Abstract
We have observed that patients on concurrent cyclosporin and phenytoin therapy required increased doses of cyclosporin to maintain therapeutic concentrations of this novel immunosuppressive drug. We have, therefore, studied the influence of phenytoin on the pharmacokinetics of oral cyclosporin in six healthy male subjects. Cyclosporin concentrations in serum and whole blood were measured by high pressure liquid chromatography (h.p.l.c.) and radioimmunoassay (RIA). Concentrations of cyclosporin in whole blood were consistently higher than corresponding values in serum. Concentrations of cyclosporin determined by RIA were also consistently higher than those determined by h.p.l.c. Irrespective of the biological fluid (serum or whole blood) or the type of drug analysis (h.p.l.c. or RIA), changes in cyclosporin kinetics following phenytoin administration exhibited similar patterns. Phenytoin significantly reduced the maximum concentration and the area under the concentration-time curve and significantly increased total body clearance of cyclosporin. There was a statistically significant reduction of cyclosporin half-life (t 1/2) in whole blood using h.p.l.c. analysis. However, there was no significant change in cyclosporin t 1/2 in serum following phenytoin administration, using either form of drug analysis. Cyclosporin metabolites 17 and 18 were measured by h.p.l.c. in whole blood samples only, since these metabolites were found almost entirely in red blood cells. Phenytoin significantly reduced the Cmax and AUC of both metabolites, but no significant change was observed in the t 1/2 of either. Phenytoin enhanced the metabolism of antipyrine which was co-administered with cyclosporin to assess oxidative enzyme activity. We conclude that patients undergoing organ transplantation should be carefully monitored if they require phenytoin or other drugs known to accelerate oxidative metabolism.
