breckenridge A. M., Park B. K., Gilmore I. T. The biliary and urinary metabolites of [3H]17 alpha-ethynylestradiol in women. Xenobiotica. 1983 Jul;13(7):421–431. doi: 10.3109/00498258309052280. [DOI] [PubMed] [Google Scholar]
- Nelson S. D., Mitchell J. R., Dybing E., Sasame H. A. Cytochrome P-450-mediated oxidation of 2-hydroxyestrogens to reactive intermediates. Biochem Biophys Res Commun. 1976 Jun 21;70(4):1157–1165. doi: 10.1016/0006-291x(76)91024-x. [DOI] [PubMed] [Google Scholar]
- Park B. K., Eichelbaum M., Ohnhaus E. E. 6 beta-hydroxycortisol excretion in relation to polymorphic N-oxidation of sparteine. Br J Clin Pharmacol. 1982 May;13(5):737–740. doi: 10.1111/j.1365-2125.1982.tb01448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trush M. A., Mimnaugh E. G., Gram T. E. Activation of pharmacologic agents to radical intermediates. Implications for the role of free radicals in drug action and toxicity. Biochem Pharmacol. 1982 Nov 1;31(21):3335–3346. doi: 10.1016/0006-2952(82)90609-8. [DOI] [PubMed] [Google Scholar]
- Wilkinson C. F., Hetnarski K., Yellin T. O. Imidazole derivatives--a new class of microsomal enzyme inhibitors. Biochem Pharmacol. 1972 Dec 1;21(23):3187–3192. doi: 10.1016/0006-2952(72)90147-5. [DOI] [PubMed] [Google Scholar]
- Williams M. C., Goldzieher J. W. Chromatographic patterns of urinary ethynyl estrogen metabolites in various populations. Steroids. 1980 Sep;36(3):255–282. doi: 10.1016/0039-128x(80)90001-x. [DOI] [PubMed] [Google Scholar]
- Williams M. C., Helton E. D., Goldzieher J. W. The urinary metabolites of 17alpha-ethynylestradiol-9alpha,11xi-3H in women. Chromatographic profiling and indentification of ethynyl and non-ethynyl compounds. Steroids. 1975 Feb;25(2):229–246. doi: 10.1016/s0039-128x(75)90135-x. [DOI] [PubMed] [Google Scholar]


