Abstract
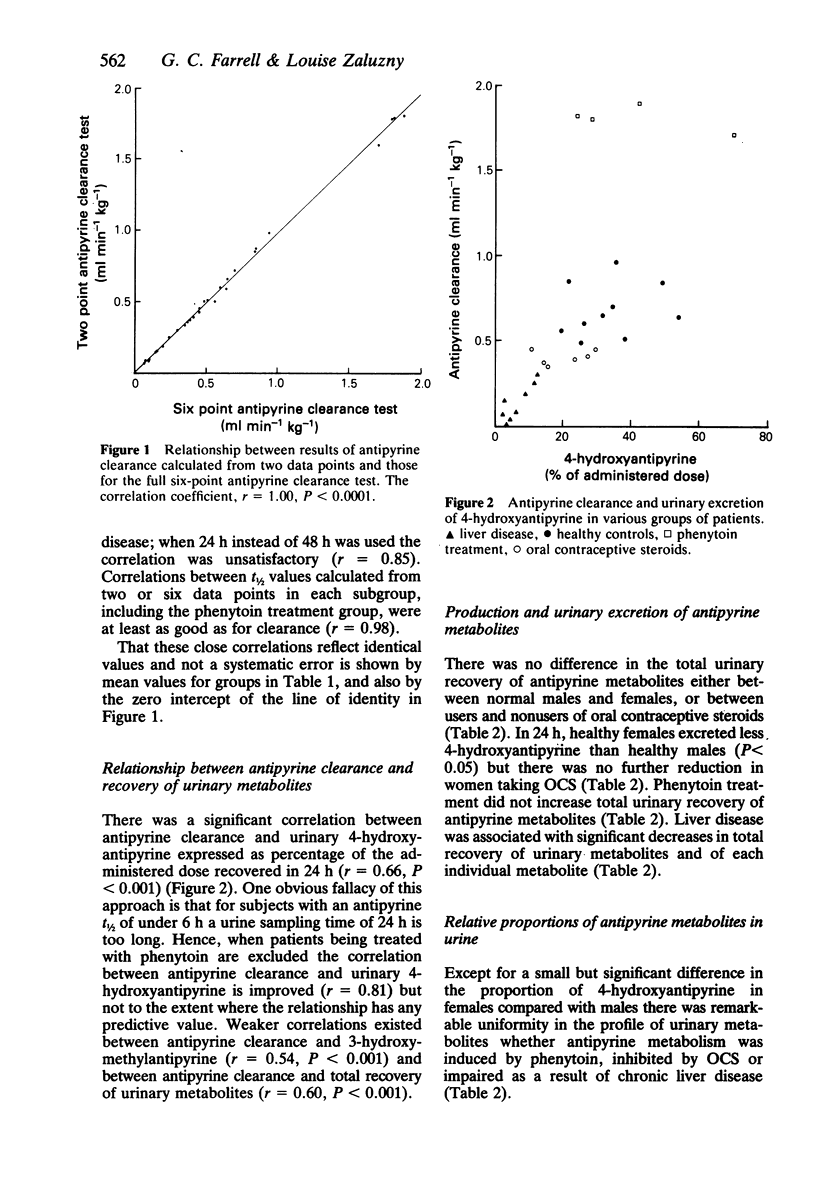
High-performance liquid chromatography (h.p.l.c.) was used to measure plasma antipyrine concentrations in sixteen healthy subjects; five males, six females taking oral contraceptive steroids (OCS) and five age-matched females not taking OCS. Following oral administration of the drug, antipyrine clearance could be determined with similar precision and accuracy from plasma concentrations at two selected times (4 h and 24 h) ('two-sample antipyrine clearance test') as from six samples taken over two to three elimination half-lives (t1/2) of the drug ('conventional antipyrine clearance test'). Provided times for plasma sampling were modified appropriately, the two-sample antipyrine clearance test also gave reliable results in four patients taking phenytoin (sampling times 4 h and 8 h) who had significantly enhanced antipyrine clearance, and in nine patients with severe liver disease (using 4 h and 48 h) in whom antipyrine clearance was impaired. Urinary excretion of antipyrine metabolites (from 0-24 h) was determined in the above groups. Antipyrine systemic clearance correlated best with the percentage of administered dose excreted as 4-hydroxyantipyrine (r = 0.66, P less than 0.001) but also with 3-hydroxymethylantipyrine (r = 0.54, P less than 0.001) and with total metabolites excreted (r = 0.60, P less than 0.001). Total antipyrine metabolites excreted in urine in 24 h were significantly different from controls only in patients with liver disease (14 +/- 7.6% of administered dose vs 62 +/- 14%, P less than 0.001). The relative proportion of antipyrine metabolites did not appear to be altered when hepatic mixed function oxidation was induced by phenytoin or inhibited by OCS or by severe liver disease.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abernethy D. R., Greenblatt D. J. Impairment of antipyrine metabolism by low-dose oral contraceptive steroids. Clin Pharmacol Ther. 1981 Jan;29(1):106–110. doi: 10.1038/clpt.1981.17. [DOI] [PubMed] [Google Scholar]
- Danhof M., Groot-van der Vis E., Breiner D. D. Assay of antipyrine and its primary metabolites in plasma, saliva and urine by high-performance liquid chromatography and some preliminary results in man. Pharmacology. 1979;18(4):210–223. doi: 10.1159/000137254. [DOI] [PubMed] [Google Scholar]
- Danhof M., Krom D. P., Breimer D. D. Studies on the different metabolic pathways of antipyrine in rats: influence of phenobarbital and 3-methylcholanthrene treatment. Xenobiotica. 1979 Nov;9(11):695–702. doi: 10.3109/00498257909042337. [DOI] [PubMed] [Google Scholar]