Full text
PDF
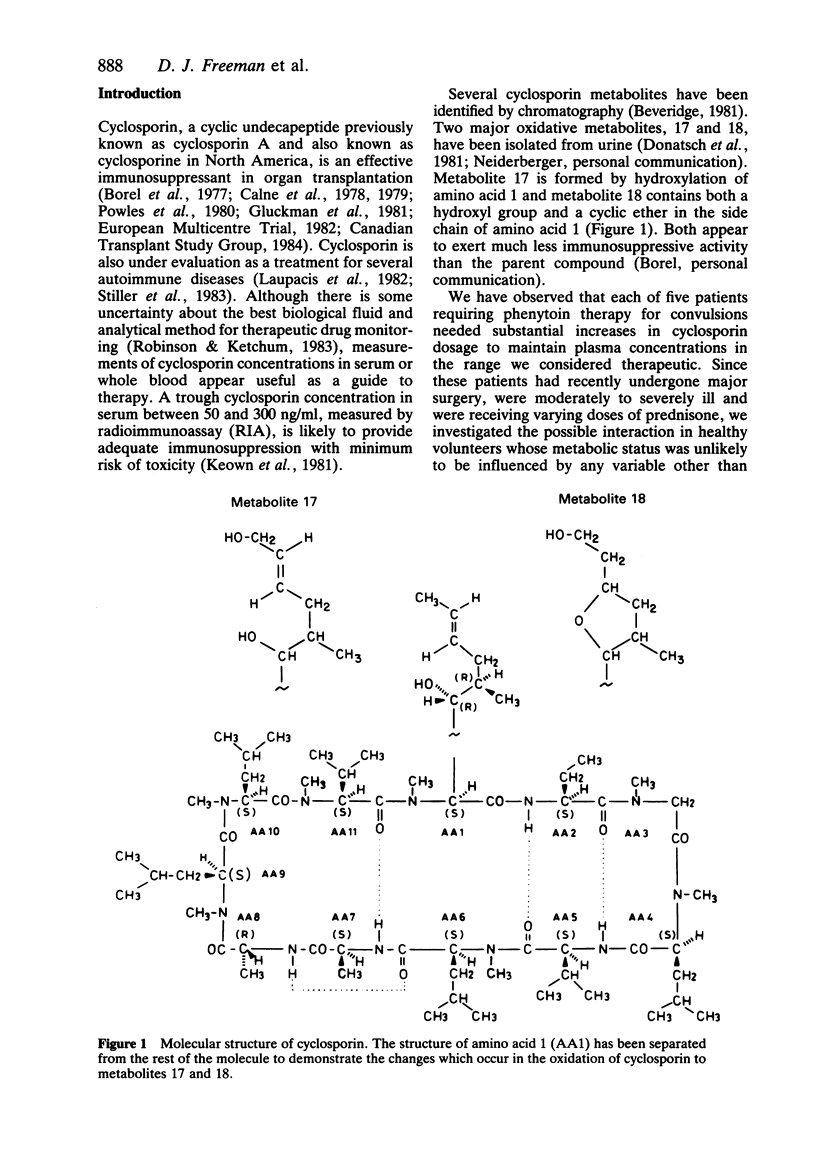


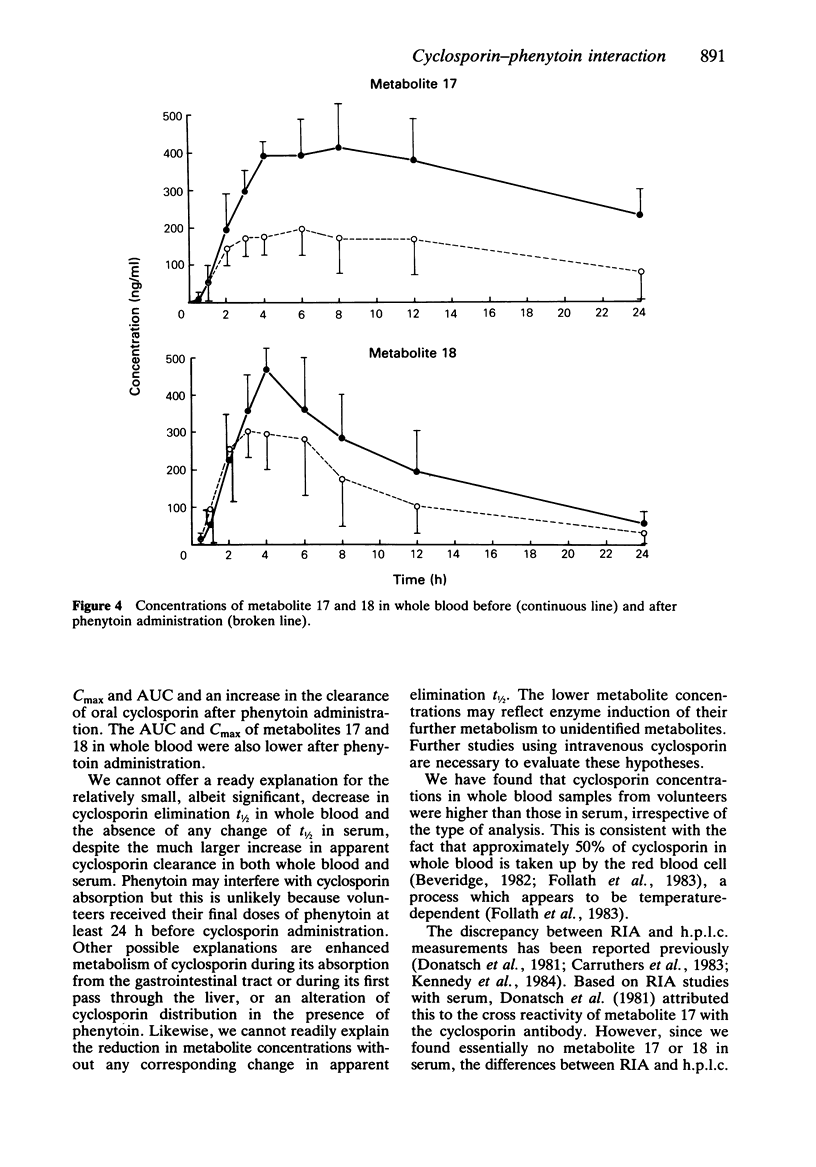
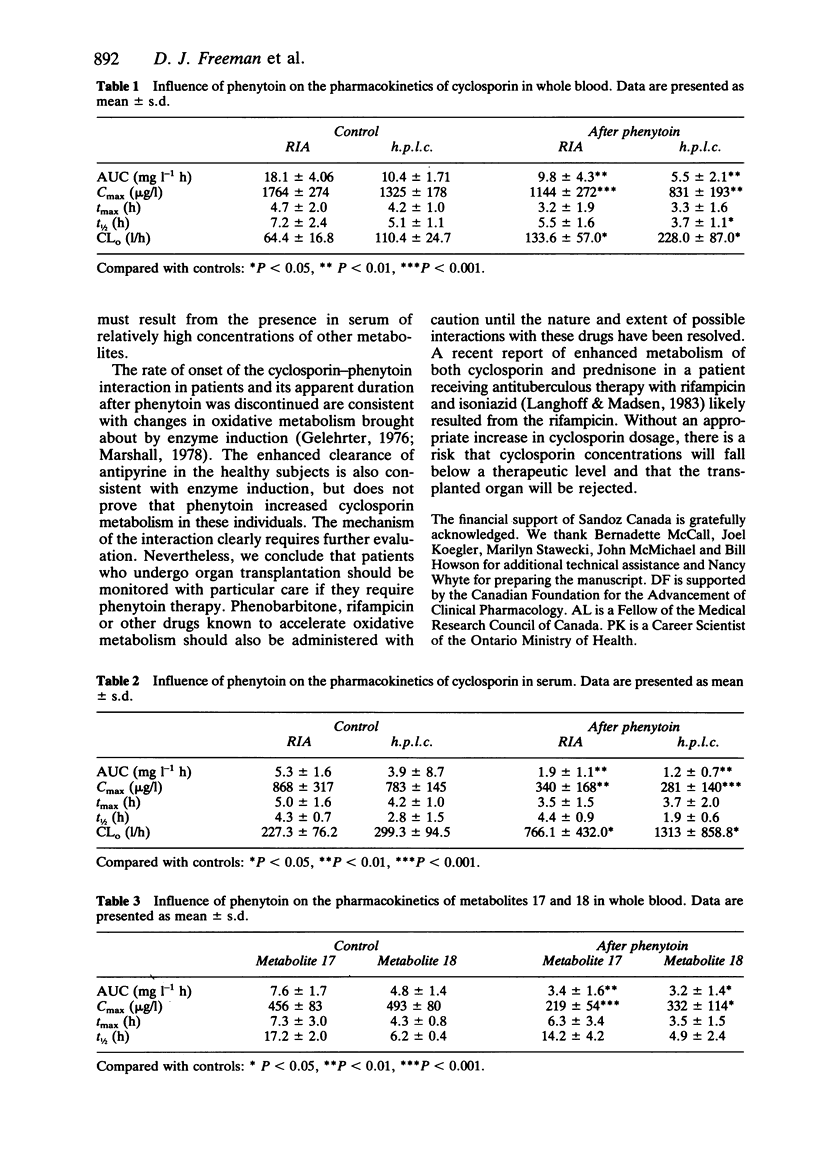

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borel J. F., Feurer C., Magnée C., Stähelin H. Effects of the new anti-lymphocytic peptide cyclosporin A in animals. Immunology. 1977 Jun;32(6):1017–1025. [PMC free article] [PubMed] [Google Scholar]
- Calne R. Y., Rolles K., White D. J., Thiru S., Evans D. B., McMaster P., Dunn D. C., Craddock G. N., Henderson R. G., Aziz S. Cyclosporin A initially as the only immunosuppressant in 34 recipients of cadaveric organs: 32 kidneys, 2 pancreases, and 2 livers. Lancet. 1979 Nov 17;2(8151):1033–1036. doi: 10.1016/s0140-6736(79)92440-1. [DOI] [PubMed] [Google Scholar]
- Calne R. Y., White D. J., Thiru S., Evans D. B., McMaster P., Dunn D. C., Craddock G. N., Pentlow B. D., Rolles K. Cyclosporin A in patients receiving renal allografts from cadaver donors. Lancet. 1978 Dec 23;2(8104-5):1323–1327. doi: 10.1016/s0140-6736(78)91970-0. [DOI] [PubMed] [Google Scholar]
- Carruthers S. G., Freeman D. J., Koegler J. C., Howson W., Keown P. A., Laupacis A., Stiller C. R. Simplified liquid-chromotographic analysis for cyclosporin A, and comparison with radioimmunoassay. Clin Chem. 1983 Jan;29(1):180–183. [PubMed] [Google Scholar]
- Donatsch P., Abisch E., Homberger M., Traber R., Trapp M., Voges R. A radioimmunoassay to measure cyclosporin A in plasma and serum samples. J Immunoassay. 1981;2(1):19–32. doi: 10.1080/01971528108062989. [DOI] [PubMed] [Google Scholar]
- Follath F., Wenk M., Vozeh S., Thiel G., Brunner F., Loertscher R., Lemaire M., Nussbaumer K., Niederberger W., Wood A. Intravenous cyclosporine kinetics in renal failure. Clin Pharmacol Ther. 1983 Nov;34(5):638–643. doi: 10.1038/clpt.1983.226. [DOI] [PubMed] [Google Scholar]
- Gelehrter T. D. Enzyme induction (third of three parts). N Engl J Med. 1976 Mar 18;294(12):646–651. doi: 10.1056/NEJM197603182941206. [DOI] [PubMed] [Google Scholar]
- Gluckman E., Arcese W., Devergie A., Boiron M. Cyclosporin-A prophylactic treatment of graft-versus-host disease in human allogeneic bone marrow transplantation: preliminary results. Transplant Proc. 1981 Mar;13(1 Pt 1):368–370. [PubMed] [Google Scholar]
- Keown P. A., Stiller C. R., Ulan R. A., Sinclair N. R., Wall W. J., Carruthers G., Howson W. Immunological and pharmacological monitoring in the clinical use of cyclosporin A. Lancet. 1981 Mar 28;1(8222):686–689. doi: 10.1016/s0140-6736(81)91971-1. [DOI] [PubMed] [Google Scholar]
- Langhoff E., Madsen S. Rapid metabolism of cyclosporin and prednisone in kidney transplant patient receiving tuberculostatic treatment. Lancet. 1983 Oct 29;2(8357):1031–1031. doi: 10.1016/s0140-6736(83)91019-x. [DOI] [PubMed] [Google Scholar]
- Laupacis A., Keown P. A., Ulan R. A., McKenzie N., Stiller C. R. Cyclosporin A: a powerful immunosuppressant. Can Med Assoc J. 1982 May 1;126(9):1041–1046. [PMC free article] [PubMed] [Google Scholar]
- Marshall W. J. Enzyme induction by drugs. Its relevance to clinical biochemistry. Ann Clin Biochem. 1978 Jan;15(1):55–64. doi: 10.1177/000456327801500115. [DOI] [PubMed] [Google Scholar]