- Danhof M., Verbeek R. M., van Boxtel C. J., Boeijinga J. K., Breimer D. D. Differential effects of enzyme induction on antipyrine metabolite formation. Br J Clin Pharmacol. 1982 Mar;13(3):379–386. doi: 10.1111/j.1365-2125.1982.tb01389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danhof M., van Zuilen A., Boeijinga J. K., Breimer D. D. Studies of the different metabolic pathways of antipyrine in man. Oral versus i.v. administration and the influence of urinary collection time. Eur J Clin Pharmacol. 1982;21(5):433–441. doi: 10.1007/BF00542332. [DOI] [PubMed] [Google Scholar]
- Døssing M., Poulsen H. E., Andreasen P. B., Tygstrup N. A simple method for determination of antipyrine clearance. Clin Pharmacol Ther. 1982 Sep;32(3):392–396. doi: 10.1038/clpt.1982.177. [DOI] [PubMed] [Google Scholar]
- Farrell G. C., Cooksley W. G., Hart P., Powell L. W. Drug metabolism in liver disease. Identification of patients with impaired hepatic drug metabolism. Gastroenterology. 1978 Oct;75(4):580–588. [PubMed] [Google Scholar]
- Farrell G., Zaluzny L., Baird-Lambert J., Cvejic M., Buchanan N. Impaired elimination of metronidazole in decompensated chronic liver disease. Br Med J (Clin Res Ed) 1983 Dec 17;287(6408):1845–1845. doi: 10.1136/bmj.287.6408.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrun L. H., Villeneuve J. P. Hypermetabolism of phenytoin as a cause of treatment failure. Clin Neuropharmacol. 1983 Mar;6(1):67–70. doi: 10.1097/00002826-198303000-00008. [DOI] [PubMed] [Google Scholar]
- McPherson G. A., Benjamin I. S., Boobis A. R., Brodie M. J., Hampden C., Blumgart L. H. Antipyrine elimination as a dynamic test of hepatic functional integrity in obstructive jaundice. Gut. 1982 Sep;23(9):734–738. doi: 10.1136/gut.23.9.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes J. C., Houston J. B. Antipyrine metabolite kinetics in phenobarbital and beta-naphthoflavone-induced rats. Drug Metab Dispos. 1983 Mar-Apr;11(2):131–136. [PubMed] [Google Scholar]
- Shargel L., Cheung W. M., Yu A. B. High-pressure liquid chromatographic analysis of antipyrine in small plasma samples. J Pharm Sci. 1979 Aug;68(8):1052–1054. doi: 10.1002/jps.2600680835. [DOI] [PubMed] [Google Scholar]
- Sjöqvist F., von Bahr C. Interindividual differences in drug oxidation: clinical importance. Drug Metab Dispos. 1973 Jan-Feb;1(1):469–482. [PubMed] [Google Scholar]
- Teunissen M. W., Srivastava A. K., Breimer D. D. Influence of sex and oral contraceptive steroids on antipyrine metabolite formation. Clin Pharmacol Ther. 1982 Aug;32(2):240–246. doi: 10.1038/clpt.1982.154. [DOI] [PubMed] [Google Scholar]
- Vesell E. S., Page J. G. Genetic control of drug levels in man: antipyrine. Science. 1968 Jul 5;161(3836):72–73. doi: 10.1126/science.161.3836.72. [DOI] [PubMed] [Google Scholar]
- Vesell E. S. The antipyrine test in clinical pharmacology: conceptions and misconceptions. Clin Pharmacol Ther. 1979 Sep;26(3):275–286. doi: 10.1002/cpt1979263275. [DOI] [PubMed] [Google Scholar]