- Niederberger W., Schaub P., Beveridge T. High-performance liquid chromatographic determination of cyclosporin A in human plasma and urine. J Chromatogr. 1980 Jun 13;182(3-4):454–458. doi: 10.1016/s0378-4347(00)81500-5. [DOI] [PubMed] [Google Scholar]
- Powles R. L., Clink H. M., Spence D., Morgenstern G., Watson J. G., Selby P. J., Woods M., Barrett A., Jameson B., Sloane J. Cyclosporin A to prevent graft-versus-host disease in man after allogeneic bone-marrow transplantation. Lancet. 1980 Feb 16;1(8164):327–329. doi: 10.1016/s0140-6736(80)90881-8. [DOI] [PubMed] [Google Scholar]
- Robinson C. A., Jr, Ketchum C. H. Monitoring of cyclosporin A: is it possible? Ther Drug Monit. 1983;5(3):371–372. doi: 10.1097/00007691-198309000-00023. [DOI] [PubMed] [Google Scholar]
- Starzl T. E., Klintmalm G. B., Porter K. A., Iwatsuki S., Schröter G. P. Liver transplantation with use of cyclosporin a and prednisone. N Engl J Med. 1981 Jul 30;305(5):266–269. doi: 10.1056/NEJM198107303050507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starzl T. E., Weil R., 3rd, Iwatsuki S., Klintmalm G., Schröter G. P., Koep L. J., Iwaki Y., Terasaki P. I., Porter K. A. The use of cyclosporin A and prednisone in cadaver kidney transplantation. Surg Gynecol Obstet. 1980 Jul;151(1):17–26. [PMC free article] [PubMed] [Google Scholar]
- Stiller C. R., Laupacis A., Dupre J., Jenner M. R., Keown P. A., Rodger W., Wolfe B. M. Cyclosporine for treatment of early type I diabetes: preliminary results. N Engl J Med. 1983 May 19;308(20):1226–1227. doi: 10.1056/NEJM198305193082012. [DOI] [PubMed] [Google Scholar]


